
Review Article
Austin J Cerebrovasc Dis & Stroke. 2016; 3(1): 1042.
Transcranial Direct Current Stimulation as a Tool in Rehabilitation of Visual Processing after Stroke: A Review
Cristino ED*, Silva JBS, Andrade MJO, Almeida NL and Santos NA
Department of Psychology, Federal University of Paraíba, Brazil
*Corresponding author: Cristino ED, Laboratory of Perception, Neuroscience and Behavior, Department of Psychology, Federal University of Paraíba, Cidade Universitária, João Pessoa, PB, Zip Code: 58051-900, Brazil
Received: May 15, 2016; Accepted: July 13, 2016; Published: July 15, 2016
Abstract
Stroke is one of the major causes of incapacity worldwide. More specifically, the visual impairments after stroke can lead to substantial losses in the activities of daily living and bring great impact upon an individual’s sense of well-being and independence. The development of novelty rehabilitation strategies to promote the recovery of visual function after stroke is of great importance. Transcranial direct current stimulation (tDCS) is a neuromodulatory technique with increasing popularity in the fields of basic research and rehabilitation. Despite the significant number of studies involving tDCS in rehabilitation after stroke, there are few published studies that specifically involve treatment of visual processing deficits. The aim of this review is to describe and discuss the research using tDCS in visual rehabilitation after stroke and to encourage future investigation on visual processing using tDCS as a tool in rehabilitation after brain lesions. Studies have pointed out that tDCS applied to the occipital cortex has demonstrated good results, with important effects on visual field rehabilitation in hemianopia, motion perception and color discrimination. Although some successes have been achieved in recent years, a lot of questions still need to be understood and others asked. All of this is in order to improve protocols used and, thus, obtain better results.
Keywords: Stroke; Rehabilitation; Visual processing; Transcranial direct current stimulation (tDCS)
Abbreviations
tDCS: Transcranial Direct Current Stimulation; ADL: Activities of Daily Living; HH: Homonymous Hemianopia; tPA: Intravenous Thrombolytic Treatment; TCI: Alteration in Transcallosal Inhibition; VRT: Visual Rehabilitation Training; EEG: Electroencephalography; fMRI: Functional Magnetic Resonance Imaging; HRP: High- Resolution Perimetry; QOL: Quality of Life
Introduction
Every year, about 16.9 million people worldwide suffer their first stroke [1]. This is an alarming number which generates a serious impact on society and on public health. Stroke survivors can experience different kinds of sequelae such as cognitive, motor and sensory perception deficits [2,3]. These consequences can lead to substantial losses in the activities of daily living (ADL) and quality of life [4,5]. More specifically, the visual impairments after stroke include eye movement disorders, perceptual deficits and visual field defects [6]. Visual field defects are a consequence of posterior strokes, and occur on one side of the visual field, usually in both eyes. This condition, called homonymous hemianopia (HH), is reported in up to 57% of patients during 3 months post event. After this period, a complete recovery of visual fields can occurs in up to 44% of cases and the partial recovery in up to 72% [7-9]. In addition, this kind of sequel can profoundly affect many important ADL, including reading, performing visual searches, driving [5,6] and navigating safely within one’s environment [10,11]. Thus, it is possible to realize that all kinds of visual deficits bring great impacts upon an individual’s sense of well-being and independence [12].
Intravenous thrombolytic treatment (tPA) has been successful in reversing visual impairments in the hyperacute phase of ischemic stroke; however, this treatment is only indicated when the neuronal tissue is not yet permanently damaged by the ischemia [13]. Nevertheless, once tissue damage has developed, spontaneous recovery is unpredictable and often incomplete [14]. Therefore, the development of novelty rehabilitation strategies to promote the recovery of visual function after stroke is of great importance [15].
Studies using human clinical trials and animal models have pointed towards evidence of the brain’s potential to reorganize itself within the context of functional recovery after injury [16-18]. Thus, identifying interventions that can promote and modulate these mechanisms seems very important to improving rehabilitation after stroke. In this context, noninvasive cortical stimulation techniques, such as transcranial direct current stimulation (tDCS), have gained prominence in neuro rehabilitation research as a reason of their potential to improve neuro plastic mechanisms associated with functional recovery [19,20].
Transcranial direct current stimulation (tDCS)
Studies that involve transcranial direct current stimulation (tDCS) are growing in frequency. In 2000, a PubMed database search using the search terms of tDCS produced only four articles. In 2013, the same search produced approximately 370 references [21]. Nowadays, three years after the study by Barry Hill, et al. [21], it is possible to find more than 2,500 studies involving tDCS in the PubMed database.
