Review Article
Austin J Cerebrovasc Dis & Stroke. 2014;1(3): 1012.
Stem Cell Therapy for Spinal Cord Injury: Cellular Options
Adeeb N1, Hose N1, Tubbs RS2 and Mortazavi MM2*
1Department of Neurological Surgery, University of Washington-Harborview Medical Center, USA
2Pediatric Neurosurgery, Children’s of Alabama, USA
*Corresponding author: Mortazavi MM, Department of Neurological Surgery, University of Washington- Harborview Medical Center, 325 Ninth Ave, Box 359766, Seattle, WA 98104, USA
Received: July 15, 2014; Accepted: August 15, 2014; Published: August 19, 2014
Abstract
Spinal cord injury is one of the most debilitating conditions, which mostly affects relatively young individuals. Following the initial insult, secondary pathologic processes including the vascular and inflammatory cascades lead to permanent cellular disruption, causing an irreparable damage. For the last two decades, replacement of the lost cellular and supporting elements at the site of the injury have become a major topic of spinal cord regenerative research. Various types of stem cells have been used as sources for these elements. However, despite the extensive advancement in the isolation, expansion, and transplantation of these stem cells, and the promising functional outcomes in animal models, they are yet to be widely used in humans, and more studies are needed to determine their safety and efficacy. In this review, we will discuss the major different types of stem cells used in SCI.
Introduction
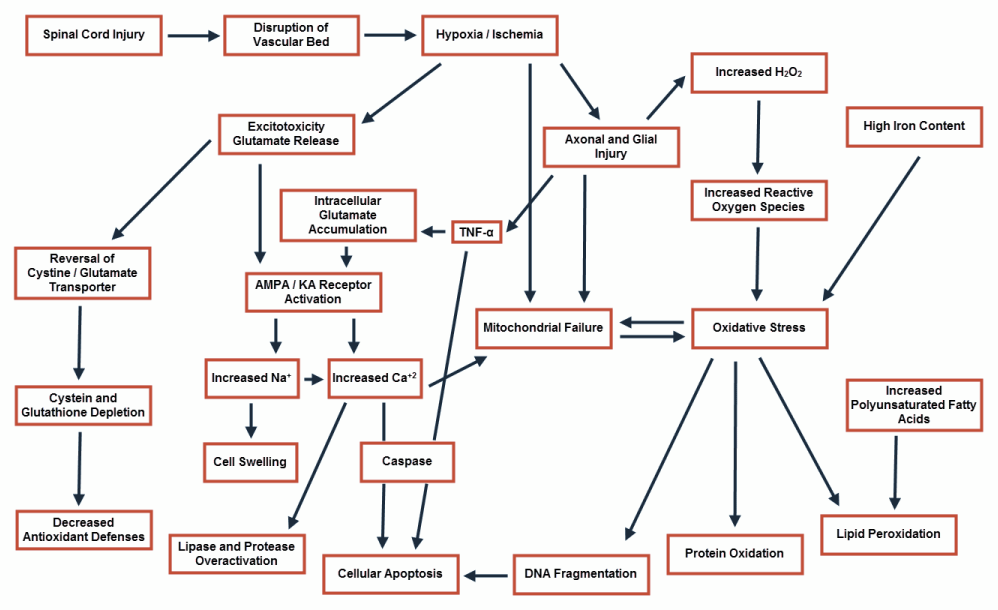
Spinal cord injury (SCI) is one of the most prevalent disabling conditions in the world. It has an annual incidence of 15-40 cases per million [1]. It most commonly affects young, and otherwise healthy, patients [2]. SCI has been divided into four types, based on the gross anatomic findings: 1- Solid cord injury, the least common type, is associated with normal appearance of the spinal cord following injury; 2- Contusion/cavity, the most common type, is associated with areas of hemorrhage and expanding necrosis and cavitation, but with no disruption of the surface of the spinal cord; 3- Laceration, where there is a clear-cut disruption of the surface anatomy; and 4- Massive compression, where the cord is macerated or pulpified to a variable degree [3]. However, such gross findings carry no significant difference in the consequent histological changes [4], which are the major determinant of the post-traumatic functional impairment. These histological changes are divided into two phases, primary and secondary. The primary phase results from the direct traumatic effect of the insult on the neural and vascular structures of the spinal cord. This phase is followed by a more insidious and deleterious phase, the secondary phase. It is associated with ischemic and inflammatory changes, leading to cellular necrosis and apoptosis, scar formation, and prolonged Wallerian degeneration [4]. (Figure 1) These changeshave become a major area of research, intending to identify possible therapeutic targets to interrupts this sequence of events. Moreover,within the last two decades, filling the post traumatic cavitation of the spinal cord with the lost tissue elements derived from stem cells has become one of the main pillars of spinal cord regeneration studies.
