
Case Report
Austin J Cerebrovasc Dis & Stroke. 2015;2(1): 1032.
Thrombolysis in a Patient with Capsular Warning Syndrome and Incidental Pineal Tumor
Bain L1, Rana A³, Bhatt P², Clarke R¹ and Reid JM¹*
1Acute Stroke Unit, Aberdeen Royal Infirmary, UK
2Department of Neurosurgery, Aberdeen Royal Infirmary, UK
3Department of Neuroradiology, Aberdeen Royal Infirmary, UK
*Corresponding author: John Reid, Acute Stroke Unit, Department of Neuroradiology, Aberdeen Royal Infirmary, UK.
Received: March 09, 2015; Accepted: March 30, 2015; Published: April 01, 2015
Abstract
There are many relative contraindications to administering intravenous thrombolysis (IVT) for acute ischemic stroke (AIS), such as rapidly improving symptoms. In addition there has been debate about whether certain stroke subtypes are less likely to respond to IVT (e.g. lacunar stroke). We describe a case with consent of a 63 year old man with Capsular Warning Syndrome (CWS), who was later identified to also have a pineal tumor. The patient was treated with IVT, but this was stopped part way through the infusion when the tumor diagnosis was realized and the patient had clinically improved. The patient’s stroke symptoms later recurred, and following discussion with neuroradiology and neurosurgical colleagues IVT was then restarted. This case emphasizes the debate about the best treatment for CWS and supports use of thrombolysis for this condition. Secondly we believe this to be the first case report of a patient with a pineal tumor receiving IVT for AIS. It highlights that for some types of brain tumors, particularly if benign or extra-axial location, IVT may be relatively safe.
Keywords: Intravenous thrombolysis; Lacunar stroke; Acute ischemic stroke; Intracranial tumors
Introduction
There are many contraindications to the use of intravenous thrombolysis (IVT) in acute ischemic stroke (AIS) including rapidly recovering symptoms and the presence of an intracranial mass lesion [1]. We describe a patient with capsular warning syndrome (CWS) who appeared to benefit from IVT without negative consequence, despite the presence of a previously unknown intracranial mass. This case highlights areas of uncertainty that can be encountered when considering use of IVT in AIS patients.
Case Presentation
A 63 year old man developed right sided weakness and dysarthria at 0545, 20 minutes after waking. His medical history was significant for untreated hypertension and current smoking (20 cigarettes/day). Upon arrival in the emergency department (ED) at 0803, there had been some partial recovery in his weakness, and National Institutes of Health Stroke Scale (NIHSS) score was 6. Blood pressure was 158/82 mmHg and he was in sinus rhythm. A non-contrast CT head scan was reviewed by a junior radiologist and the consultant stroke physician. This was thought to show ventriculomegaly but no cause for this noted. There was no hemorrhage, early ischemic change or other abnormality. The patient had no symptoms of headache or papilledema. Following discussion of the risks and benefits of IVT, alteplase (initial 10% bolus dose and total dose 0.9 mg/kg over 60 minutes) was initiated with consent at 0846. Approximately 25 minutes through the alteplase infusion (0910), further radiological review of the CT scan by a consultant neuroradiologist suggested the scan demonstrated a pineal tumor (Figure 1A). At this point, the patient’s symptoms had fully resolved (NIHSS = 0) and therefore, in view of the possible risks associated with IVT and the new radiological interpretation, the alteplase infusion was stopped. At 1025 the patient developed recurrent flaccid right sided weakness, with mild dysarthria and facial droop (NIHSS = 8). A repeat CT scan was unchanged. His case was further discussed with the on-call neurosurgical consultant who was of the opinion that the pineal tumor was likely benign and longstanding, and felt the risks of intracerebral hemorrhage from IVT were probably negligible. Following further discussion with the patient, and in view of recurrent disabling weakness it was decided to continue and complete the alteplase infusion. His symptoms had fully resolved on completion of the alteplase infusion and he was transferred to the acute stroke unit at 1200. At 1230 he developed a further flaccid right hemi paresis resolving after one hour. There was one final episode of severe right arm weakness at 0030 which resolved after one hour. 24 hours after presentation, only a mild right arm and leg drift was present (NIHSS = 2) and he was started on Aspirin 300mg daily. T2-weighted cranial MRI showed a wedged-shaped hyperintensity in the posterior limb of the left internal capsule, with restriction on diffusion weighted images typical of recent infarct, and no intracranial hemorrhage (Figure 1B). ECG, 24-hour cardiac monitor and carotid duplex ultrasound were within normal limits. He was discharged home after a further 24 hours and initiated on Aspirin 300 mg/day and Simvastatin 40 mg at night. NIHSS was 0 at one week following presentation and modified Rankin score (mRS) 0 at 3 months. Further neurosurgical review is planned with follow-up imaging to determine if the pineal tumor is changing.
