Research Article
Austin J Cerebrovasc Dis & Stroke. 2014;1(2): 1007.
The Utility of Intracranial Plaque Imaging Using 3-Tesla Magnetic Resonance Imaging for Middle Cerebral Artery Atherosclerosis
Kurisu K1, Shichinohe H1*, Abumiya T1, Kazumata K1, Nakayama N1, Terae S2 and Houkin K1
1Department of Neurosurgery, Hokkaido University Graduate School of Medicine, Japan
2Department of Radiology, Hokkaido University Graduate School of Medicine, Japan
*Corresponding author: Shichinohe H, Department of Neurosurgery, Hokkaido University Graduate School of Medicine, North 15, West 7, Kita-ku, Sapporo, Japan
Received: July 07, 2014; Accepted: July 14, 2014; Published: July 16, 2014
Abstract
Middle cerebral artery atherosclerosis often causes severe ischemic stroke. Plaque imaging techniques for extracranial carotid lesions with conventional magnetic resonance imaging are widely used to prevent subsequent stroke. However, the plaque imaging for intracranial atherosclerosis with the conventional magnetic resonance imaging is limited because of the poor imaging quality. We hypothesized that the use of high resolution magnetic resonance imaging might resolve the problem and the acquired image might be useful to estimate intra-cranial plaque component. Therefore, the aim of this study is to clarify the utility of a plaque imaging technique using high-resolution magnetic resonance imaging in patients with middle cerebral artery atherosclerosis. In 6 patients (mean, 62; range, 36-75), plaque imaging using T1- and T2-weighted black blood (BB) methods were performed with a 3 tesla scanner without a contrast medium. In each patient, the ability to identify any plaque characteristic was assessed, and the plaques were evaluated on the basis of their signal intensity. For result, plaque images were obtained with enough quality to evaluate plaque components in 5 patients. In a symptomatic case, the plaque included a high-intensity lesion in the T1-weighted black blood method, and this was considered a vulnerable plaque. The plaques in the other cases were iso-intensity and were considered non vulnerable plaques. In a representative case, sudden cardiac arrest occurred 6 months after the stroke. Unfortunately, transient global ischemia caused a severe cerebral infarction on the side of the untreated MCA stenosis. Our results showed that intracranial plaque imaging is useful to estimate plaque histology. In future, this method will be helpful to determine the optimal treatment, including endovascular surgery, for these patients.
Keywords: Intracranial plaque imaging; Middle cerebral artery stenosis; Middle cerebral artery atherosclerosis; High-resolution magnetic resonance imaging; Black blood methods
Abbreviations
MCA: Middle Cerebral Artery; MRI: Magnetic Resonance Imaging; HR: High-resolution; TOF: Time-of-flight; BB: Black-blood; TSE: Turbo Spin Echo; TR: Repetition Time; TE: Echo Time; FOV: Field of View; ETL: Echo Train Length; NEX: Number of Excitations
Introduction
It is well known that middle cerebral artery (MCA) atherosclerosis is an important cause of cerebral ischemic stroke [1-3]. It has been reported that subsequent strokes occurred in 1 or 2 years in approximately 25% cases of severe MCA stenosis [4,5]. Thus, the prevention of cerebral ischemic events is important for patients with MCA atherosclerosis.
Plaque imaging techniques that use magnetic resonance imaging (MRI) for imaging extracranial carotid lesions are widely used for subsequent stroke prophylaxis. It is known that plaque characteristics are closely related to clinical presentations and subsequent ischemic events [6-9]. On the basis of these facts, plaque imaging has been the standard for assessing plaque characteristics and for choosing the optimal therapeutic strategy for carotid artery lesions [8,10]. In patients with MCA stenosis, however, plaque evaluations with conventional MRI have been of limited utility because of the low resolution of the vessel walls of small arteries. Although high-resolution imaging with high-field MRI scanners may be expected to enable detailed visualization of the atherosclerotic plaques that are located in intracranial arteries, there have been only a few studies regarding the feasibility of high-resolution MRI (HR-MRI) for the examination of MCA atherosclerosis [11-16]. In the present study, we aimed to clarify the utility of intracranial plaque imaging using HR-MRI in patients with MCA atherosclerosis.