Figure 1 : Mechanism of cellular damage following spinal cord injury.
Stem cells
Stem cells are non-differentiated cells with high proliferation and differentiation potentials, and with self-renewal and regenerative capability. Stem cells are classified according to their potency into totipotent, pluripotent, multipotent, oligopotent, and unipotent, based on how many types of differentiated cells, and of what germ layers, the stem cells are capable to produce [5]. Of these different types of stem cells, embryonic stem cells (ESCs) and adult stem cells (ASCs) have received most attention. The ESCs are pluripotent cells that arise from the inner cell mass of the blastocyst during early stage of development. They may give rise to any type of cells from the yetto- be formed three germ layers: ectoderm, mesoderm, and endoderm. After tissue differentiation in children and adults, most of these tissues retain a population of stem cells that give rise to the specific line of cells of the retaining organ. These cells are named the ASCs. For unknown reason, ASCs, do not exist in some tissues including brain (exist in some subependymal areas and hippocampus and a few other areas described below), spinal cord, heart, and kidneys, with minor exceptions [5,6].
For long time, the process of tissue differentiation into adult somatic cells was considered unidirectional, and pluripotent stem cells could only be obtained from specific sites, including developing embryos and adult bone marrow. In 2006, Takahashi and Yamanaka [7] were able to go back in cellular time by reprogramming adult somatic cells into pluripotent cells similar to the ESCs using defined pluripotency-related transcription factors (i.e. Oct3/4, Sox2, c-Myc, and Klf4). These newly formed pluripotent stem cells were called the induced pluripotent stem cells (iPSCs) [8]. However, despite the extensive, rapid, and important advancements associated with these cells, their generation has been associated with increased chromosomal aberrations and genetic deletions, and therefore, higher risk of carcinoma [7].
Cellular transplantation
Stem cells at different stages of maturation have been used for cellular transplantation. These cells have mainly included the neural stem cells, oligodendrocytes and their progenitors, motor neurons and their progenitors, mesenchymal stem cells, and bone marrow stem cells (Table 1).
Neural stem cells
Neural stem cells (NSCs) are multipotent cells that are able to differentiate into neuronal and non-neuronal cells (e.g. oligodendrocytes and astrocytes), and thus, can replace multiple lost elements at the site of SCI. The sources of human NSCs are divided into embryonic and adult depending on the development level at the time of isolation. Embryonic sources include the fetal neural tissue and the ESCs. The adult sources include the adult neural tissue and the iPSCs.
Human NSCs derived from fetal neural tissue
The most significant breakthrough in human NSCs isolation from fetal neural tissue was made by Svendsen et al. [9,10]. For the first time, these stem cells were capable of migration and differentiation into neurons and glial cells (oligodendrocytes and astrocytes) after transplantation into rat brains. Thereafter, multiple studies have attempted to isolate these cells from fetal brain [11-13] and spinal cord [14], and enhance their expansion, maturation, and survival using various neurotrophic factors, including epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), leukemia inhibitory factor (LIF), cilliary neurotrophic factor (CNTF), and brain-derived growth factor (BDGF).
Transplantation of these human NSCs has been carried out on animal models of SCI, including mice [15], rats [16,17], and common marmosets [18]. It has not applied on humans. In all these studies, cellular transplantation was performed during acute stage of injury (less than 2 weeks), and was associated with signs of cellular proliferation and migration, with significant gradual functional improvement.