The technique
The method tDCS is a non-invasive neuromodulation method for delivering low-intensity polarizing electrical currents to the brain cortex [22]. The use of two electrodes (anode and cathode) placed on the scalp can favor neuronal activity [23]. These electrodes are often large (25–35 cm²), and the current intensity varies between 1 and 2 mA [24]. The current flows from the anode to the cathode electrode, and depending on the electrode positioning, may modulate the resting membrane potential of neurons to be closer or more distant from the firing threshold [22,24]. In general, the anode electrode produces an excitatory modulation while the cathode electrode produces an inhibitory effect. However, some studies point to results with the opposite outcome.
The main mechanism which explains tDCS effects is their capacity to modulate the resting membrane potentials of the stimulated area. In synthesis, is possible to claim that anodal tDCS can causes the resting membrane potential to become more positive, and, as a result, it makes the cell more responsive. On the other hand, the cathodal stimulation will lead to cell hyperpolarization, what difficult the neural impulse [25,26]. To elucidate this action, a study has delivered calcium and sodium channel blockers and noted the in activation of anodal tDCS effects. For more information, see Nitsche, et al [25, 26]. Other studies propose, as mechanism of action, which tDCS alters the levels of gamma-aminobutyric acid (GABA) and glutamate and it can alter the cortical excitability [27-29].
Moreover, it is important to highlight that tDCS effects seem to be site specific, so, moving the electrodes just a few centimeters can dramatically alter the results. Despite this, effects of tDCS are not site limited, what means that the stimulation can affect different areas and not only focus under the electrodes [22-24,30,31]. The safety profiles, reasonable cost, and promising findings have contributed to highlighting the technique [21]. As a therapeutic tool, tDCS has been used, for example, in rehabilitation in Parkinson’s disease [32,33], Alzheimer’s disease [34,35] and chronic pain [36,37].
tDCS in stroke
Studies evaluating the effects of tDCS on stroke have presented well-structured protocols and consistent results. In addition, a variety of sequelae resulting from stroke have shown improvements after different stimulation protocols of tDCS. As an example, it is possible to mention improvements on aphasia [38,39], cognitive functions [40,41] and motor rehabilitation [41-44].
The neurobiological basis of functional recovery after neuro stimulation in stroke patients has their foundation in the interhemispheric imbalance theory that occurs after vascular injury [45]. The interhemispheric imbalance theory suggests an alteration in transcallosal inhibition (TCI), in which inhibition exerted from the ipsilesional hemisphere (lesioned) on the contralesional hemisphere (intact) is weaker than inhibition exerted from the contralesional hemisphere on the ipsilesional hemisphere [46-48]. Restoring the interhemispheric balance by modulating brain activity can be achieved through the use of tDCS [24,26,49,50].
Literature search strategy
Despite the significant number of studies involving tDCS in rehabilitation after stroke, there are only a few published studies that specifically involve treatment of visual processing. Thus, the aim of this review is to describe and discuss the research using tDCS in visual rehabilitation after stroke.
For this purpose, three databases were consulted: PubMed, Scopus and Web of Science for the years 1996-2016. The search terms used were: “tDCS stroke visual function”, “Stroke tDCS visual cortex”, “Stroke tDCS contrast sensitivity” and “Stroke tDCS human color discrimination”. All articles selected were published in English. As a final result, we found 6 studies that involve neuromodulation by tDCS on specific visual losses after stroke. We opted to exclude studies about hemineglect, since this deficiency involves a complexity of functions beyond visual processing. A flowchart of the systematic review (Figure 1) summarizes the literature search strategy.
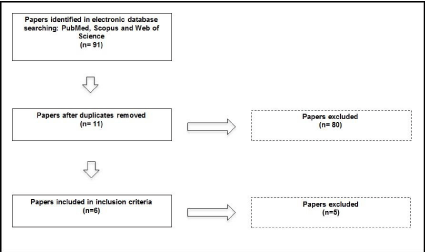
Figure 1: Flowchart of the systematic review.
tDCS in the rehabilitation of visual processing after stroke
tDCS and visual restoration therapy (VRT) in hemianopic patients: One of the first studies that involves tDCS and visual rehabilitation after stroke was developed by Halko, et al. [51]. This study combined fMRI to characterize regional changes in brain activity with an individualized, high-resolution computational model of brain current flow in a patient who underwent a successful combined visual rehabilitation and tDCS training protocol. For the authors, this study provides the first report demonstrating correlative effects of regional brain current flow during tDCS with brain activity characterized by fMRI.
