
Special Article - Stroke Recovery and Rehabilitation
Austin J Cerebrovasc Dis & Stroke. 2016; 3(2): 1045.
Therapeutic Efficacy and Mechanism of Action Assessment of AT1 Blocker Telmisartan with Calcium Channel Blocker Nimodipine and Cox Inhibitor Aspirin in Global Ischemic Mice Model
Justin A¹, Divakar S¹, Ramamoorthy V² and Ramanathan M³*
¹Department of Pharmacology, JSS College of Pharmacy, India
²Department of Biotechnology, PSG College of Technology, India
³Department of Pharmacology, PSG College of Pharmacy, India
*Corresponding author: Ramanathan M, Department of Pharmacology, PSG College of Pharmacy, Peelamedu, Coimbatore, Tamilnadu, India
Received: June 30, 2016; Accepted: August 10, 2016; Published: August 12, 2016
Abstract
The pathogenesis involved in the cerebral ischemia is broad and treatment approach towards single target of ischemic pathological events may not appropriate to prevent the further disease progression. Considerate and development of combination therapy for cerebral ischemia with two or more potential agents targeting different molecular events of cerebral ischemia may fetch effective therapy. Therefore, the present study is planned to assess the therapeutic efficacy of combination of AT1 blocker telmisartan (TM) with calcium channel blocker nimodipine (NM) (or) COX inhibitor aspirin (ASP) in global ischemic mice model. Global ischemia in mice was induced by occlusion of both common carotid arteries followed by reperfusion injury, and then respective treatments were made. The therapeutic efficacy of different drug combinations was evaluated through motor and muscle co-ordination tests, cerebral blood perfusion, neurotransmitter, cytokines and brain angiotensin II peptide level measurements in brain. Gene expression study (NF-κB, GSK-3β, EAAT-2, AT1 & AT2) and cresyl violet, synaptophysin, GFAP staining were carried out in hippocampus region of brain to support the research findings. Results indicate that TM and NM restored the CBF, improved the muscle and motor co-ordination, attenuated glutamate, aspartate and GABA release and EAAT2 expression. Further TM and NM treatments regulated the release of inflammatory cytokines, GSK-3β. ASP could control only inflammatory mediators release during ischemic condition. The AT1 receptor expression was down regulated with TM treatment. TM has shown synergistic effect with NM and with aspirin it was observed only in few parameters. Positive correlation with glutamate clearance and cytokine levels were observed. The study can be concluded that Ang II/AT. pathway mediated neuroprotection during ischemic reperfusion injury. TM with NM has shown better synergistic response than TM and ASP combination. NM by preventing calcium entry has shown neuroprotective activity. Cytokines and excitotoxicity are interlinked, clearance of glutamate attenuated the inflammatory mediator response and not vice versa.
Keywords: Glutamate; Interleukins; Behaviour; Stroke; GABA; GSK3β
Abbreviations
Ang II: Angiotensin II; ANOVA: Analysis of Variance; ASP: Aspirin; AT: Angiotensin Receptors; AT1: Angiotensin II Receptor 1; AT2: Angiotensin II Receptor 2; BBB: Blood Brain Barrier; BCCAo: Bilateral Common Carotid Artery; BSA: Bovine Serum Albumin; Ca2+: Calcium; CBF: Cerebral Blood Flow; cDNA: Complementary Deoxyribonucleic Acid; CMC: Carboxy Methyl Cellulose; COX: Cyclooxygenase; DEPC: Diethylpyrocarbonate; EAA: Excitatory Amino Acid: EAAT-2: Excitatory Amino Acid Transporter- 2; ELISA: Enzyme-Linked Immunosorbent Assay; GABA: Gamma- Aminobutyric Acid; GFAP: Glial Fibrillary Acidic Protein; GSK- 3β: Glycogen Synthase Kinase-3β; HPTLC: High Performance Thin Layer Chromatography; i.p: Intraperitoneal; i.v: Intravenous; IHC: Immunohistochemistry; IL: Interleukin; IR: Ischemic Reperfused; MCAo: Middle Cerebral Artery Occluded; mRNA: Messenger Ribonucleic Acid; Na+: Sodium; NF-κB: Nuclear Factor- κB; NM: Nimodipine; NMDA: N-Methyl-D-Aspartate Receptor; PPAR-γ: Peroxisome Proliferator-Activated Receptor Gamma; PBS: Phosphate Buffered Saline; qPCR: Quantitative Polymerase Chain Reaction; ROS: Reactive Oxygen Spices; RH: Relative Humidity; RNase: Ribonuclease; RT-PCR: Real Time - Polymerase Chain Reaction; SEM: Standard Error of Mean; TLR: Toll Like Receptor; TM: Telmisartan; TNF-α: Tumor Necrosis Factor-α
Introduction
Understanding the combination therapy in the treatment of disease is required due to multiple etiology of pathogenesis. Reports indicate that in acute ischemic stroke experimental model combination of neuroprotective drugs have shown better neuroprotective activity than the individual drug treatment [1]. The pathophysiology of cerebral ischemic stroke is a complex phenomenon; it involves energy failure, ionic imbalance, excitotoxicity through excitatory amino acids (EAA), activation of inflammatory pathways, oxidative damage by reactive oxygen species (ROS) and apoptosis leading to irreversible neuronal loss [2].
