
Case Report
Austin J Cerebrovasc Dis & Stroke. 2016; 3(1): 1040.
Hemorrhagic Dissection of the P1 Segment of Posterior Cerebral Artery Treated with Stent-Assisted Coil Embolization: A Case Report
Nishimuta Y1,2*, Tomosugi T2, Kubo F1,2, Ito N1,2, Hirahara K2, Nagayama T3, Tokimura H1 and Arita K1
1Department of Neurosurgery, Kagoshima University, Japan
2Department of Neurosurgery, Kagoshima City Hospital, Japany
3Department of Neurosurgery, Atsuchi Neurosurgical Hospital, Japan
*Corresponding author: Yosuke Nishimuta, Department of Neurosurgery, Graduate School of Medical and Dental Sciences, Kagoshima University, 8-35-1 Sakuragaoka, 890-8544, Kagoshima, Japan
Received: April 17, 2016; Accepted: May 11, 2016; Published: May 13, 2016
Abstract
Dissecting aneurysms of posterior cerebral artery (PCA) are very rare. We report a case with hemorrhagic dissecting aneurysm of the P1 segment of PCA treated by stent-assisted coiling. An otherwise healthy 47-year-old woman had sudden severe headache and vomiting. Brain CT showed subarachnoid hemorrhage thicker in the left ambient and prepontine cistern. 3D CTA revealed a fusiform dilatation of the P1 segment of left PCA. Because surgical intervention involved high risks for treatment-related morbidity and mortality, the patient was treated conservatively with antihypertensive and sedatives for seven days. Her clinical course was uneventful, and the irregularity of the PCA wall improved on magnet resonance angiogram (MRA) two weeks after the onset. One year after onset, however, MRA revealed recurrence of PCA dissection. The aneurysm was successfully treated by stent-assisted coiling using trans-cell technique without rebleeding. The aneurysm has not recurred on the latest DSA at 6 months after the treatment. Endovascular treatment with “stent assist” seems a reasonable choice for treating dissecting aneurysm involving the P1 segment of PCA.
Keywords: Stent-assisted coil embolization; Hemorrhagic P1 dissection; Posterior cerebral artery
Introduction
Hemorrhagic dissecting aneurysms of cerebral artery tend to rebleed and the prognosis after rebleeding is poor. Hence, proactive treatments are given to prevent rebleeding. Posterior cerebral artery (PCA) aneurysms are quite rare, comprising approximately 1% of all intracerebral aneurysms [1]. Dissecting aneurysms account for about 35% of PCA aneurysms [2].
Endovascular treatment has been utilized as a modality of treatment of this rare entity [3]. But the proper choice of the treatment and the long term results are yet to be clear. We here report a case of hemorrhagic dissecting aneurysm of the P1 segment of PCA which was successfully treated with stent-assisted coiling.
Case Presentation
A 47-year-old homemaker woman with high blood pressure, which had been well controlled by medication, underwent magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) studies (Figure 1A) due to pulsatile tinnitus in her left ear lasting for 1 week. The studies showed no abnormality. No irregular caliber of the left P1 segment was visible. She felt chronic tension headache since then. Seven days after the first MR studies, she was found to be standing in stuporous state in the shower room. She was lethargic with GCS 14 (E3, V5, M6) and high blood pressure (170/80 mm Hg) at the emergency room, computed tomography (CT) scan showed diffuse subarachnoid hemorrhage (SAH) which was thicker in the left side of ambient and prepontine cistern (Figure 2). Digital subtraction angiography (DSA) demonstrated fusiform enlargement and irregularity of the P1 segment of left PCA (Figure 1B arrow). The patient’s level of consciousness became normal after a week-long blood control using antihypertensives and small dose of sedatives. MRA at two weeks after admission showed smoothening of wall of the P1 segment (Figure 1C arrow heads).
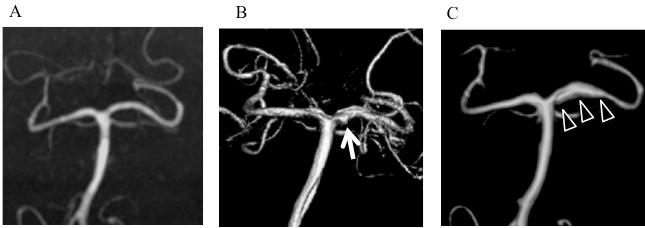
Figure 1: Initial changes in configuration of left posterior cerebral artery (PCA)
A. Initial magnetic resonance angiogram (MRA) showed no abnormality.
B. Three-dimensional digital subtraction angiogram (3D-DSA) after subarachnoid hemorrhage (SAH) showed a fusiform dilatation of proximal end of the P1
segment of left PCA (arrow).