Therefore, the study count was with a 61-year-old patient, righthanded, female, diagnosed with a right homonymous hemianopia following a left posterior cerebral artery stroke in the chronic phase of recovery. As part of her rehabilitative training, the patient underwent 2 half-hour sessions, 3 days a week during 3 months of Visual Restoration Therapy combined with concurrent tDCS.
During the procedure, she was instructed to detect and respond to a series of light stimuli presented primarily within the border between the areas of affected and unaffected vision. In conjunction with visual rehabilitation training (VRT), tDCS was delivered concurrently using an electrode configuration designed to up regulate occipital cortex excitability, and known to enhance visuoperceptual functioning in healthy participants in which it was previously used [23,52,53]. For this purpose, two electrodes (5 × 7 cm; 35 cm surface area) were used, with the anode electrode placed overlying the Oz position, and the reference (cathode) electrode placed over Cz (vertex), following the 10-20 International EEG coordinate system. With this configuration, it was possible to bilaterally stimulate both the lesioned and nonlesioned hemispheres. The electrodes were then connected to a battery operated unit delivering a continuous 2 mA current for the entire duration of VRT training. The current was delivered continuously throughout the 30-minute training sessions.
The results indicate that the association of tDCS with TRV over three months led to visual field improvements equivalent to the ones expected for a six-month treatment with VRT alone. The authors also point out that the changes in the fMRI signal found during treatment were significantly correlated with the modeled electric field induced by tDCS, supporting the role of tDCS as a visual rehabilitation booster.
After the first case study showed promising results, the same research group tried to strengthen the adequacy of the tDCS and VRT combination for rehabilitation of the visual field. For this purpose, Plow, et al. [54] developed a study that aimed to standardize a protocol for promoting visual rehabilitative outcomes in poststroke hemianopia, by combining occipital cortical tDCS with VRT. The study had a small sample which was comprised of two patients. Patients 1 and 2 (both women, aged 61 and 62 years, respectively) were both diagnosed with right-sided hemianopic visual field loss resulting from ischemic stroke, and were in the chronic phase of visual recovery. Patient 1 was randomly assigned to receive VRT combined with active tDCS, whereas patient 2 received VRT combined with sham tDCS. Both patients underwent an identical VRT and tDCS protocol used by Halko, et al. [51]. In patient 1, anodal tDCS was delivered to the occipital cortex during VRT training, whereas in patient 2, sham tDCS with VRT was performed. Specifically in patient 1, once the current was adjusted to the 2 mA/min target, it was sustained throughout the duration of VRT training. For patient 2, the current was ramped down (to zero) after initial habituation. Thus, both patients remained unaware as to whether they were receiving active or sham tDCS. Outcomes were assessed at baseline (pretest) and at monthly intervals until completion of the 3-month training period (post-test). Visual field evaluations were applied by using high-resolution perimetry (HRP) and functional outcomes measures. The authors also incorporated fMRI to identify patterns of activation associated with recovery of function. The fMRI data were collected at baseline and at post-test for patient 1.
As a result, the high-resolution perimetry revealed a greater shift in visual field borders for patient 1 versus patient 2. Patient 1 also showed greater recovery of function in activities of daily living (ADL). Nevertheless, contrary to the expectation, patient 2 perceived greater subjective improvement in visual field despite objective highresolution perimetry results that indicated otherwise. In patient 1, visual function recovery was associated with functional magnetic resonance imaging activity in surviving peri-lesional and bilateral higher-order visual areas. The results corroborate the hypothesis that the combination of visual rehabilitative training and noninvasive brain stimulation leads to an increase in functional visual recovery compared with visual rehabilitation alone.
In a subsequent study, Plow, et al. [55] investigated the same protocol (VRT and tDCS), but with a larger sample. The authors used a sample of 8 patients with unilateral postchiasmal visual field loss following stroke or brain damage, who were in the chronic phase of recovery. Participants were randomly assigned to 1 of 2 arms: VRT with active tDCS or VRT with sham tDCS. The outcome measures included objective and subjective changes in visual field, recording of visual fixation performance, and vision-related activities of daily living (ADLs) and quality of life (QOL).