Since the pathological events of cerebral ischemia are multifaceted, treatment with single agent may not have good therapeutic value. Treatment with angiotensin receptor 1 (AT1) antagonist, telmisartan (TM) and calcium channel blocker, nimodipine (NM) have shown synergistic neuroprotective activity in middle cerebral artery occluded (MCAo) focal ischemic rat model [3]. It was attributed due to control of both inflammation and excitotoxicity in the neurons after ischemic insult, but the molecular events behind the neuroprotective activity of TM and NM combination has not been elucidated.
TM has been focused in the treatment of cerebral ischemia because of its central anti-inflammatory property and high BBB permeation [4,5]. Earlier studies have shown that TM (5 mg/kg) treatment suppressed the cerebral injury in a murine model of transient focal ischemia through its anti-inflammatory effects through AT1 receptor blockade and PPAR-γ (Peroxisome proliferatoractivated receptor gamma) agonist property [5]. Interestingly, TM exerted neuroprotection without altering the blood pressure at low dose whereas at high dose have shown neuroprotection along with reduced blood pressure in cerebral ischemic condition [6]. NM was developed initially for the treatment of high blood pressure, later it has been investigated in the various cerebrovascular related problems such as subarachnoid hemorrhage, cerebral resuscitation, head injury and ischemic stroke [7]. NM ameliorated the glutamate induced excitotoxicity in MCAo occluded ischemic rats with remarkable elevation of energy metabolites, this mechanism was mediated by reduction of Ca2+-dependent NMDA receptors activation and central vasodilation property of NM [8,9]. Li, et al. [10] have proposed attenuation of inflammatory reactions and oxidative stress with NM. Amyloid-β induced IL-1β production from microglia was inhibited with NM which further supports the anti-inflammatory property of NM [11].
ASP is useful in the management of cerebral ischemic patients due to its anti-inflammatory and anti platelet aggregation activity [12]. Clinical observations have shown that early treatment of ASP and continuing treatment in acute ischemic patients have reduced the risk of early re-occurrence of stroke [13]. Tissue plasminogen activator (t-PA) as thrombolytic agent has to be administered within critical time period of 4 after ischemic insult. Unfortunately, t-PA only restore the cerebral blood flow (CBF), but it fails to prevent the excitotoxicity, oxidative stress and inflammation which are the major events in ischemic pathology; this could be the reason for less therapeutic outcome in ischemic patients [14,15]. Hence, drugs which can control the molecular pathological events in the neurons after ischemic insult can be a better drug of choice in ischemic stroke patients. Multiple approaches with different therapeutic agents which are already in clinical will give a new therapeutic avenue in cerebral ischemia.
intensity of neuroprotective effect was evaluated through monitoring motor and muscle co-ordination, measurement of cerebral blood perfusion in ischemic mice, neurotransmitters quantification, and evaluation of cytokines and angiotensin II (Ang II) peptide level in brain. Further the above mentioned parameters were evaluated by correlation analysis between the different treatment groups. To investigate the molecular mechanism of the combination therapy in ischemic conditions, the gene expression of NF-κB (nuclear factor- κB), GSK-3β (glycogen synthase kinase-3β), EAAT-2 (excitatory amino acid transporter- 2), AT1 & AT2 (angiotensin receptors) were studied. The immunohistochemistry staining of synaptophysin, GFAP (glial fibrillary acidic protein) were carried out in hippocampus region of brain to understand the protective effect of the drugs.