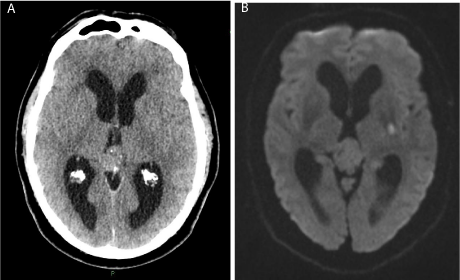
Figure 1: A) Non-contrast axial computed tomography brain scan on admission to the Emergency Department, demonstrating calcified pineal tumor and relative
ventriculomegaly; B) Diffusion weighted MRI brain scan 24 hours post thrombolysis showing DWI +ve lesion in the posterior limb of the left internal capsule.
Discussion
This case highlights two areas of uncertainty when treating AIS; the patient who is rapidly improving or fluctuating (e.g. CWS), and whether it can be safe in some circumstances to give thrombolysis in the presence of an intracranial tumour.
Capsular warning syndrome
This case fulfils criteria for CWS as first described by Donnan and colleagues over 20 years ago [2], with at least three recurrent stereotyped transient ischemic attacks (TIAs) that are purely motor, sensory or both. CWS affects at least two regions of face, arm and leg simultaneously, in the absence of cortical symptoms, within a 24 hour period. The attacks can fluctuate dramatically, e.g. between a flaccid hemiparesis and normality. The majority of CWS patients (42-71%) go on to develop a capsular infarct [2, 3], with permanent neurological deficit [4], but despite this most have a favorable outcome [3]. CWS rarely localises to the pons [5]. CWS can present treatment difficulties to the stroke physician; the optimal treatment remains controversial including use of anticoagulation [2, 6, 7], antiplatelets [8-11] and thrombolysis [3, 11-13]. The evidence is limited to case reports and small population based studies and so there is lack of consensus on the best treatment strategy. The pathogenesis of CWS is complex and not fully understood. Common hypotheses include hemodynamic impairment [2], vasospasm, artery-to-artery embolism [14], or peri-infarct depolarization [14]. More recently, a cardio-embolic etiology of CWS has been reported [3]. Whether all these mechanisms are amenable to thrombolysis is debated. CWS is considered a distinct entity, but there is evidence that thrombolysis improves outcome across a variety of stroke subtypes including lacunar stroke syndromes and strokes with minor severity [15-17].
In the case of our patient the diagnosis of CWS was made after completion of thrombolysis, since he continued to fluctuate. Whilst our patient’s neurological status was improving at presentation, we were keen to consider thrombolysis knowing that one third of patients with early recovery go on to develop neurological worsening after withholding thrombolysis [18]. Most medico-legal cases involving stroke thrombolysis relate to failure to treat, rather than adverse side effects associated with its use [19]. In our view there should be a bias in favor of administering thrombolysis in cases where there is some uncertainty of benefit, if there is no clear precedent to withhold treatment.
A recent multicentre study [3] from Spain collected data on 42 patients with CWS over a 10 year period. 8 of 12 CWS patients who received thrombolysis had favourable outcomes (mRS 0-2) at 3 months. This was comparable to the remainder of patients who were treated with combinations of anticoagulants, anti-platelet agents and vasopressors. There could be a rationale for a multi-centre trial of thrombolysis in CWS. However meta-analyses and case series suggest that lacunar stroke syndromes such as occur in CWS do benefit from thrombolysis [15-17], so a trial may be considered unethical.