As a result, the VRT and tDCS group demonstrated significantly greater expansion in visual field and improvement on ADLs compared with the VRT and sham group. However, the same unexpected result found in Plow, et al. [54] was observed: the subjective perception of visual field change was greater in the VRT and sham group. In addition, QOL did not change for either group. To investigate the stability of the effects as a result of the intervention, the authors compared performance on ADLs and impact on QOL and participation in life roles between the posttest and 6-month follow-up in 5 patients. Improvements appeared to be stable, because no significant difference was observed during this follow-up period. Again, it was possible to conclude that the combination of occipital cortical tDCS with visual field rehabilitation appears to enhance visual functional outcomes compared with visual rehabilitation alone. TDCS may enhance inherent mechanisms of plasticity associated with training.
After these previous reports pointed out that tDCS delivered to the occipital cortex enhances visual functional recovery when combined with rehabilitative training in patients with hemianopia, Plow, et al. [56] evaluated the temporal sequence of effects of tDCS on visual recovery, as they appear over the course of training and across different indicators of visual function. Therefore, the same intervention protocol used by Plow, et al. [54] and Plow, et al. [55] was applied in Twelve Patients previously diagnosed with unilateral post-chiasmal visual field loss due to stroke or surgical trauma. All Patients were in the chronic stage of recovery. The participants were randomized to one of two possible study arms: VRT combined with active tDCS or VRT combined with sham tDCS.
Primary outcome measures shift in visual field border and stimulus detection accuracy within the affected hemifield were collected at baseline (pretest), with monthly interim intervals, and at posttest (3 months). The secondary outcome measures contrast sensitivity and reading performance, and was collected at pretest and posttest time-points only. The results were compared between patients randomized to either VRT combined with active tDCS, or VRT paired with sham tDCS.
The results had shown that active tDCS combined with VRT accelerated the recovery of stimulus detection as between-group differences appeared within the first month of training. In contrast, a shift in the visual field border was only evident at posttest (after 3 months of training). The method of tDCS did not present effects on contrast sensitivity or reading performance. The results presented suggest that tDCS may affect the magnitude and sequence of visual recovery differentially, in a manner that is task-specific and related to the visual rehabilitative training strategy employed.
tDCS effects on motion perception and color discrimination: Studies suggest that tDCS effects on visual perception of stroke patients may not be limited to improvements in visual field border or contrast sensitivity. Olma, et al. [57] developed a study where motion perception was investigated in the unaffected hemifield of subjects with unilateral visual cortex lesions. Twelve subjects participated in the study, all had a history of ischemic stroke and chronic homonymous visual field defects. Subjects were, thus, in comparable chronic post-stroke phases.
The study followed a within-subject, repeated-measures, crossover design, comprising two blocks, each with 5-days of stimulation per week with daily measurements, then two follow-up measurements at 2 and 4 weeks. Motion perception was tested through computerized campimetric tests of color and motion detection, and automated threshold perimetry before and after stimulation sessions. Each subject received both anodal and sham intervention, and the two stimulation conditions were counterbalanced between blocks: six subjects received anodal tDCS in the first block, while six received it in the second block.
The anode (5 cm x 5 cm anodal electrode) was applied over the calcarine sulcus (ipsilateral to the lesion, aided by 1.5 T Magnetom Vision MRI scanner-guided neuronavigation) and the reference (7 cm x 5 cm reference cathode electrode) was placed over Cz (vertex), based on the 10-20 International EEG Coordinate System. Anodal and sham tDCS were administered for 20 min during stimulation sessions. Anodal tDCS was applied at a current of 1.5 mA, giving a current density of 0.06 mA/cm2.
Serial anodal tDCS over the visual cortex resulted in an improvement in motion perception. This effect was still measurable at 14-day and 28-day follow-up measurements. Thus, this may represent evidence for long-term tDCS-induced plasticity, and has implications for the design of studies examining the time course of tDCS effects in the visual system.
Another within-subject, sham-controlled, double-blinded study showed an important result about color discrimination. Dargie, et al. [58] evaluated whether serial anodal tDCS can induce long-lasting improvements in color discrimination in the unimpaired visual hemifield of patients with occipital stroke. The same protocol used in Olma, et al. [57] was used in twelve chronic stroke patients with unilateral visual cortex lesions. Campimetric testing of age-matched color discrimination was performed in the unaffected hemifield before and after each stimulation session, and at 14-and 28-day follow-ups. No significant difference was seen between the baselines of anodal and sham conditions. However, in the anodal condition, color discrimination was improved compared to sham on day 5 and at two and four-week follow-ups.