Materials and Methods
Chemicals and reagents
Telmisartan (TM) and nimodipine (NM) gift samples was obtained from Zydus Cadila, India. Aspirin (ASP) was purchased from Sigma Aldrich, USA. Triton X-100 was supplied from HiMedia Laboratories, India. TriZol reagent, benzamidine, phenylmethylsulfonyl fluoride, penzethonium chloride, and aprotinin were procured from Sigma Aldrich, USA. cDNA Reverse Transcription kit and DEPC water were purchased from Life technologies, USA. SYBR Premix Ex Taq II was obtained from Takara, USA. RNase-Free Water was supplied from Qiagen, India. Monoclonal mouse anti-GFAP and anti-synaptophysin clone SY38 antibodies were procured from Dako, Denmark. All other chemicals, reagents and solvents were of analytical grade unless mentioned.
Experimental animals
Swiss albino male mice (25-35 g) were used in the study. The mice were supplied from the central animal house facilities, PSG Institute of Medical Sciences and Research, Coimbatore, India. All the animals were housed in a separate polypropylene cage in a good ventilated room and animals were maintained at 25±2°C temperature and 55% relative humidity (RH) conditions with a 12 hr light/ dark cycle. The animals had free access to food and water ad libitum. All the experimental animals were acclimatiser at least for 7 days prior to the experiment to adapt to the laboratory conditions. All the animal experimental procedures were carried out according to the “Guide for the Care and Use of Laboratory Animals” (Indian Council of Medical Research). Institutional Animal Ethical Committee (IAEC), PSG Institute of Medical Sciences and Research, Coimbatore, India approved the study protocol (proposal authorisation number-158/ PO/BC/99/CPCSEA/163).
Experimental design
This study includes six groups and each group consist of 9 mice. The six groups are as follows, the first group was ischemic reperfused (IR) group, the second, third and fourth groups were telmisartan (5 mg/kg) [TM5], nimodipine [NM5] and aspirin (100 mg/kg) [ASP100] treated groups. The fifth group was treated with TM5 and NM5 combination, whereas sixth group was administered with TM5 and ASP100 combination. The treatment groups and experimental design are represented in Figure 1.
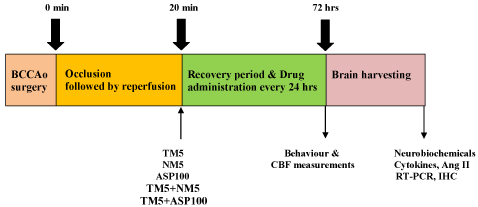
Figure 1: Experimental design.
Drug preparations and administration
TM, NM and ASP were suspended in 0.3% carboxymethyl cellulose (CMC) for oral administration. After the reperfusion injury, the treatment was continued as once daily dose in the morning to respective groups until the study was completed.
Surgical procedure
Global ischemia in mice was induced by occlusion of bilateral common carotid artery (BCCAo) model. The mice were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and placed on the operating table. Mid incision was made in anterior neck region exposing the right and left common carotid arteries. Both the common carotid arteries were occluded with the help of micro vascular clips. The restriction of blood flow through the arteries was confirmed visually. The animals were then left for 20 min after the occlusion and the occlusions in the both arteries were relaxed by removing the micro vascular clips to ensure the reflow of the blood (reperfusion injury). Throughout the surgical procedure, body temperature of the mice was maintained at 37±0.5°C using thermostatically controlled heating blanket. Post-operative care includes the application of neomycin topical ointment in the incision made. Lidocaine was applied locally in the incision to reduce the pain, and 5 ml of sterile isotonic saline was injected subcutaneously to prevent dehydration during recovery. The behavioral assessments were made after 72 hrs of ischemic reperfusion (IR) [16,17].
Locomotor activity
Motor co-ordination in mice was evaluated by keeping the mice in actophotometer (Microteknik, India, 45x45 cm) for 5 min. The movement of the animal directly correlates with the cut off a beam of light falling on a photocell. The number of cut off (count) was displayed digitally and recorded [18]. The count indicates the number of ambulations made by the mice within a 5 min time duration.