Materials and Methods
Subjects
Intracranial plaque imaging for MCA atherosclerosis was performed in patients who had been diagnosed with MCA stenosis using time-of-flight magnetic resonance angiography (TOF-MRA). For all patients, CTA was also performed to diagnose MCA atherosclerosis and to evaluate degree of stenosis. The subjects in the present study included patients with symptomatic MCAatherosclerosis as well as those with asymptomatic lesions. Routine MRI (T1-, T2-, and diffusion-weighted imaging), MRA, and plaque imaging were performed in all patients who gave informed consent.
Imaging protocol
MRI examinations were performed with a 3T MRI system (Achieva 3.0-T TX series; Philips Medical Systems, Best, The Netherlands) and an 8-channel phased array coil. Both the raw TOF-MRA data and reconstructed blood vessel data were used for determining the position of the subsequent black-blood MRI (BB-MRI) scans. The BB technique with pre-regional saturation pulses of 80-mm thickness was used to saturate incoming arterial flow for all BB-MRI scans. Sagittal and coronal images were acquired in each patient. T1-weighted BB-MRI was acquired with a 2-dimensional turbo spin echo (TSE) sequence with the following imaging parameters: repetition time (TR)/echo time (TE), 500/16 ms; field of view (FOV), 100 × 100 mm; matrix size, 256 × 241; echo train length (ETL), 7; slice thickness, 3 mm; interslice gap, 0.3 mm; number of excitations (NEX), 8; and acquisition time, 2 min 19 s. For T2-weighted BB-MRI scans, the TSE sequence with a TR/TE of 3,000/80 ms was used. Scans were performed with the following parameters: FOV, 100 × 100 mm; matrix size, 256 × 230; ETL, 23; slice thickness, 3 mm; interslice gap, 0.3 mm; and NEX, 8. The scan time was 3 min 6 s for sagittal images and 1 min 33 s for coronal images.
Evaluation of plaque imaging
In each patient, the BB-MRI imaging quality was evaluated on the basis of the method reported by Ryu et al. that used the conspicuity of the vessel margins and lumen as well as the wall architecture [14]. The degree of imaging quality was assessed as follows: good, architecture was visualized in detail and the lumen and outer boundary of the artery were clearly defined; moderate, outer boundary and/or lumen partially obscured; and poor, outer boundary and lumen not identifiable [14].
For images with good or moderate imaging quality, the plaque signal intensity was characterized. Interpretation of the plaque signal intensity was made with reference to the adjacent gray matter of the brain parenchyma. The scans were evaluated by mutual agreement between 2 neurosurgeons (K. K., H. S.) [14,17]. When intraplaque high-intensity was observed in a T1-weighted image, we estimated the lesion to be a vulnerable plaque, including those with a lipid-rich necrotic core or intraplaque hemorrhage, similar to that observed in carotid plaques [10,18,19].
Results
Clinical data
A summary of the clinical data for each case is illustrated in Table 1. The intracranial plaque imaging was performed in 6 patients with MCA atherosclerotic occlusive disease (mean age, 62 years; range, 36-75 years). Three of the 6 patients (50%) had been diagnosed with symptomatic MCA atherosclerosis, and the other patients (50%) had asymptomatic lesions. In the symptomatic cases, 2 patients (Cases 4 and 6) suffered from cerebral infarction, whereas the third patient (Case 3) developed transient ischemic attacks due to stenosis in the horizontal portion of MCA (M1). The lesions were located on M1 in 5 cases and on the insular portion of MCA (M2) in the sixth (asymptomatic) case.