Table 1 summarizes the stimulation parameters and outcome measures for each of the cited studies conducted with tDCS for visual processing rehabilitation after stroke.
Reference
Location of target electrode (international 10-20 system)
Locationofreturnelectrolde
Stimulationparameters
Stimulationtypes
tDCSprotocol
Samplesize
MainResult
Halko, et al.
[45]
Oz, 35 cm2
Cz, 35 cm2
2.0 mA for
30 min
(twice a day), 3 sessions a
week over 3 months
Anodal
Online
Case study
Visual field improvements equivalent to the ones expected for a six-month treatment with VRT alone
Plow, et al.
[48]
Oz, 35 cm
2
Cz, 35 cm2
2.0 mA for
30 min
(twice a day), 3 sessions a
week over 3 months
Anodal, sham
Online
Twoparticipants
Greater shift in visual field borders and recovery of function in ADL for patient 1 versus patient 2
Plow, et al.
[49]
Oz, 35 cm
2
Cz, 35 cm2
2.0 mA for
2.0 mA for
30 min
(twice a day), 3 sessions a
week over 3 months
Anodal, sham
Online
Eightparticipants
Greater expansion in visual field and improvement on ADLs compared with the VRT and sham group
Plow, et al.
[50]
Oz, 35 cm
2
Cz, 35 cm2
2.0 mA for
30 min
(twice a day), 3 sessions a
week over 3 months
Anodal, sham
Online
Twelveparticipants
Active tDCS accelerated the recovery of stimulus detection within the first month of training. Shift in the visual field border was only evident at posttest
Olma, et al.
[51]
*MRI-derived
V1, 25 cm
2
Cz, 35 cm2
1.5 mA for
20 min (5
consecutivdays)
Anodal, sham
Offline
Twelveparticipants
Improvement in motion perception. This effect was still measurable at 14-day and 28-day follow-up measurements.
Dargie, et al. [52]
*MRI-derived
V1, 25 cm
2
Cz, 35 cm2
1.5 mA for
20 min (5
consecutivdays
Anodal, sham
Offline
Twelveparticipants
In the anodal condition, colour discrimination was improved compared to sham on day 5 and at two and four-week follow-ups.
Studies in chronological order
*studies that do not employ the International 10–20 electrode placement system.
Table 1: Parameters and details for each study discussed in the section “tDCS in the rehabilitation of visual processing after stroke”.
Discussion
Despite the few studies involving tDCS in the rehabilitation of visual functions after stroke, it is important to recognize that, in just five years, some relevant achievements have been made both to the development of basic research and for clinical research. The study by Halko, et al. [51] was the first to propose that tDCS can be used in rehabilitation of visual processing in patients in the chronic phase of stroke. Thus, the first successful intervention protocol on visual rehabilitation after stroke was proposed and, in addition, the brain changes resulting from the application of tDCS in V1 were characterized. This study was of great importance in order to initiate research in this specific field, and provide theoretical and practical insights for future studies.
Plow, et al. [54-56] gave continuity to the studies on the subject and confirmed the hypothesis that tDCS can lead to an expansion of the visual field in patients with hemianopia in the chronic phase of stroke. Moreover, the results indicate a temporal stability of the obtained effects. By adding other objective measures, such as contrast sensitivity and reading skills, as well as subjective measures such as quality of life and functional performance, these studies supplemented the knowledge about the effects of tDCS in visual rehabilitation. Olman, et al. [57] and Dargie, et al. [58] indicated that the effects of tDCS visual rehabilitation was not limited to improvements in the visual field or contrast sensitivity, and reported that the anodic tDCS on the visual cortex can result in improvement in motion perception and color discrimination.
Despite the successes achieved in recent years, some questions and gaps still need to be understood, and new questions must be asked. All of this in order to improve protocols and, thus, obtain better results.
A first point worth mentioning is the lack of studies involving visual processing rehabilitation in the sub-acute phase of stroke. Even though most of the studies on stroke in chronic phase show good results for tDCS, it is possible that even better results can arise if therapy is applied in the early stages after the stroke. It is known that spontaneous recovery tends to occur in the first weeks [59] and that it is an active process of brain plasticity, which tends to reach a plateau between 3 and 6 months after the stroke [60]. Early application of tDCS can therefore exploit the window of the best response in the brain, thus, reaching better results. In addition, longitudinal studies may be important in order to identify the effects of tDCS at the different stroke recovery phases.