Rotarod test
Rotarod test was performed to assess the muscle co-ordination activity of ischemic animals using rotarod apparatus (Microteknik, India, 5 compartment systems). The experimental animals were placed for 5 min on the rotating rod at a speed of 20 rpm. The time spent by animals on the rotating rod was recorded to assess the muscle co-ordination and grip strength of the animal [19].
Laser doppler image analyzer
After rotarod test, the mice were subjected to mild anaesthesia. Skin incision was made above the skull region and the skull was exposed to measure the cerebral blood flow using Laser Doppler image analyzer (Moor-FLPI, UK) technique. The blood flow intensity was expressed as flux mean and it was measured in the mid brain region as per manufacturer guidelines. The colour patterns in the brain images are represent as red – high blood flow; yellowish orange – low blood flow and blue – no blood flow respectively.
Brain isolation
Excessive ether anesthesia was given to all the animals for euthanasia and their brains were removed. Brain samples were suspended in buffer solution (1% Triton X-100, aprotinin 200 U/ ml, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride, 1 mM benzamidine and 1 mM ethylenediaminetetraacetic acid) and then centrifuged at 14,000 x g for 30 min at 4°C and the supernatant was separated for Ang II and cytokines estimation. Three brain samples were used for each analysis.
Neurotransmitters and neurobiochemicals estimation
The neurotransmitters glutamate, aspartate, and GABA (gamma- Aminobutyric acid) were estimated using high performance thin layer chromatography (HPTLC) [3].
ELISA
The total brain Ang II peptide content in the brain homogenate was estimated by using angiotensin II ELISA kit according to manufacturer instructions (Sigma-Aldrich, USA). The proinflammatory cytokines IL-1β and IL-6 level in different brain regions were quantified using ELISA kits as per manufacturer’s instruction (Quantikine and Invitrogen).
RT-PCR
The total RNA from brain samples were isolated using TriZol reagent (Sigma-Aldrich, USA) and quantified using Nano Drop (Thermo Scientific, USA). Then total RNA was converted to cDNA using high-capacity cDNA Reverse Transcription Kit (Life technologies, USA). A two step qPCR was performed using Step One Plus instrument. The cDNA, converted from 2 μg of RNA was used for qRT-PCR. The SYBR green master mix was purchased from Takara, USA. The forward and reverse primers for all the genes were obtained from Sigma, India (Table 1). The primers were designed using Primer3 software. The reaction mixture was prepared and allowed for qRT-PCR cycles. The PCR running conditions were as follows SYBR green master mix activation (30 sec, 95°C); initial denaturation (5 sec, 95°C), and annealing-elongation (30 sec, 60°C) respectively. The gene expression was calculated by ΔΔCt method using β-actin as internal control [20].
Gene
Primers (sequence)
Base pairs
β-actin
Forward 5’-ACCATGTACCCAGGCATTGCT-3’
Reverse 5’- TCCACACAGAGTACTTGCGCT-3’
113
EAAT-2
Forward 5’-TAACTCTGGCGGCCAATGGAAAGT-3′
Reverse 5’-ACGCTGGGGAGTTTATTCAAGAAT-3′
113
GSK-3β
Forward 5’-CGGGACCCAAATGTCAAACT-3’
Reverse 5’-CTGAATCCGAGCATGTGGAG-3’
126
NF-κB*
Forward 5’-CTTCCGAATTTGGCGTCCTTC-3’
Reverse 5’-GGGGACAGCGACACCTTTTA-3’
218
AT1
Forward 5’-AGTTGGGAGGGACTGGATGA-3’
Reverse 5’-GTTAAGTCCGGGAGAGCAGC-3’
149
AT2
Forward 5’-TGATGCCTTCTTGGGGGTAA-3’
Reverse 5’-GGAACTGTGCCCAGAAATGC-3’
120
*annealing-elongation (60 sec, 60°C)
Table 1: The forward and reverse primers for all the genes were obtained from Sigma, India.
Correlation study
Correlation analysis study was done for EAAT-2 mRNA expression vs. glutamate /or cytokines levels in the brain with respect to each treatments (IR, TM5, NM5, ASP100, TM5+NM5 and TM5+ASP100) were made.
