Case Report
Austin J Cerebrovasc Dis & Stroke. 2014;1(2): 1008.
Mitral Valve Mass Presenting as Recurrent Ischemic Strokes
Kimbrough TA1, Sherma AK2 and Martin-Schild S21*
1Department of Medicine, Tulane University, USA
2Department of Neurology, Tulane University Hospital, USA
*Corresponding author: Martin-Schild S, Department of Neurology, Stroke Program, Tulane University Hospital, 1430 Tulane Avenue #8065, New Orleans, LA 70112, USA
Received: June 28, 2014; Accepted: July 22, 2014; Published: July 24, 2014
Abstract
Mitral valve masses are an important cause of ischemic stroke in young individuals. The differential diagnosis for valvular cardiac mass includes infective endocarditis, sterile endocarditis and primary cardiac tumors. Given the high risk of recurrent ischemic events with mitral valve pathology, prompt identification and treatment is necessary in order to minimize significant longterm neurological sequelae. Here we present the case of a 28 year-old woman with a past medical history of mitral valve myxoma status-post surgical resection found to have a new mitral valve mass of unclear etiology upon recurrent bilateral ischemic stroke.
Keywords: Cardiac myxoma; Mitral valve mass; Recurrent ischemic stroke
Abbreviations
TTE: Transthoracic Echocardiogram; TEE: Transesophageal Echocardiogram; tPA: Tissue Plasminogen Activator; ANA: Antinuclear Antibody; PAS: Periodic Acid-Schiff stain; AFP: Acid- Fast Stain
Case Presentation
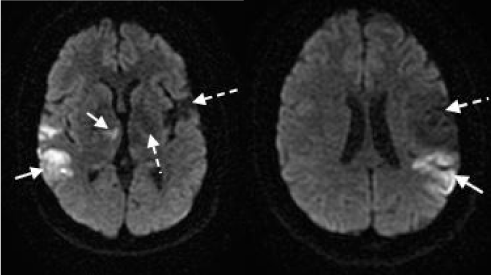
A 28-year-old woman with a history of four prior ischemic events and mitral valve myxoma status-post surgical resection presented to our facility with sudden onset severe aphasia, dysarthria and disconjugate gaze. The patient was given intravenous tissue plasminogen activator 175 minutes from last seen normal. Subsequent MRI revealed acute bilateral ischemic infarcts involving the right thalamus, right parietal lobe and left posteroparietal region, along with gliosis involving the left thalamus, right occipital region and left middle cerebral artery distribution consistent with patient’s known prior infarcts (Figure 1).
Figure 1 : Bilateral ischemic infarcts on diffusion-weighted MRI. Acute ischemic infarcts can be seen involving the right thalamus, right occipital region and left posteroparietal region (solid white arrows). Gliosis consistent with known prior infarcts can also be visualized in the left thalamus and involving a large section of the left middle cerebral artery distribution (dashed white arrows).
This patient initially presented to our facility in 2009 at age 23 with right-sided weakness and aphasia. At that time, the patient was diagnosed with an acute ischemic infarct secondary to an occlusion of the anterior temporal branch of the left middle cerebral artery as identified on formal catheter angiogram. The patient was also noted to have two remote infarcts involving the left thalamus and right occipital region, of which the patient was unaware but had experienced referable symptoms without formal diagnosis. The patient had no known family history of stroke, vasculitis or cardiac pathology, and denied any personal medical history or use of tobacco/intravenous drugs. A full stroke work-up at the time of initial admission, including transthoracic echocardiogram, transesophageal echocardiogram, catheter angiography, CT angiography and a hypercoagulability workup failed to elucidate a clear etiology. Due to the strong suspicion of an underlying cardio embolic source, the patient was discharged on warfarin along with maximal standard medical therapy for secondary stroke prevention.
The patient was again admitted to the hospital 14 months after her initial presentation with new onset left-sided weakness and worsening aphasia. The patient was treated with intravenous tPA, which was halted upon return of the patient’s INR at 3.1. No adverse effects were noted secondary to administration of tPA in the context of the patient’s elevated INR. Her deficits resolved completely. Transthoracic echocardiogram revealed a mass on the mitral valve with mild mitral regurgitation; of note, there was no evidence of an intra cardiac mass on TTE six months prior to admission. Followup TEE visualized a 3.3 x 4.4 cm non-pedunculated mass on the atrial side of the posterior leaflet of the mitral valve consistent with a mitral fibroelastoma. The differential diagnosis for the mass included mitral valve myxoma, valvular lipoma, hematic cyst, Libman-Sacks endocarditis, maranteric endocarditis and subacute infective endocarditis. The patient was afebrile throughout her hospital admission and multiple blood cultures were negative. Repeat hypercoagulability workup, including ANA, was again negative. A chest/abdomen/pelvis computed tomography scan was negative for extra cardiac masses.