Another relevant issue is that few studies explore the effects of tDCS in contrast sensitivity function and color discrimination after stroke. In this review, only 2 of the 6 studies found addressed this issue and have yielded inconclusive results. Plow, et al. [56] suggests, based on the results, that the effects of tDCS are only observed in specific functions related to the concomitant training, in this case, VRT to expand the visual field. However, widespread improvements are not observed in the visual processing as they are in contrast sensitivity. On the other hand, Dargie, et al. [58] found results that suggest improvements in color discrimination as a tDCS effect, even in the absence of specific training for this skill.
Given the limited amount of specific studies and the lack of consensus of findings [56,58], new studies evaluating the effects of tDCS in basic visual functions after stroke seem relevant, since this technique has already shown efficacy in healthy patients. In various experimental trials, Costa, et al. [61,62] identified effects of the anode and cathode tDCS on color discrimination and contrast sensitivity in healthy participants. Studies like this should be replicated in patients with brain lesions to assess how isolated tDCS can act in the recovery of specific visual functions. Furthermore, it is known that the contrast sensitivity function can be altered in patients with stroke even when the lesion has not occurred in occipital areas [63-65]. In this sense, it seems justified to evaluate the effects of tDCS in basic visual functions not only in patients with occipital lesions, but also seeking to develop studies investigating their effect in patients with injuries in different areas.
Conclusion
The set of results described and the considerations suggested converge to one main conclusion: tDCS is an important technique in the context of visual rehabilitation in stroke patients and, therefore, more studies need to be encouraged. Furthermore, the development of studies on visual models is important as an objective tool to be used as a diagnostic method for visual perceptual disorders, and monitoring of the evolutionary process of stroke patients
References
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA et al. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014; 383: 245-254.
- Protopsaltis J, Kokkoris S, Korantzopoulos P, Milionis HJ, Karzi E, Anastasopoulou A, et al. Prediction of long-term functional outcome in patients with acute ischemic non-embolic stroke. Atherosclerosis. 2009; 203: 228-235.
- Chestnut C, Haaland KY. Functional significance of ipsilesional motor déficits after unilateral stroke. Arch Phys Med Rehabil. 2008; 89: 62-68.
- Paula M, Pinto K, Lúcia M. Relação entre depressão e disfunçãocognitiva em pacientes após acidente vascular cerebral: um estudo. Psicologia Hospitalar. 2008; 6: 21-38.
- Radanovic M. Características do atendimento de pacientes com acidente vascular cerebral em hospital secundário. Arquivos de Neuropsiquiatria. 2005; 58: 99-106.
- Rowe FJ, Wright D, Brand D, Jackson C, Harrison S, Maan T, et al. A prospective profile of visual field loss following stroke: prevalence, type, rehabilitation, and outcome. Biomed Res Int. 2013; 2013:719096.
- Searls DE, Pazdera L, Korbel E, Vysata O, Caplan LR. Symptoms and signs of posterior circulations ischemia in the New England Medical Center Posterior Circulation register. ArchNeurol. 2012; 69; 346-351.
- Tao WD, Liu M, Fisher M, Wang DR, Li J, Furie KL, et al. Posterior versus anterior circulation infarction: how different are the neurological deficits?. Stroke. 2012; 43: 2060-2065.
- Ali M, Hazelton C, Lyden P, Pollock A, Brady M. Recovery from post stroke visual impairment: evidence from a clinical trials resource. Neurorehabil Neural Repair. 2013; 27: 133-141.
- Kerkhoff G. Neurovisual rehabilitation: Recent developments and future directions. Am J Ophthalmol 2000; 130: 687-688.
- Romano JG. Progress in rehabilitation of hemianopic visual field defects. Cerebrovasc Dis. 2009; 27: 187-190.
- Wagenbreth C, Franke GH, Sabel BA, Gall C. Impairments of vision and health-related quality of life in stroke patients with homonymous visual field defects depend on severity of visual function loss. Klin Monbl Augenheilkd.2010; 227: 138-148.
- Strbian D, Soinne L, Sairanen T, Scheperjans F, Salonen O, Palomäki M, et al. Intravenous thrombolysis in ischemic stroke patients with isolated homonymous hemianopia. Acta Neurol. Scand. 2012; 126: 17-19.