Histopathology and immunohistochemistry (IHC) studies
Cresyl violet staining: The mice were sacrificed by excessive anaesthesia and the brain was isolated quickly and immersed in 10% formalin for fixation. After removing lipid debris by soaking in alcohol, the brain was embedded in paraffin wax. Five μm thin coronal sections were taken, processed and stained with 0.1% cresyl violet. The sections were dehydrated in increasing ethanol concentrations. The stained brain sections were observed under a binocular light microscope (40X) and photographed to assess the extent of neurodegeneration and neuroprotection by different treatments [8].
Synaptophysin: The integrity of synapse in ischemic brain regions were measured by performing IHC staining of synaptophysin. After isolation of brain, it was fixed quickly in 10% formalin. 5 μm thin paraffin sections were made and IHC was done using a FLEX monoclonal mouse anti-synaptophysin clone SY38 antibody (Dako, Denmark). The staining protocol was followed as per the instructions given by the manufacturer. The active synapse was evaluated under a binocular light microscope (40X) and photographed [3].
Glial Fibrillary Acidic Protein (GFAP): About 5 μm thin paraffin sections of different brain regions were stained with monoclonal mouse anti-GFAP (Dako, Denmark) (1:300) using immunoperoxidase techniques. The paraffin sections of the mouse brain were deparaffinised and hydrated with distilled water. The sections containing antigenic sites were exposed by incubating them in a microwave oven with antigen retrieval solution (trisodium citrate, pH 6.2) for 20 min at 96°C. Following retrieval, the slides were cooled with distilled water for 5 min. Subsequently the slides were washed with phosphate buffered saline (PBS) in each step. Then the slides were treated with 3% hydrogen peroxide to decrease the endogenous peroxidise activity. The slides were than incubated with 1.5% bovine serum albumin (BSA) for blocking the non-specific binding sites. The immunoreactivity was detected with a biotinylated goat anti-mouse IgG (for GFAP) secondary antibody at 1:100 dilution and Immuno Cruz mouse ABC staining kit and it was evaluated under a binocular light microscope (40X) and photographed [21].
Quantification of neurons: The quantification of cresyl violet positive neurons, synaptophysin stained synapse and GFAP positive neurons in the hippocampus CA1 region of IR and the effect of different treatments in ischemic conditions were made by counting the stained neurons. Three different hippocampus sections were taken for quantification of each staining. The positive neurons in each section were counted at five different regions using the soft ware program provided with the microscope.
Statistical analysis
Data were expressed as mean±SEM. Statistical significance between the groups were analyzed by one way ANOVA followed by Dunnett multiple comparison tests using GraphPad Prism, 4.03 (San Diego, USA). Probability levels less than 0.05 (p<0.05) were fixed as the criterion for statistical significance.
Results
Effect of TM combination on locomotor activity in global ischemic mice model
Treatment with TM5 (p<0.05), NM5 (p<0.05), TM5+NM5 (p<0.001) and TM5+ASP100 (p<0.001) have shown increased number of ambulation in ischemic mice. The combination of TM5 with NM5 (p<0.05) or ASP100 (p<0.01) have significantly increased the locomotor activity in ischemic mice in comparison to TM5 alone treated mice. NM5, TM5 and ASP100 treated mice exhibited similar locomotor activity (Figure 2).
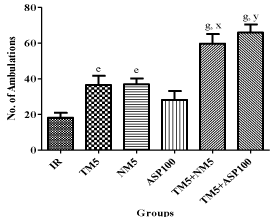
Figure 2: Effect of TM combination on locomotor activity in global ischemic
mice model.
Data are expressed as mean ±SEM. Superscript e, g denotes p<0.05,
p<0.001 vs. IR; x, y denotes p<0.05, p<0.01 vs. TM5 respectively significance
with one way ANOVA followed by Dunnett-t test. The abbreviations indicate
as IR- ischemic reperfused; TM5- telmisartan (5 mg/kg); NM5-nimodipine (5
mg/kg) and ASP100 – Aspirin (100 mg/kg) respectively.
Effect of TM combination on muscle co-ordination in global ischemic mice model
TM & NM treated ischemic mice have shown significant improvement in the muscle co-ordination. ASP100 failed to improve the muscle co-ordination in ischemic mice. The time spent in rotating rod have been increased significantly in ischemic mice treated with TM5+NM5 (p<0.001) and TM5+ASP100 (p<0.05) in comparison to TM5 alone treated mice (Figure 3).