Case
Age/gender
clinical presentation
lesion location
imaging quality
plaque intensity
plaque evaluation
T1WI
T2WI
1
71/F
asymptomatic
Lt M1 stenosis
good
iso
iso~high
nonvulnerable
2
36/F
asymptomatic
Rt M2 stenosis
poor
3
45/F
symptomatic (TIA)
Lt M1 occlusion
moderate
iso
iso
nonvulnerable
4
74/F
symptomatic (infarction)
Lt M1 stenosis
good
iso
iso
nonvulnerable
5
71/F
asymptomatic
Rt M1 stenosis
good
iso
iso
nonvulnerable
6
75/M
symptomatic (infarction)
Rt M1 stenosis
good
high
high
vulnerable
Table 1: Clinical data and magnetic resonance imaging (MRI) findings from 6 patients with middle cerebral artery (MCA) atherosclerosis.
Image quality
In terms of imaging quality, both T1- and T2-weighted images with the BB technique were satisfactory enough to evaluate the plaque components in 5 (83.3%) of the 6 cases. In these, the images of 4 cases were considered as having good quality for the visualization of plaque components. Although the images of a case (Case 3) were assessed as being of moderate quality, motion artifacts might have contributed to the decreased imaging quality. In contrast to the cases with good or moderate quality for visualization, only 1 lesion (Case 2) was assessed as being of poor quality. In this case, the plaque of the MCA atherosclerosis was located on M2.
Imaging evaluation
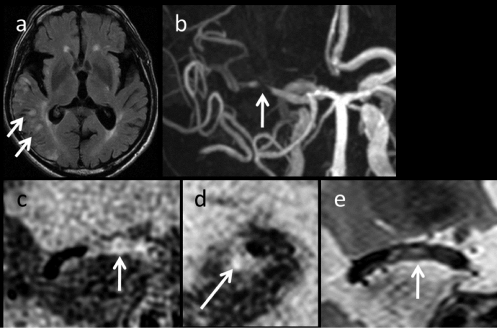
In the 5 cases with good or moderate imaging quality, the obtained images were evaluated. In a symptomatic case (Case 6) who suffered from a cerebral infarction due to artery-to-artery embolism (Figure 1a and 1b) the MCA plaque included a high-intensity lesion in T1- weighted BB scans (Figure 1c and 1d). Because the high-intensity plaque in T1-weighted images was estimated to be an intraplaque hemorrhage, it was evaluated as a vulnerable plaque. In the other cases, an isointense plaque was seen in T1-weighted BB MRI images. They were estimated to be non vulnerable plaques. In T2-weighted images, however, the plaques in the 2 cases were high-intensity (Figure 1e) and those in 3 cases were iso-intensity (Table 1).
Figure 1 : Case 6.Fluid-attenuated inversion recovery (FLAIR) image (a) revealing an acute cerebral infarct as high-intensity lesions (arrows). Magnetic resonance angiography (MRA) (b) revealed severe stenosis in the horizontal portion of the right middle cerebral artery (MCA) (indicated with an arrow). T1-weighted black-blood magnetic resonance imaging (BB-MRI) scans (c: a coronal view, d: a sagittal view) and a coronal view of T2-weighted BB-MRI (e) scans clearly showed a plaque located in the arterial wall of MCA (arrows).
Illustrative case (Case 4)
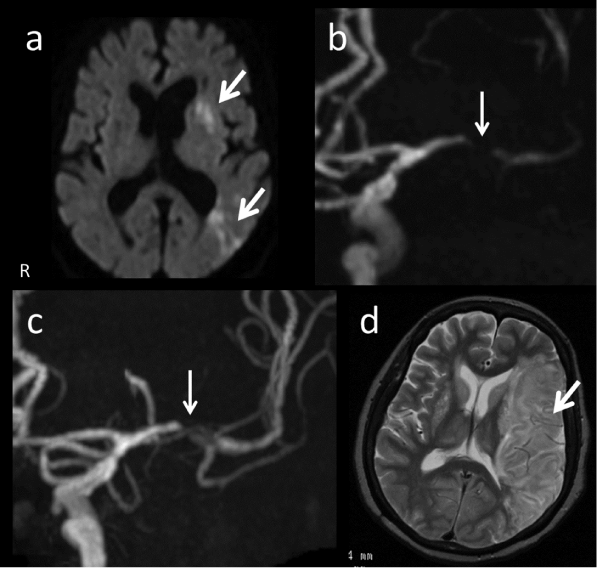
The patient was a 74-year-old woman. She had a sudden onset of speech impairment and left-sided paralysis. Her past history included dyslipidemia and hypertension, and she had taken medication. When the patient visited our hospital, a neurological examination revealed that she suffered from right hemiparesis and total aphasia. In the neuroradiological findings, diffusion-weighted images (DWI) showed an acute cerebral infarct in the left deep white matter (Figure 2a), and MRA revealed a nearly occlusive lesion in left M1 (Figure 2b). Because thrombolysis of the MCA occlusive lesion was indicated, the intravenous infusion of recombinant tissue plasminogen activator was performed, after which her neurological deficits disappeared immediately. However, severe stenosis in left M1 was still observed after the thrombolysis (Figure 2c).