C. 3D-DSA performed two weeks after SAH showed smoothening of wall of the P1 segment (arrowheads).
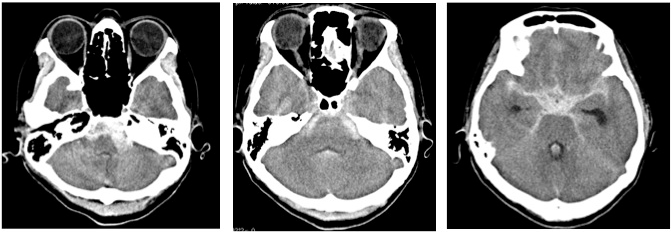
Figure 2: Emergency computed tomography (CT) scan showed diffuse SAH with left sided thickness.
Because MRAs studied at 3, 6 and 9 months after the SAH attack did not show any significant change in the artery and surgical intervention involved high risks for treatment-related morbidity and mortality, the patient was treated conservatively with antihypertensive. But MRA at 12 months after the SAH attack revealed apparent bulging of the wall at the same position of left PCA (Figure 3A). As the recurrence of the aneurysm seemed to be an indication of the proactive treatment and the degree of bulging was greater than the initial one, we decided to treat this aneurysm. We chose the endovascular treatment rather than trapping because of hypoplasia of left posterior communicating artery (Pcom). Stent-assisted coiling technique was employed due to broad aneurysmal neck and that the circumference of the artery is dysplastic. Left vertebral artery was chosen as an access route. DSA just before the embolization showed irregular contrast filling of the aneurysmal lumen (Figure 3B arrow).
The tip of Prowler Select Plus microcatheter (Codman & Shurtleff, Raynham, MA, USA) was positioned into the left PCA. Then, the tip of Excelsior SL-10 microcatheter (Stryker, Neurovascular, Fremont, CA, USA) was introduced into the aneurysmal lumen. And a CODMAN Enterprise TM VRD stent (4.5mm x 28mm, Codman & Shurtleff, Raynham, MA, USA) was introduced through the Prowler Select Plus microcatheter and deployed in the arterial lumen from the P2 segment of PCA to the midpoint of basilar artery (Figure 3C arrowheads). But, at this moment, the tip of SL-10 microcatheter was dislodged from the aneurysmal lumen due to wide aneurysmal neck. So, the tip of SL-10 microcatheter was re-introduced into the aneurysmal lumen through a cell of the stent (trans-cell technique). Finally, CERECYTE Delta Plush coils (4 mm x 6 cm, 3 mm x 4cm, 2 mm x 3 cm, Codman & Shurtleff, Raynham, MA, USA) were inserted into the lumen through the microcatheter (Figure 3C). Although slight body filling was seen after the placement of three coils, we assumed to leave a slight filling into the aneurismal dome because of the introduction of another coil.
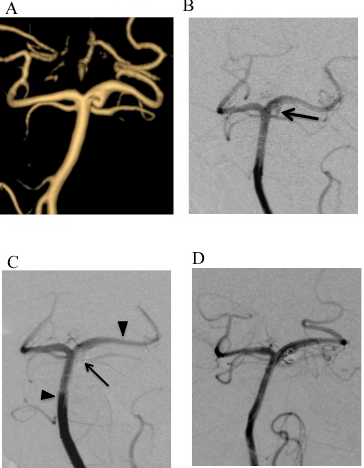
Figure 3: Changes in the dissecting aneurysm of left PCA along with the time
course of treatment
A. MRA at 12 months after SAH demonstrated reemergence of the localized
bulging of the wall of PCA.
B. DSA just before the embolization showed irregular contrast filling of the
aneurysm (arrow).
C. After the placement of three coils, DSA showed slight body filling of the
aneurysmal lumen (arrow). Two arrowheads indicate the proximal and distal
ends of stent.
D. DSA at 6 months after the embolization showed complete obliteration of
the aneurysmal lumen.
The postoperative course was uneventful. There was not an ischemic stroke on the post op MRI in the posterior perforating arteries territory. The follow-up DSAs at 3 and 6 months after the embolization showed complete obliteration of aneurysmal lumen (Figure 3D).