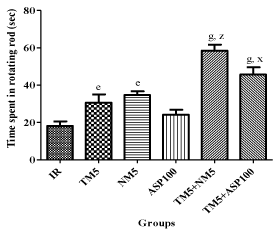
Figure 3: Effect of TM combination on muscle co-ordination in global
ischemic mice model.
Data are expressed as mean±SEM. Superscript e, g denotes p<0.05, p<0.001
vs. IR; x, z denotes p<0.05, p<0.001 vs. TM5 respectively significance with
one way ANOVA followed by Dunnett-t test. The abbreviations indicate as
IR- ischemic reperfused; TM5- telmisartan (5 mg/kg); NM5-nimodipine (5 mg/
kg) and ASP100 – Aspirin (100 mg/kg) respectively.
Effect of TM combination on cerebral blood flow in global ischemic mice model
In comparison to ischemic mice, significant increase in the CBF was observed with TM5 (p<0.01), NM5 (p<0.001) and its combination (p<0.001). In comparison to TM5 treated ischemic mice, the combination with NM5 have shown increased flux mean in laser doppler analyzer. Aspirin did not improve the CBF in comparison to IR mice. Combination of TM5 with ASP100 has not shown significant improvement in the cerebral blood flow in comparison to TM5 treated mice (Figure 4A and B).
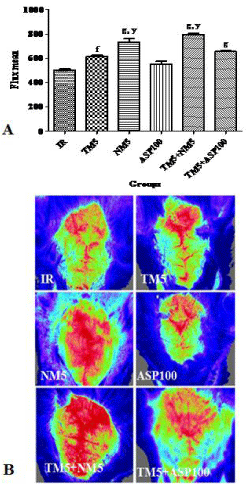
Figure 4: Effect of TM combination on CBF in global ischemic mice model.
[A] represents the flux mean value of CBF and [B] represents the Laser
Doppler blood perfusion image of brain. Data are expressed as mean±SEM.
Superscript f, g denotes p<0.01, p<0.001 vs. IR; y denotes p<0.01 vs. TM5
respectively significance with one way ANOVA followed by Dunnett-t test.
The abbreviations indicate as IR- ischemic reperfused; TM5- telmisartan
(5 mg/kg); NM5-nimodipine (5 mg/kg) and ASP100 – Aspirin (100 mg/kg)
respectively.
Effect of TM combination on brain glutamate level in global ischemic mice model
In cortex and striatum, the glutamate level was significantly reduced with all the treatments groups except ASP100. NM5 (p<0.05) treatment and its combination with TM5 (p<0.01) have significantly reduced the glutamate level in hippocampus region in comparison to IR mice. Ischemic mice administered with TM5+NM5 combination significantly reduced the cortex (p<0.05), and striatum (p<0.01) glutamate level in comparison to TM5 treatment (Table 2).
Groups
Glutamate (µmoles/g tissue)
Aspartate (µmoles/g tissue)
GABA (µmoles/g tissue)
Cortex
Striatum
Hippo campus
Cortex
Striatum
Hippo campus
Cortex
Striatum
Hippo campus
IR
11.12±0.30
11.55±1.02
8.77±0.36
9.57±0.28
10.02±0.05
9.55±0.33
8.59±0.11
8.54±0.15
7.85±0.19
TM5
7.34±0.42g
8.45±0.43e
7.42±0.29
7.69±0.10f
6.76±0.56g
6.74±0.19f
6.95±0.16g
6.19±0.29g
6.14±0.09
NM5
6.81±0.11g
7.09±0.48g
7.15±0.34e
6.49±0.40g
5.89±0.22g
5.81±0.38g
5.75±0.26g,x
5.72±0.09g
5.88±0.13e
ASP100
9.82±0.21
10.65±0.46
9.93±0.38
9.19±0.17
9.05±0.32
7.99±0.31
8.08±0.24
7.75±0.47
8.20±0.82
TM5+NM5
6.01±0.18g,x
6.06±0.10g,y
6.35±0.36f
5.79±0.48g,x
4.82 ±0.52g
4.60±0.68g,x
4.87±0.17g,z
5.06±0.20g
4.45±0.22g
TM5+ASP100
6.86±0.19g
7.08±0.11g
8.28±0.15
7.19±0.27f
6.88±0.34g
6.28±0.28g
6.39±0.15g
5.74±0.14g
5.94±0.19e
Data are expressed as mean±SEM. Superscript e, f, g denotes p<0.05, p<0.01, p<0.001 vs. IR; x, y,z denotes
p<0.05, p<0.01, p<0.001 vs. TM5 respectively.