Figure 2 : Case 4. Diffusion-weighted imaging (a) revealed an acute cerebral infarct as a hyper intense lesion in the left deep white matter (arrows). MRA (b) revealed a near occlusion in the horizontal portion (arrow) of the left MCA and the recanalization after thrombolysis (an arrow in c). T2-weighted images (d) after the sudden cardiac arrest revealed a large cerebral infarction in the left hemisphere.
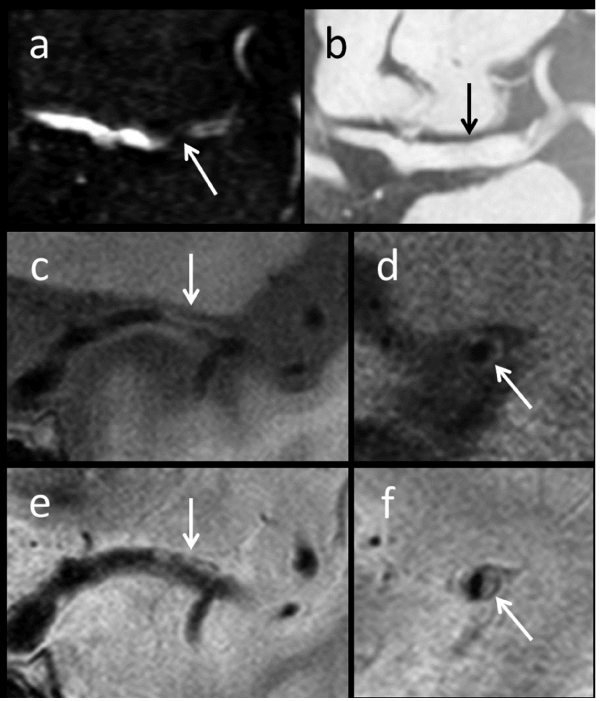
Further examination of the MCA atherosclerosis with HR-MRI was performed in the subacute phase. The lumen of the stenotic lesion in M1 revealed a flow image in the raw image of TOF-MRA, although no information about the arterial wall was obtained (Figure 3a). However, an axial 3-dimensional constructive interference in steady state (3D-CISS) image clearly showed the boundary of the outer membrane of MCA (Figure 3b). Subsequently, we performed plaque imaging with the usage of black-blood technique. Coronal view of T1-weighted (Figure 3c) BB-MRI showed the plaque in arterial wall of MCA so clearly that the degree of imaging qualities was assessed as good. It was observed that the plaque protruded irregularly towards the arterial lumen. Sagittal view of T1-weighted (Figure 3d) BB-MRI at the level of stenosis also demonstrated that the plaque was surrounding the arterial lumen. As for the plaque component, the plaque showed homogenous iso-intensity compared with the adjacent gray matter in both T1- and T2- (Figures 3c, e: coronal view, and 3d, f: sagittal view) weighted BB-MRI. We considered the plaque as non-vulnerable plaque. The patient had antithrombotic medication and discharged about 1 month after the stroke.