Discussion
About 40% of PCA dissecting aneurysms present with SAH [4]. The most frequent site is P1-P2 segment, involved in about 50% of PCA dissecting aneurysms [4]. The preference to the proximity could be related to the vicinity to the tentorial edge [5]. In this case, there was no history of head injury and hypertension was well controlled by medication.
The treatment strategy for PCA dissecting aneurysm has not been established mainly due to the paucity of cases. Conservative treatment followed by regular follow-up can be chosen because the prognosis, even in hemorrhagic case, is not bad when the aneurysm does not grow [4,6]. Some recommend early intervention as in dissecting aneurysm of other sites, instead [7].
Including ours, only 8 patients, 7 women and one man, of nontraumatic hemorrhagic P1 dissecting aneurysm have been reported (Table 1) [3,4,7,8] Patients’ ages ranged from 23 to 52 years with a median of 41.5 years. They occurred more predominantly on the right side; 6 on right vs. 2 on left. Seven patients presented with SAH and one presented with intracerebral and intraventricular hematoma. Four patients were conservatively treated; the other three patients underwent aggressive treatment including direct surgery or endovascular procedure, all of them led to obstruction of the affected PCA.
Author (year)
Age (y)
Sex
Hemorrhage
Side
Location
Treatment
Follow-up (month)
Recurrence
Outcome
1
[8]
38
F
SAH
Rt.
P1
conservative therapy
12
no
GR
2
[8]
44
F
SAH
Lt.
P1
conservative therapy
6
no
GR
occipital infarction
3
[7]
52
F
SAH
Rt.
P1
conservative therapy
1
no
death
(spasm)
4
[7]
28
F
SAH
Rt.
P1
conservative therapy
5
yes
GR
5
[4]
47
F
SAH
Rt.
P1-2
proximal ligation
36
yes
mRS1
(sensory disturbance)
6
[3]
39
M
SAH
(HKG: 5)
Rt.
P1
parent artery occlusion
1
no
mRS5
7
[3]
23
F
ICH, IVH
(HKG: 5)
Rt.
P1
trapping
N.M.
no
death
8
present case
46
F
SAH
(HKG: 2)
Lt.
P1
conservative therapy→IVR
6
yes→cure
mRS1
F: female, M: male, Rt.: right, Lt.: left
SAH: Subarachnoid Hemorrhage; ICH: Intracerebral Hemorrhage; IVH: Intraventricular Hemorrhage; HKG: Hunt and Kosnik Grade; GR: Good Recovery; N.M.: Not Mentioned; mRS1: Modified Rankin Scale 1 (no significant disability despite symptoms); mRS5: Modified Rankin Scale 5 (severe disability); IVR: Interventional Radiology
Table 1: Reported cases of dissecting P1 aneurysm presenting with hemorrhage.
Assessment of existence of major perforators and the extent of collateral circulation through Pcom must be needed for the proper selection of treatment modality especially for P1 dissecting aneurysm. The most secure method to prevent rebleeding from dissecting aneurysm should be trapping the dissected arterial segment. This might, however, cause hemianopsia in some proportion of patients, [2] especially in those with hypoplastic Pcom. More serious sequel may derive from the occlusion of perforators arising from the P1 segment. According to Kaya’s anatomical study on fresh cadaver, 94 thalamoperforating branches were identified in 27 P1 segments (mean 3.35 branches per segment) [9] Occlusion of these arteries will cause variety of syndromes including hemiplegia, ataxia and oculomotor paresis. The syndromes include well known bithalamic bilateral paramedian thalamic infarctions manifesting as various levels of compromised consciousness, which is caused by occlusion of Percheron’s artery in which a single dominant thalamoperforating artery supplies the bilateral medial thalami with variable contribution to the rostral midbrain [10]. Intensive occlusion of proximal P1 portion may also lead to the infarcts of area irrigated by these perforators.
Hence, the obliteration of aneurysmal lumen with conservation of parent artery flow should be targeted whenever possible. Recently stent-assisted coiling has been exploited for intracranial dissecting aneurysms including those of vertebral artery (VA) [11-15].
For deploying microcatheter into aneurysmal lumen in stentassisted For deploying microcatheter into aneurysmal lumen in stentassisted coiling, we first employed “jailing technique” which has advantage of fixing microcatheter [16]. But dislodging of microcatheter after expansion of the stent urged us to employ “transcell technique” as a second choice. Although this technique harbors risks of access difficulty and moving stent, we could successfully catheterize aneurysmal lumen again without stent displacement.