Intracranial tumors and thrombolysis
The management of this patient was further complicated by an incidental finding of an intra-cranial mass on the initial CT scan. No study has looked at incidental findings on CT during acute stroke, although the incidence of ischemic stroke in patients with primary brain tumours is 1.3% [20]. Typically intracranial tumors are a contraindication to thrombolysis, largely as this formed part of the exclusion criteria in the early stroke trials [21] due to fears of an increased risk of bleeding. There may be a reporting and publication bias to report favorable outcomes in AIS patients with intracranial tumors treated with thrombolysis. Case reports have formed the only body of evidence regarding off-label use of thrombolysis in this way [22]. A recent large retrospective US study identified 416 (0.34% total) thrombolysed AIS patients with a primary brain tumor (both benign and malignant) [23]. The presence of a tumor did not adversely affect outcome per se (i.e. no difference in hemorrhage rate, mortality or rate of discharge home). However, outcomes were worse for malignant brain tumor subtypes and intraparenchymal tumors. This suggests that careful consideration of tumor location and pathology may allow delivery of thrombolysis to some patients with brain tumors. In our case, we were confident the pineal mass was benign after seeking both expert neuroradiological and neurosurgical opinions. However without confirmatory histology there is always uncertainty solely from a radiological diagnosis. The peripherally dispersed calcification and well circumscribed nature of the lesion as seen in our patient are radiologically characteristic of a benign pineocytoma [24, 25]. Pineal region tumors (all sub-types considered) are rare, accounting for less than 1% of intracranial tumors [24]. The pineal mass was initially missed by an experienced stroke physician who viewed the CT scan in the emergency department and reflects the rarity of these lesions. Of note the patient had no symptoms attributable to the tumor. This case appears to be the only published case of thrombolysis for AIS in a patient with a pineal tumor. One study has looked at the ability of stroke physicians and radiologists of varying experience to interpret early ischemic changes in CT scans in AIS [26]. Improved detection was associated with later imaging from symptom onset, taking more time to interpret images, and being a neuroradiologist. This highlights the benefit that experienced radiological opinion can provide to thrombolysis decision making, although is not always available after hours or at weekends in many centres.
Conclusion
The case highlights two controversial areas regarding thrombolysis in AIS, CWS and the presence of an incidental tumor on the acute CT brain scan. Whilst the optimum management of patients with CWS remains unknown, thrombolysis appears to be an acceptable therapeutic option. Patients will continue to be treated on an individual basis until further evidence is produced. The most feared complication of thrombolysis is intra-cranial hemorrhage. However, patients with an intracranial neoplasm may not necessarily be at higher risk of bleeding, particularly if the histological subtype is benign and the tumor is extra-axial. This can be challenging given the time pressures in assessing AIS, and will often require the prompt advice of a neuroradiologist and neurosurgeon. It is notable that AIS patients with conventional contraindications (e.g. very mild or severe stroke, glucose level and blood pressure) seem to respond just as well to IVT as patients without contraindications [27]. In carefully selected patients, thrombolysis administration may be beneficial despite the presence of an intracranial mass.
References
- Jauch EC, Saver JL, Adams HP Jr, et al. American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013; 44: 870-947.
- Donnan GA, O'Malley HM, Quang L, Hurley S, Bladin PF. The capsular warning syndrome: pathogenesis and clinical features. Neurology. 1993; 43: 957-962.
- Camps-Renom P, Delgado-Mederos R, Martínez-Domeño A, Prats-Sánchez L, Cortés-Vicente E, Simón-Talero M, et al. Clinical characteristics and outcome of the capsular warning syndrome: a multicenter study. Int J Stroke. 2014.
- Donnan GA, O’Malley HM, Quang L, Hurley S. The capsular warning syndrome: The high risk of early stroke. Cerebrovasc Dis. 1996; 6: 202–207.
- Muengtaweepongsa S, Singh NN, Cruz-Flores S. Pontine warning syndrome: case series and review of literature. J Stroke Cerebrovasc Dis. 2010; 19: 353-356.