Figure 3 : Case 4 (cont.). Raw image of time-of-flight (TOF) MRA (a) showed the lumen of the stenotic MCA (arrow). Three-dimensional constructive interference in steady state (3D-CISS) scan (b) clearly shows the boundary of the outer membrane of MCA (arrow). Coronal views of T1-weighted (c) and T2-weighted (e) BB-MRI scans clearly show the plaque located in the arterial wall of MCA (arrows). Sagittal views of T1-weighted (d) and T2-weighted (f) BB-MRI scans demonstrated the plaque in the arterial wall (arrows).
However, she had sudden cardiac arrest due to an acute myocardial infarction and was transferred to the emergency department of our hospital 6 months after the stroke. Cardiopulmonary resuscitation was successful, but global ischemia occurred during the cardiac arrest. Unfortunately, a large cerebral infarction developed in her left cerebral hemisphere, which was the side of the MCA atherosclerosis (Figure 2d).
Discussion
Our present study demonstrated that the BB technique with 3T MRI was valuable for plaque imaging in patients with MCA atherosclerosis. Our case series showed that intracranial plaque imaging was satisfactory enough to evaluate the plaque components, except only 1 case with a plaque located in M2. In the evaluation of plaque imaging, the plaque in a symptomatic case was diagnosed as vulnerable because of a high-intensity lesion that was revealed in a T1-weighted image; the other cases were diagnosed with non vulnerable plaques.
Atherosclerotic lesions in MCA are an important cause of cerebral ischemic events [1-3]. They have various clinical presentations, which are determined by multiple factors, including not only the degree of stenosis and the status of collateral circulation but also the plaque morphology and pathology [4,15,20]. Recent reports have shown the feasibility of HR-MRI in patients with MCA atherosclerosis for evaluating plaque morphology [11-16]. Ryu et al. have reported that HR-MRI with the BB technique is useful for evaluating the degree of stenosis and plaque components in patients with MCA stenosis [14]. Moreover, Xu et al. [15] have suggested that high intraplaque signal intensity in T1-weighted images indicate intraplaque hemorrhage, which is a well-known predictor of symptomatic ischemic events, such as carotid lesions. In addition, we estimated the lesion with intraplaque high-intensity in a T1-weighted image as a vulnerable plaque that included a lipid-rich necrotic core or intraplaque hemorrhage, which is similar to carotid lesions. It is well known that the plaque imaging of carotid lesions strongly correlates with the histological features [10,21]. Although it was obscure whether the obtained images of MCA plaques really represent their histological features, a recent study of autopsies has revealed that MCA plaques have the same basic structure and components as plaques in other peripheral arteries [18]. Several studies have also suggested that MCA plaques display similar findings to carotid atherosclerotic plaques when observed by HR-MRI [15,22]. Thus, plaque imaging for MCA atherosclerosis is expected to be a new valuable technique for estimating histological features. In the present study, a high-intensity MCA plaque was found in T1-weighted BB-MRI in a symptomatic case. It is natural that the images are considered vulnerable plaques. However, further investigations are required to elucidate the correlations between the radiological and histological features of lesions in MCA atherosclerosis.
Considering the treatments available for MCA atherosclerosis, it is most important to prevent ischemic stroke. In a recent randomized control trial (SAMMPRIS trial) [23], which compared the therapeutic efficiency between aggressive medical management and percutaneous transluminal angioplasty and stenting (PTAS) in 451 patients with MCA stenosis, the risk of stroke was significantly higher in the patients who were treated with PTAS than in those treated with medical therapy. Therefore, the efficacy and safety of interventional treatment have been doubted for patients with MCA stenosis. In the study, however, plaque characteristics were not taken into consideration [23]. Because plaque characteristics are closely related to clinical presentation and subsequent ischemic stroke in carotid plaques, this factor would also be important for patients with MCA atherosclerosis. For example, 1 case in our present study (case 5) had a plaque component that was estimated to be non vulnerable, and it may be considered as low risk for PTAS. Thus, PTAS would be a more favorable treatment to prevent subsequent stroke if the plaque characteristics that were estimated by intracranial plaque imaging are taken into consideration for therapeutic indications, such as with extracranial carotid plaques. Very recently, wingspan stent system (Stryker, Japan) which was developed to treat intracranial atherosclerotic lesion was approved in our country and become available. In future, the establishment of new therapeutic strategies that include intracranial plaque imaging is warranted.