- Ali M, Hazelton C, Lyden P, Pollock A, Brady M. Recovery from poststroke visual impairment: evidence from a clinical trials resource,Neurorehabil. Neural Repair.2013; 27: 133-141.
- Plow E, Maguire S, Obretenova S, Pascual-Leone A, Merabet LB. Approaches to rehabilitation for visual field defects following brain lesions. Expert Rev Med Devices. 2009; 6: 291-305.
- Wandell BA, Smirnakis SM. Plasticity and stability of visual field maps in adult primary visual cortex. Nat Rev Neurosci. 2009; 10: 873-884.
- Nudo R. Functional and structural plasticity in motor cortex: Implications for stroke recovery. Phys Med RehabilClin N Am. 2003; 14: S57-S76.
- Wu CW, Kaas JH. Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. J Neurosci.1999; 19: 7679-7697.
- Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of recovery mechanisms after stroke. Neurorehabil Neural Repair. 2010; 24: 125-135.
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007; 3: 383-393.
- Berryhill ME, Peterson DJ, Jones KT, Stephens JA. Hits and misses: leveraging tDCS to advance cognitive research. Front Psychol. 2014; 5: 800.
- Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct Current stimulation. J Physiol.2000; 527: 633-639.
- Kraft A, Roehmel J, Olma MC, Schmidt S, Irlbacher K, Brandt SA. Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Exp Brain Res. 2010; 207: 283-290.
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art. Brain Stimul. 2008; 1: 206-223.
- Nitsche M, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiology. 2003; 553: 293-301.
- Nitsche M, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation-technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003; 56: 255-276.
- Nitsche M., Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Frommann K, et al. GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. European J Neurosci. 2004; 19: 2720-2726.
- Nitsche MA, Niehaus L, Hoffmann KT, Hengst S, Liebetanz D, Paulus W, et al. MRI study of human brain exposed to weak directcurrent stimulation of the frontal cortex. Clin Neurophysiol. 2004; 115: 2419-2423.
- Stagg C, Best J, Stephenson M, O'Shea J, Wylezinska M, Kincses Z, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neuroscience. 2009; 29: 5202-5206.
- Costa TL, Lapenta OM, Boggio PS, Ventura DF. Transcranial direct current stimulation as a tool in the study of sensory-perceptual processing. Atten Percept Psychophys.2015; 77: 1813-1840.
- Nitsche M, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003; 15: 619-626.
- Boggio PS, Ferrucci R, Rigonatti S, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J. Neurol. Sci. 2006; 249: 31-38.
- Fregni, F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti S, et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov. Disord. 2006; 21: 1693-1702.
- Boggio PS, Valasek CA, Campanha C, Giglio AC, Baptista NI, Lapenta OM, et al. Non-invasive brain stimulation to assess and modulate neuro plasticity in Alzheimer’s disease. Neuropsychol Rehabil. 2011; 21: 703-716.
- Boggio PS, Ferrucci R, Mameli F, Martins D, Martins O, Vergari M, et al. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul. 2012; 5: 223-230.
- Fregni F, Boggio P, Lima M, Ferreira M, Wagner T, Rigonatti S, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006; 122: 197-209.
- Lefaucheur J, Antal A, Ahdab R, Ciampi De Andrade D, Fregni F, Khedr E, et al. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. BrainStimul. 2008: 1; 337-344.
- Shah PP, Szaflarski JP, Allendorfer J, Hamilton RH. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Front Hum Neurosci. 2013; 7: 888.
- Fama ME, Turkeltaub PE. Treatment of poststroke aphasia: current practice and new directions. Semin Neurol. 2014; 34: 504-513.
- Jo JM, Kim YH, Ko MH, Ohn SH, Joen B, Lee KH. Enhancing the working memory of stroke patients using tDCS. Am J Phys Med Rehabil. 2009; 88(5): 404-9.
- Plow E, Cunningham D, Beall E, Jones S, Wyant A, Bonnett C, et al. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: study protocol for a randomized controlled trial. Trials. 2013; 14: 331.
- Simis M, Adeyemo B, Medeiros L, Miraval F, Gagliardi R, Fregni F. Motor cortex-induced plasticity by noninvasive brain stimulation: a comparison between transcranial direct current stimulation and transcranial magnetic stimulation. Neuroreport. 2013; 24: 973-975.
- Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011; 25: 819-829.