Human NSCs derived from adult neural tissue
Adult sources for human NSCs isolation include the subependymal zone and the hippocampus. Other areas may also include the spinal cord, striatum, and neocortex. Proliferation of these cells has been induced using the EGF and bFGF. Withdrawal of these factors leads to NSCs differentiation [19]. Following transplantation into rats during acute stage of SCI, the NSCs were able to migrate and differentiate into neurons and glial cells, with significant locomotor recovery [19,20]. During chronic stage of SCI (after 8 weeks), no signs of functional improvement was recorded [20], which supports the therapeutic window theory. No trials were conducted on humans.
The therapeutic window theory proposes the presence of a window period at early stages of SCI, before more deleterious secondary changes progress. These changes will progressively limit the cellular migration to the site of injury, and suppresses their maturation and differentiation, and hence, results in a very limited functional improvement [21-23].
Human NSCs derived from ESCs and iPSCs
Isolation and transplantation of human NSCs derived from ESCs and iPSCs has only scantly been reported in the literature, probably due to the complexity of the procedure, and presence of other less complicated source for NSCs, with similar outcomes. In all the reported studies, transplantation of these cells into mice [24,25] and rats [26] spinal cord was performed at acute stage of injury, and resulted in cellular migration and differentiation, and significant functional improvement. No studies were reported on humans.
Oligodendrocytes and motor neurons
SCI is associated with significant loss of motor neurons (MNs), oligodendrocytes (OLs), and their progenitors. This loss is a major contributing factor to the functional deterioration following injury. Therefore, replacement of these elements has become a main research target. In most studies, human OLs and MNs are derived from ESCs, and few studies have recently isolated these cells from iPSCs.
Transplantation of these MNs [27,28] and OLs [21,29-32] into animal models of SCI at acute stage of injury was associated with effective migration and integration, and resulted in significant locomotor improvement. No studies were conducted on human.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent stem cells that can be derived from the bone marrow and umbilical cord in adults and neonates, respectively. They are able to differentiate into any cell of the mesenchymal linage, including osteoblasts, chondrocytes, adipocytes, and stroma. They are also able to Trans-differentiate into cells of endodermal and ectodermal lineages such as hepatocytes and neurons, respectively [33,34]. These mesenchymal cells have the capacity to increase tissue preservation and decrease cyst and injury size [35]. They are also capable of producing various neurotrophic factors that promotes neuronal survival and functional recovery. Moreover, MSCs constitute an autologous source for stem cell engraftment, and can be expanded from relatively small amounts of bone marrow aspirates, and therefore represents an attractive source for cellular transplantation [33,34]. There was also no evidence of tumor formation following MSCs transplantation.
Pal et al. [36] have administered autologous bone marrow MSCs (BM-MSCs) intrathecally into 30 patients with chronic complete SCI. Patients were divided into two groups based on the time of injury as less or more than 6 months. In patients with less than 6 months of injury, minimal improvement of motor and sensory functions was noticed, but was not sufficient to elicit positive motor and somatosensory evoked potentials, or to produce MRI changes. Patients, however, had improvement of daily activities and quality of life, and recovery of bladder and bowel sensation and control. In patients with more than 6 months of injury, no improvement was detected.
Kishk et al. [37] have also used intrathecal route for BM-MSCs administration in 43 patients with complete and incomplete SCI of subacute and chronic duration (less than 6 months). Over a period of 6 months, only minimal sensory and motor improvement was noticed, including bladder and bowel control, compared to control group.
Bhanot et al. [35] have used a combination of intrathecal followed by intramedullary BM-MSCs administration in 13 patients with chronic complete SCI of more than 8 weeks duration. Over 1 year of follow-up, only 1 patient had slight motor recovery, 2 patients had patchy improvement in pin prick sensation below the level of injury, and 1 patient subjectively developed sensation of bladder fullness.
Park et al. [38] have used intramedullary route for BM-MSCs administration in 10 patients with subacute and chronic SCI of more than 4 weeks duration. Their method included multiple intramedullary injections, 2 above the cavity and 3 into the cavity, and using the fibrin glue to seal the site of injection. Over 6 months of follow-up, 7 patients with complete SCI had no functional or MRI improvement. The remaining 3 patients with incomplete SCI, showed motor improvement, and were followed for another 30 months. Over this period, these patients had remarkable motor and functional recovery. For the first time, electrophysiological study, assessed by motor and somatosensory evoked potentials, and MRI, showed significant improvement. This promising result, compared to previous studies might be related to many factors, including the presence of incomplete SCI in patients with functional recovery, and the use of direct intramedullary route with multiple injections into the site of injury.