This is the first reported case of hemorrhagic P1 dissection which was successfully treated with stent-assisted coil embolization. To know the exact efficacy and safety of this maneuver, longitudinal clinical and DSA follow-up seems necessary.
Conclusion
Stent-assisted coiling was chosen for the treatment of recurrent dissecting aneurysm of the P1 segment of PCA. Complete obliteration of aneurysm and patency of parent artery was achieved using transcell technique, which was confirmed on the follow-up DSAs. Stentassisted embolization has a possibility of being the first choice of treatment for PCA dissection along with advancement of related techniques and instruments.
References
- Hallacq P, Piotin M, Monet J. Endovascular occlusion of the posterior cerebral artery for the treatment of P2 segment aneurysms: retrospective review of a 10-year series. AJNR Am J Neuroradiol. 2002; 23: 1128-1136
- Ciceri EF, Klucznik RP, Grossman RG, Rose JE, Mawad ME. Aneurysms of the posterior cerebral artery: Classification and endovascular treatment. AJNR Am J Neuroradiol. 2001; 22:27-34.
- Wang H, Du Rose, Stary J, Gkogas C, Kim D, Day A, et al. Dissecting Aneurysms of the posterior cerebral artery: Current endovascular/surgical evaluation and treatment strategies. Neurosurgery. 2012; 70: 1581-1588.
- Shinno K, Nagahiro S, Nishide S. A clinical analysis of posterior cerebral artery dissection with special reference to management and outcome. Surg Cereb Stroke. 2010; 38: 95-100.
- Lazinski D, Willinsky RA, TerBrugge K, Montanera W. Dissecting aneurysms of the posterior cerebral artery: angioarchitecture and review of the literature. Neuroradiology. 2000; 42: 128-133.
- Inoue T, Nishimura S, Hayashi N, Numagami Y, Takazawa H, Nishijima M. Postpartum dissecting aneurysm of the posterior cerebral artery. J Clin Neurosci. 2007; 14: 576-581.
- Ono K, Inohara T, Shirotani T, Shimizu A, Ooigawa H, Muraoka Y. Posterior cerebral artery dissection. Jpn J Neurosurg. 2001; 10: 711-717.
- Pozatti E, Padovani R, Fabrizi A, Sabattini L, Gaist G. Benign arterial dissections of the posterior circulation. J Neurosurg. 1991; 75: 69-72.
- Kaya AH, Dagcinar A, Ulu MO, Topal A, Bayri Y, Ulus A, et al. The perforating branches of the P1 segment of the posterior cerebral artery. J Clin Neurosci. 2010; 17: 80-84.
- Spiotta AM, Wheeler AM, Smithason S, Hui F, Moskowitz S. Comparison of techniques for stent assisted coil embolization of aneurysms. J Neuro Interv Surg. 2012; 4: 339-344.
- Kim BM, Shin YS, Kim SH, Ihn YK, Kim DI, Kim DJ, et al. Incidence and risk factors of reccurence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke. 2011; 42: 2425-2430.
- Chen YA, Qu RB, Bian YS, Zhu W, Zhang KP, Pang Q. Stent placement to treat ruptured vertebral dissecting aneurysms. Interv Neuroradiol. 2013; 19: 479-482.
- Joo JY, Ahn JY, Chung YS, Han IB, Chung SS, Yoon PH, et al. Treatment of intra-and extracranial arterial dissections using stents and embolization. Cardiovasc Intervent. Radiol. 2005; 28: 595-602.
- Lubicsz B, Collignon L, Lefranc F, Bruneau M, Brotchi J, Baleriaux D, et al. Circumferential and fusiform intracranial aneurysms: reconstructive endovascular treatment with self expandable stent. Neuroradiology. 2008; 50: 499-507.
- Kim MJ, Chung J, Kim SL, Roh HG, Kwon BJ, Kim TH, et al. Stenting from the vertebral artery to the posterior inferior cerebellar artery. AJNR Am J Neuroradiol. 2012; 33: 348-352.
- Lazzaro NA, Wright B, Castillo M, Fischbein NJ, Glastonbury CM, Hildenbrand PG, et al. Artery of Percheron Infarction: Imaging Patterns and Clinical Spectrum. AJNR. 2010; 31: 1283-1289.