Given the patient’s presentation of recurrent ischemic stroke while supratherapeutic on coumadin, the patient was deemed to be at high risk for future ischemic insults and elected to undergo surgical removal of the mass. The operation revealed multi centric disease, with a larger 3x4 mm mass flanked by two smaller masses on the posterior leaflet of the mitral valve. Inspection of the anterior leaflet and atrial cavity revealed no further abnormalities. Histopathology of the samples was consistent with a diagnosis of mitral myxoma. Following the surgical resection of the mitral myxoma, the patient was taken off anticoagulation therapy and was followed with serial transthoracic echocardiograms which were negative for intra cardiac masses three years postoperatively.
Six months prior to the patient’s most recent admission, she presented to the emergency department with severe nausea and vomiting upon ingestion of food or medications. Per patient, her symptoms persisted for 2-3 months with only mild symptomatic relief from anti emetics. Retrospectively, the patient’s clinical presentation is concerning for possible chronic mesenteric ischemia secondary to subacute cardioembolic occlusion. Patient also endorsed intermittent palpitations for several months and had been referred for a Holter monitor and repeat echocardiography shortly before admission.
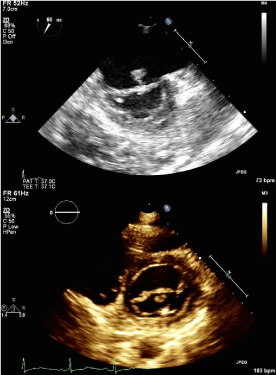
Following her current admission with a diagnosis of acute bilateral ischemic stroke, TTE revealed a mobile pedunculated 8x8 mm mass on the atrial aspect of the anterior mitral valve leaflet; diagnosis was confirmed with TEE (Figure 2). The patient was immediately placed on a heparin drip and was taken to surgery eight days after admission for excision of the suspected recurrent mitral valve myxoma. A 12 mm sessile mass was excised from the mid portion of the anterior leaflet. Intraoperative frozen section and follow-up permanent section returned as fibrinopurulent vegetation without evidence of myxoid stroma. Tissue bacterial culture was negative for aerobic and anaerobic organisms. Initial PAS and AFB stains were also negative; final cultures were pending at time of publication. The patient had no evidence of active infection throughout her hospital stay and blood cultures were negative. The patient’s anti-ß2 glycoprotein I IgG antibody level was found to be newly elevated at 54units/ ml from the single prior level of 13units/ml in 2009. The patient’s Russell viper venom test, anti-cardiolipin IgG/IgM/IgA antibodies and phosphatidylserine IgG/IgM/IgA antibodies were within normallimits.
Figure 2 : Visualization of intracardiac mass attached to atrial side of anterior leaflet of mitral valve. In panel a, a pedunculated 8x8 mm mass attached to the atrial aspect of The mitral valve can be visualized via transesophageal echocardiogram. The mass can be seen in cross-section on transthoracic echocardiogram in the parasternal short axis view (panel b).
The patient tolerated the procedure without complications and was discharged to inpatient rehabilitation seven days postoperatively. At the time of publication, the patient continued to experience significant residual expressive aphasia, right sided hemianesthesia and neuropathic pain, and mild right sided hemiparesis.
Discussion
Valvular cardiac masses are a rare but important cause of stroke, notably in young patients [1,2]. The differential diagnosis for cardiac masses includes primary cardiac tumors (myxoma, fibroelastoma, lipoma, hematic cysts, sarcoma), infective endocarditis and sterile endocarditis (Libman-Sacks endocarditis, maranteric endocarditis), with papillary fibroelastoma being the most common non-infectious etiology [1,3-6]. While myxomas are overall the most common primary cardiac tumor, the majority of myxomas are located intraatrial. Valvular myxomas are rare, with an estimated incidence of 1.5-6.1% [5,7-11]. In their respective literature reviews, Sadeghi et al. documented 25 cases of mitral valve myxoma from 1974-2000, with Yuan documenting 55 cases from 2006-2011 [7,9]. Patients with mitral valve masses appear to be at relatively increased risk for thromboembolic events secondary to the dynamic movement of the valvular leaflets resulting in chronic instability and agitation of the valvular mass, with associated increased risk of both tumor fragment embolization and embolization of adherent thrombi [3,4,6,8,11,13]. Urgent surgical resection is the treatment of choice for all intra cardiac masses, as anticoagulation is not entirely protective against recurrent cardioembolic events (as seen in this case) due to the risk of tumor fragment embolization [1-8,10,13].Common neurological complications of cardiac myxomas and other types of benign valvular masses (notably papillary fibroelastoma) include ischemic infarct/transient ischemic attack, syncope, headache and seizures; other sequelae include myocardial infarction, congestive heart failure and sudden death [1,2,5,6,8,9,12]. The lifetime risk of ischemic stroke in untreated myxoma patients has been estimated as high as 82% [2]. Recurrence rates for cardiac myxoma vary widely in the literature, ranging between 0.4-7% for sporadic myxoma and up to 25% for familial myxoma and patients with Carney complex, which this patient had no evidence of [1,2,5,8,9,12]. The average time to recurrence was 3.9 years upon Sadeghi et al. literature review, although recurrences have been reported up to 14 years postoperatively [7]. Of note, the Sadeghi review did identify multi centric disease upon initial resection, such as seen in this case, as a risk factor for tumor recurrence [5,7].