Bone marrow stem cells
The bone marrow stem cells (BMSCs) are used as an entire bone marrow aspirate containing a mixed population of cells, including MSCs, hematopoietic stem cells, endothelial progenitor cells, macrophages, lymphocytes, and marrow stromal cells. This combination provides the injured spinal cord with the established neuroprotective effect of its components [39-42]. They also have the advantages of being autologous and have low teratogenicity. Cellular administration can be done through intrathecal, intramedullary, intravenous, or intra-arterial routes.
Park et al. [43] have used intramedullary administration of autologous BMSCs, combined with granulocyte macrophage-colony stimulating factor (GM-CSF) in 6 patients with acute complete SCI of less than 2 weeks duration (one patient received only GM-CSF). GM-CSF induces growth of hematopoietic stem cells, and prevents cellular apoptosis of hematopoietic and neuronal cells. It also enhances BMSCs survival within the spinal cord, and induces excretion of neurotrophic cytokines, including BDGF. Four patients (including the one with GM-CSF only) showed significant motor and sensory recovery, 3 to 7 months after transplantation.
Yoon et al. [44] have used combined intramedullary administration of BMSCs and GM-CSF in 35 patients with complete SCI. Patients were divided into three groups, based on timing of transplantation: acute (less than 2 weeks), subacute (2-8 weeks), and chronic (more than 8 weeks). Over 10 weeks of follow-up, moderate functional recovery was noticed in acute and subacute SCI groups.
Sykova et al. [45] have used BMSCs transplantation via either intravenous or intra-arterial routes in 20 patients with acute and chronic complete SCI. Over 3 months of follow-up, only minimal motor and sensory recovery was noticed in 5 out of 7 acute patients and in 1 out of 13 chronic patients. These patients were mainly from the intra-arterial group.
Geffner et al. [46] have used different routes of BMSCs transplantation in 8 patients with acute and chronic complete SCI. These routes include intramedullary, intrathecal, and intravenous. Over two years of follow-up, minimal functional recovery was noticed mainly in patients with acute SCI.
Conclusion
From the previous studies, we can notice that despite the promising results in animals, cellular transplantation of NSCs, OLs, and MNs in humans with SCI is strictly limited, due to the fear of immunologic reaction, rejection, and tumor formation. On the other hand, the use of autologous MSCs and BMSCs represent a safe, feasible, and reliable method of cellular transplantation for SCI treatment, and hence, are the main source of transplant applied in humans. However, in addition to the rarity of these clinical trials, there is a significant variation in the methods applied, and controversial outcomes are sometime reported, which makes it difficult to compare their results. More studies with longer follow-ups are definitely needed, and a standardized method for reporting functional outcome should also be applied.
References
- Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol. 2010; 6: 363-372.
- Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S, et al. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004; 15: 415-436.
- Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004; 21: 429-440.
- Mortazavi MM, Verma K, Harmon OA, Griessenauer CJ, Adeeb N, Theodore N, et al. The microanatomy of spinal cord injury: A review. Clin Anat. 2014.
- Cherian E, Nandhini G, Kurian A. Stem Cells. 1 ed: Jaypee Brothers Medical Pub.; 2011.
- Kiessling AA. Human Embryonic Stem Cells: An Introduction to the Science and Therapeutic Potential: Jones and Bartlett Publishers, Inc.; 2003.
- Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011; 11: 268-277.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126: 663-676.
- Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, et al. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol. 1997; 148: 135-146.
- Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998; 85: 141-152.
- Carpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, et al. In vitro expansion of a multipotent population of human neural progenitor cells. Exp Neurol. 1999; 158: 265-278.
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999; 156: 71-83.
- Zhang Q, Liu G, Wu Y, Sha H, Zhang P, Jia J, et al. BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules. 2011; 16: 10146-10156.