- Farrar J, Donnan GA. Capsular warning syndrome preceding pontine infarction. Stroke. 1993; 24: 762.
- Frey JL. Capsular warning syndrome. Neurology. 1994; 44: 195-196.
- Fahey CD, Alberts MJ, Bernstein RA . Oral clopidogrel load in aspirin-resistant capsular warning syndrome. Neurocrit Care. 2005; 2: 183-184.
- Kawano H, Nakajima M, Inatomi Y, Yonehara T, Ando Y. Loading dose of clopidogrel in combination with other antithrombotic therapy for capsular warning syndrome. J Stroke Cerebrovasc Dis. 2014; 23: 1265-1266.
- Asil T, Ir N, Karaduman F, Cagli B, Tuncel S. Combined antithrombotic treatment with aspirin and clopidogrel for patients with capsular warning syndrome: a case report. Neurologist. 2012; 18: 68-69.
- Tassi R, Cerase A, Acampa M, D'Andrea P, Guideri F, Lo Giudice G, et al. Stroke warning syndrome: 18 new cases. J Neurol Sci. 2013; 331: 168-171.
- Vivanco-Hidalgo RM, Rodriguez-Campello A, Ois A, Cucurella G, Pont-Sunyer C, Gomis M, et al. Thrombolysis in capsular warning syndrome. Cerebrovasc Dis. 2008; 25: 508-510.
- González Hernández A, Fabre Pi Ó, Cabrera Naranjo F, López Veloso A.C. Trombólisis intravenosa con activador tisular del plasminógeno recombinante en los síndromes de alarma vascular. Neurología. 2014; 29: 334-338.
- Staaf G, Geijer B, Lindgren A, Norrving B. Diffusion-weighted MRI findings in patients with capsular warning syndrome. Cerebrovasc Dis. 2004; 17: 1-8.
- Emberson J, Lees KR, Lyden P, et al. Stroke Thrombolysis Trialists' Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014; 384: 1929-1935.
- Lahoti S, Gokhale S, Caplan L, Michel P, Samson Y, Rosso C, et al. Thrombolysis in ischemic stroke without arterial occlusion at presentation. Stroke. 2014; 45: 2722-2727.
- Shobha N, Fang J, Hill MD. Do lacunar strokes benefit from thrombolysis? Evidence from the Registry of the Canadian Stroke Network. Int J Stroke. 2013; 8 Suppl A100: 45-49.
- Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001; 56: 1015-1020.
- Liang BA, Zivin JA. Empirical characteristics of litigation involving tissue plasminogen activator and ischemic stroke. Ann Emerg Med. 2008; 52: 160-164.
- Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG. Descriptive epidemiology of primary brain and CNS tumours: results from the Central Brain Tumour Registry of the United States, 1990-1994. Neuro Oncol. 1999; 1: 14-25.
- Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998; 352: 1245-1251.
- Kreisl TN, Toothaker T, Karimi S, DeAngelis LM. Ischemic stroke in patients with primary brain tumors. Neurology. 2008; 70: 2314-2320.
- Etgen T, Steinich I, Gsottschneider L. Thrombolysis for ischemic stroke in patients with brain tumors. J Stroke Cerebrovasc Dis. 2014; 23: 361-366.
- Murthy SB, Moradiya Y, Shah S, Shastri A, Bershad EM, Suarez JI. In-hospital outcomes of thrombolysis for acute ischemic stroke in patients with primary brain tumors. J Clin Neurosci. 2015; 22: 474-478.
- Smith AB, Rushing EJ, Smirniotopoulos JG. From the archives of the AFIP: lesions of the pineal region: radiologic-pathologic correlation. Radiographics. 2010; 30: 2001-2020.
- Wardlaw JM, von Kummer R, Farrall AJ, Chappell FM, Hill M, Perry D. A large web-based observer reliability study of early ischaemic signs on computed tomography. The Acute Cerebral CT Evaluation of Stroke Study (ACCESS). PLoS One. 2010; 5: e15757.
- Frank B, Grotta JC, Alexandrov AV, Bluhmki E, Lyden P, Meretoja A, et al. Thrombolysis in stroke despite contraindications or warnings? Stroke. 2013; 44: 727-733.