Conclusion
In conclusion, plaque imaging with 3-T MRI and the BB technique was useful to estimate plaque histology in patients with MCA atherosclerosis. Although the question of whether the obtained images correlated with plaque histology in these patients is still unclear, this method may be helpful to determine the optimal treatments for each of these patients.
References
- Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke; a journal of cerebral circulation. 2008; 39: 2396-2399.
- Hallevi H, Chernyshev OY, El Khoury R, Soileau MJ, Walker KC, Grotta JC, et al. Intracranial atherosclerosis is associated with progression of neurological deficit in subcortical stroke. Cerebrovasc Dis. 2012; 33: 64-68.
- Kim SJ, Ryoo S, Kim GM, Chung CS, Lee KH, Bang OY. Clinical and radiological outcomes after intracranial atherosclerotic stroke: a comprehensive approach comparing stroke subtypes. Cerebrovascular diseases. 2011; 31: 427-434.
- Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006; 113: 555-563.
- Kern R, Steinke W, Daffertshofer M, Prager R, Hennerici M. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. 2005; 65: 859-864.
- Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2007; 244: 64-77.
- Saito H, Kuroda S, Hirata K, Magota K, Shiga T, Tamaki N, et al. Validity of dual MRI and F-FDG PET imaging in predicting vulnerable and inflamed carotid plaque. Cerebrovasc Dis. 2013; 35: 370-377.
- Mono ML, Karameshev A, Slotboom J, Remonda L, Galimanis A, Jung S, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis. 2012; 34: 343-350.
- Prati P, Tosetto A, Casaroli M, Bignamini A, Canciani L, Bornstein N, et al. Carotid plaque morphology improves stroke risk prediction: usefulness of a new ultrasonographic score. Cerebrovascular diseases. 2011; 31: 300-304.
- Gao T, Zhang Z, Yu W, Zhang Z, Wang Y. Atherosclerotic carotid vulnerable plaque and subsequent stroke: a high-resolution MRI study. Cerebrovasc Dis. 2009; 27: 345-352.
- Chung GH, Kwak HS, Hwang SB, Jin GY. High resolution MR imaging in patients with symptomatic middle cerebral artery stenosis. Eur J Radiol. 2012; 81: 4069-4074.
- Li ML, Xu WH, Song L, Feng F, You H, Ni J, et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3T. Atherosclerosis. 2009; 204: 447-452.
- Niizuma K, Shimizu H, Takada S, Tominaga T. Middle cerebral artery plaque imaging using 3-Tesla high-resolution MRI. J clin neurosci. 2008; 15: 1137-1141.
- Ryu CW, Jahng GH, Kim EJ, Choi WS, Yang DM. High resolution wall and lumen MRI of the middle cerebral arteries at 3 tesla. Cerebrovasc Dis. 2009; 27: 433-442.
- Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010; 212: 507-511.
- Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009; 72: 627-634.
- Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz MI. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J neuroimaging. 2011; 21: 159-161.
- Chen XY, Wong KS, Lam WW, Zhao HL, Ng HK. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis. 2008; 25: 74-80.
- Degnan AJ, Gallagher G, Teng Z, Lu J, Liu Q, Gillard JH, et al. MR angiography and imaging for the evaluation of middle cerebral artery atherosclerotic disease. AJNR Am J Neuroradiol. 2012; 33: 1427-1435.
- Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alters stroke risk in intracranial atherosclerosis. Ann Neurol. 2011; 69: 963-974.
- Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005; 112: 3437-3444.
- Klein IF, Lavallée PC, Touboul PJ, Schouman-Claeys E, Amarenco P. In vivo middle cerebral artery plaque imaging by high-resolution MRI. Neurology. 2006; 67: 327-329.
- Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 365: 993-1003.