- Quinn SM, Walters WM, Vescovi AL, Whittemore SR. Lineage restriction of neuroepithelial precursor cells from fetal human spinal cord. Journal of neuroscience research. 1999; 57: 590-602.
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005; 102: 14069-14074.
- Emgård M, Holmberg L, Samuelsson EB, Bahr BA, Falci S, Seiger A, et al. Human neural precursor cells continue to proliferate and exhibit low cell death after transplantation to the injured rat spinal cord. Brain Res. 2009; 1278: 15-26.
- Tarasenko YI, Gao J, Nie L, Johnson KM, Grady JJ, Hulsebosch CE, et al. Human fetal neural stem cells grafted into contusion-injured rat spinal cords improve behavior. J Neurosci Res. 2007; 85: 47-57.
- Iwanami A, Kaneko S, Nakamura M, Kanemura Y, Mori H, Kobayashi S, et al. Transplantation of human neural stem cells for spinal cord injury in primates. J Neurosci Res. 2005; 80: 182-190.
- Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001; 167: 27-39.
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006; 26: 3377-3389.
- Faulkner J, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl Immunol. 2005; 15: 131-142.
- Almad A, Sahinkaya FR, McTigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011; 8: 262-273.
- Sharp J, Keirstead HS. Therapeutic applications of oligodendrocyte precursors derived from human embryonic stem cells. Curr Opin Biotechnol. 2007; 18: 434-440.
- Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells. 2012; 30: 1163-1173.
- Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. PNAS. 2011; 108: 16825-16830.
- Hatami M, Mehrjardi NZ, Kiani S, Hemmesi K, Azizi H, Shahverdi A, et al. Human embryonic stem cell-derived neural precursor transplants in collagen scaffolds promote recovery in injured rat spinal cord. Cytotherapy. 2009; 11: 618-630.
- Erceg S, Ronaghi M, Oria M, Rosello MG, Arago MA, Lopez MG, et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010; 28: 1541-1549.
- Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007; 25: 1931-1939.
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005; 25: 4694-4705.
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005; 49: 385-396.
- Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010; 28: 152-163.
- Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, et al. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Molecular and cellular neurosciences. 2007; 34: 310-323.
- Sandner B, Prang P, Rivera FJ, Aigner L, Blesch A, Weidner N, et al. Neural stem cells for spinal cord repair. Cell Tissue Res. 2012; 349: 349-362.
- King C, Patel S, Rameshwar P. The Role of Human Postnatal Bone Marrow-Derived Mesenchymal Stem Cells and Their Importance in Growth, Spinal Cord Injury and Other Neurodegenerative Disorders. In: Preedy VR, editor. Handbook of Growth and Growth Monitoring in Health and Disease: Springer. 2012.
- Bhanot Y, Rao S, Ghosh D, Balaraju S, Radhika CR, Satish Kumar KV, et al. Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg. 2011; 25: 516-522.
- Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. 2009; 11: 897-911.
- Kishk NA, Gabr H, Hamdy S, Afifi L, Abokresha N, Mahmoud H, et al. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil Neural Repair. 2010; 24: 702-708.
- Park JH, Kim DY, Sung IY, Choi GH, Jeon MH, Kim KK, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012; 70: 1238-1247.
- Mehler MF, Rozental R, Dougherty M, Spray DC, Kessler JA. Cytokine regulation of neuronal differentiation of hippocampal progenitor cells. Nature. 1993; 362: 62-65.
- Chong ZZ, Kang JQ, Maiese K. Hematopoietic factor erythropoietin fosters neuroprotection through novel signal transduction cascades. Journal of cerebral blood flow and metabolism. 2002; 22: 503-514.
- Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, et al. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002; 69: 687-691.
- Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen S, et al. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res. 2005; 80: 611-619.
- Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005; 11: 913-922.
- Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007; 25: 2066-2073.
- Syková E, Homola A, Mazanec R, Lachmann H, Konrádová SL, Kobylka P, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006; 15: 675-687.
- Geffner LF, Santacruz P, Izurieta M, Flor L, Maldonado B, Auad AH, et al. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: comprehensive case studies. Cell Transplant. 2008; 17: 1277-1293.