Due to the high risk of recurrent ischemic events in patients with mitral valve masses, a high index of suspicion must be maintained when presented with a young patient with acute stroke or transient ischemic attack. In addition to the standard stroke work-up and a hypercoagulability panel, young patients without a clear etiology should receive a transesophageal echocardiogram secondary to the risk of missing a mass <5 mm with standard transthoracic echocardiogram [1,2,9,10]. The timing of surgical resection and the use of preoperative anticoagulation is controversial in patients presenting with an acute ischemic event. However, in a stroke patient without additional risk factors for hemorrhagic conversion, we believe that every effort should be made to decrease the patient’s risk of potentially disabling to fatal recurrent embolic events by maintaining the patient on anticoagulation until the patient can be taken for definitive treatment via surgical mass excision. Valvuloplasty and annuloplasty following mass excision is preferred over valvular replacement in order to avoid lifelong anticoagulation in this relatively young patient population [7,9,10]. Postoperatively, patients should be monitored for recurrence with yearly transesophageal echocardiogram [1,2,7,9,10,12].
In summary, we present a case of a young woman with recurrent cryptogenic embolic ischemic strokes who was diagnosed with a mitral valve mass. Four years following resection, with normal yearly surveillance TTE, she experienced another embolic stroke and recurrence of the mitral valve mass was detected. The work-up of recurrent embolic stroke should include TEE, particularly in young stroke patients. A mitral valve mass is a very important biomarker for risk of cardioembolic stroke and systemic embolization and requires invasive intervention to prevent recurrence. Due to the low incidence of mitral valve myxoma and low recurrence rate, thereis no evidenced-based recommendation for the interval between surveillance cardiac imaging examinations, but TEE is likely necessary to identify recurrence while asymptomatic.
References
- O'Rourke F, Dean N, Mouradian MS, Akhtar N, Shuaib A. Atrial myxoma as a cause of stroke: case report and discussion. CMAJ. 2003; 169: 1049-1051.
- Ekinci EI, Donnan GA. Neurological manifestations of cardiac myxoma: a review of the literature and report of cases. Intern Med J. 2004; 34: 243-249.
- Colucci V, Alberti A, Bonacina E, Gordini V. Papillary fibroelastoma of the mitral valve. A rare cause of embolic events. Tex Heart Inst J. 1995; 22: 327-331.
- Edwards FH, Hale D, Cohen A, Thompson L, Pezzella AT, Virmani R. Primary cardiac valve tumors. Ann Thorac Surg. 1991; 52: 1127-1131.
- Martìn-Suàrez S, Botta L, Dell'Amore A, Camurri N, Mikus E, Leone O, et al. Mitral valve myxoma involving both leaflets. Cardiovasc Pathol. 2007; 16: 189-190.
- Keeling IM, Oberwalder P, Anelli-Monti M, Schuchlenz H, Demel U, Tilz GP, et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg. 2002; 22: 971-977.
- Cetin G, Gursoy M, Ugurlucan M, Uzunhasan I, Hatemi AC, Tireli E, et al. Single-institutional 22 years experience on cardiac myxomas. Angiology. 2010; 61: 504-509.
- Sadeghi N, Sadeghi S, Karimi A. Mitral valve recurrence of a left atrial myxoma. Eur J Cardiothorac Surg. 2002; 21: 568-573.
- Yoon JH, Kim JH, Sung YJ, Lee MH, Cha MJ, Kang DY, et al. Cardiac myxoma originating from the anterior mitral valve leaflet. J Cardiovasc Ultrasound. 2011; 19: 228-231.
- Yuan SM. Mitral valve myxoma: clinical features, current diagnostic approaches, and surgical management. Cardiol J. 2012; 19: 105-109.
- Choi BW, Ryu SJ, Chang BC, Choe KO. Myxoma attached to both atrial and ventricular sides of the mitral valve: report of a case and review of 31 cases of mitral myxoma. Int J Cardiovasc Imaging. 2001; 17: 411-416.
- Murphy DP, Glazier DB, Krause TJ. Mitral valve myxoma. Ann Thorac Surg. 1997; 64: 1169-1170.
- Aroca A, Mesa JM, Dominguez F, Oliver JM, Ramirez U, Centeno JE. Multiple recurrence of a "sporadic" (non-familial) cardiac myxoma. Eur J Cardiothorac Surg. 1996; 10: 919-921.