- Andrade SM, Santos NA, Fernández-Calvo B, Boggio PS, Oliveira EA, Ferreira JJ. Stroke Treatment Associated with Rehabilitation Therapy and Transcranial DC Stimulation (START-tDCS): a study protocol for a randomized controlled trial. Trials. 2016; 17: 56.
- Takeuchi N, Izumi S. Noninvasive Brain Stimulation for Motor Recovery after Stroke: Mechanisms and Future Views. Stroke Res Treat. 2012; 2012:584727.
- Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002; 125: 1896-1907.
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke.Ann Neurol. 2004; 55: 400-409.
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996; 144: 160-170.
- Hesse MD, Sparing R, Fink GR. Ameliorating spatial neglect with noninvasive brain stimulation: from pathophysiological concepts to novel treatment strategies. Neuropsychol Rehabil. 2011; 21: 676-702.
- Cunningham D, Machado A, Janini D, Varnerin N, Bonnett C, Yue G, et al. The assessment of inter-hemispheric imbalance using imaging and non-invasive brain stimulation in patients with chronic stroke. Arch Phys Med Rehabil. 2015; 96: S94-S103.
- Halko M, Datta A, Plow E, Scaturro J, Bikson M, Merabet L. Neuroplastic changes flowing rehabilitative training correlate with regional electrical field induced with tDCS. NeuroImage. 2011; 57: 885-891.
- Antal A, Kincses TZ, Nitsche MA, Paulus W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp Brain Res. 2003; 150: 375-378.
- Antal A, Kincses T, Nitsche M, Bartfai O, Paulus W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: directelectrophysiological evidence. Invest Ophthalmol Vis Sci. 2004; 45: 702-707.
- Plow E, Obretenova S, Halko M, Kenkel S, Jackson M, Pascual-Leone A, et al. Combining visual rehabilitative training and noninvasive brain stimulation to enhance visual function in patients with hemianopia: a comparative case study. PM R. 2011; 3: 825-835.
- Plow EB, Obretenova SN, Fregni F, Pascual-Leone A, Merabet LB. Comparison of Visual Field Training for Hemianopia With Active Versus Sham Transcranial Direct Cortical Stimulation. Neurorehabil Neural Repair. 2012; 26: 616-626.
- Plow EB, Obretenova SN, Jackson ML, Merabet LB. Temporal profile of functional visual rehabilitative outcomes modulated by transcranial direct current stimulation. Neuromodulation. 2012; 15: 367-373.
- Olma M, Dargie R, Behrens J, Kraft A, Irlbacher K, Fahle M, et al. Long-term effects of serial anodal tDCS on motion perception in subjects with occipital stroke measured in the unaffected visual hemifield. Front Hum Neurosci. 2013; 7: 314.
- Dargie R, Olma M, Behrens J, Kraft A, Irlbacher K, Fahle M, et al. P 155. Serial anodal tDCS over V1 induces long-term effects on colour discrimination in V4 measured in the unimpaired hemifield of patients with occipital stroke. Clinical Neurophysiology. 2013; 124: e138.
- Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke. 2006; 37: 2348-2353.
- Leciana MA, Gutiérrez-Fernández M, Romano M, Cantú-Brito C, Arauz A, Olmos LE, et al. Strategies to improve recovery in acute ischemic stroke patients: Iberoamerican Stroke Group Consensus. Int J Stroke. 2014; 9: 503-513.
- Costa TL, Nagy BV, Barboni MT, Boggio PS, Ventura DF. Transcranial direct current stimulation modulates human color discrimination in a pathway-specific manner. Front Psychiatry. 2012; 3: 78.
- Costa TL, Hamer RD, Nagy BV, Barboni MT, Gualtieri M, Boggio PS, et al. Transcranial direct current stimulation can selectively affect different processing channels in human visual cortex. Exp Brain Res. 2015; 233:1213-1223.
- Santos NA, Andrade SM. Visual contrast sensitivity in patients with impairment of functional Independence after stroke. BMC Neurol. 2012; 12: 90.
- Santos NAD, Andrade SM, Fernández-Calvo B. Detection of spatial frequency in brain-damaged patients: Influence of hemispheric asymmetries and hemineglect. Front Hum Neurosci. 2013; 7: 92.
- Park S, Koh E, Choi H, Ko M. A double-blind, sham controlled, pilot study to assess the effects of the concomitant use of transcranial direct current stimulation with the computer assisted cognitive rehabilitation to the prefrontal cortex on cognitive functions in patients with stroke. J Korean Neurosurg. 2013; 54: 484-488.