Table 2: Effect of different treatments on brain glutamate and aspartate level in global ischemic mice model.
Effect of TM combination on brain aspartate level in global ischemic mice model
Administrations of TM5, NM5, TM5+NM5 and TM5+ASP100 groups have shown significant reduction of aspartate level in cortex, striatum and hippocampus regions in comparison to ischemic mice. ASP100 treatment fails to reduce the aspartate level in all the three regions of ischemic mice. The aspartate level in cortex (p<0.05) and hippocampus (p<0.05) region of ischemic brain was remarkably decreased with combination therapy of TM5 and NM5 in comparison to TM5 treatment (Table 2).
Effect of TM combination on brain GABA level in global ischemic mice model
The cortex and striatum GABA level (p<0.001) was significantly decreased with TM5, NM5, TM5+NM5 and TM5+ASP100 treatments. NM5 (p<0.05), ASP (p<0.05), TM5+NM5 (p<0.001) and TM5+ASP100 (p<0.05) treatments significantly reduced the GABA level in hippocampus region in comparison to vehicle treated IR group. Co-administration of TM5 and NM5 in ischemic reperfused mice have shown significant reduction in GABA level in cortex region (p<0.001). Treatment with NM5 (p<0.05) significantly reduced the cortex GABA level as compared to TM5 treatment. ASP100+TM5 treatment did not produce any significant reduction in brain GABA level in comparison to IR vehicle mice (Table 2).
Effect of TM combination on brain IL-1β level in global ischemic mice model
All the treatment groups have shown reduction of IL-1β level in striatum region, whereas in hippocampus region ASP100 fails to decrease the IL-1β level in comparison to IR mice. Significant reduction in IL-1β level in hippocampus region (p<0.05) was observed with TM5+NM5 combination in comparison to TM treated mice. All other treatments did not show any significant effect in the reduction of IL-1β level in comparison to TM5 treatment (Table 3).
Groups
IL-1β (pg/ml)
IL-6 (pg/ml)
Striatum
Hippocampus
Striatum
Hippocampus
IR
91.28±1.89
109.54±5.01
953.32±48.07
1035.10±33.58
TM5
62.69±4.86g
71.87±4.29f
629.54±9.42g
696.88±10.28g
NM5
60.28±4.95g
79.22±4.93e
588.57±22.90g
699.75±5.28g
ASP100
69.27±2.54f
85.55±3.10
603.02±7.68g
729.22±24.45g
TM5+NM5
43.64±2.04g
41.19±4.61g,x
471.38±13.81g,z
434.73±14.25g,z
TM5+ASP100
50.89±5.80g
61.59±9.65g
575.27±22.02g
609.62±6.19g,y
Data are expressed as mean±SEM. Superscript e, f, g denotes p<0.05, p<0.01 p<0.001 vs. IR; x, y, z denotes p<0.05, p<0.01, p<0.001 vs. TM5 respectively.
Table 3: Effect of different treatments on brain IL-1β and IL-6 level in global ischemic mice model.
Effect of TM combination on brain IL-6 level in global ischemic mice model
In comparison to vehicle treated IR mice there is a significant reduction of IL-6 level (p<0.001) in striatum and hippocampus regions with all the treatments. Treatment of TM5+NM5 in ischemic mice have shown significant attenuation of IL-6 level in striatum (p<0.001) and hippocampus (p<0.05) regions in comparison to TM5 treated mice. Treatment with TM5+ASP100 have reduced the IL-6 level in hippocampus region (p<0.01) in comparison to TM5 treated ischemic brain regions (Table 3).
Effect of TM combination on brain Ang II level in global ischemic mice model
Brain Ang II peptide level was significantly reduced in ischemic mice treated with TM5 (p<0.001), NM5 (p<0.01) and combination groups (p<0.001). In comparison to TM5, combination of TM5 with NM5 (p<0.01) has significantly reduced the brain Ang II peptide level. Aspirin failed to show any effect in this parameter (Figure 5).
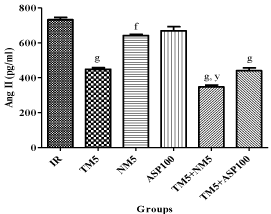
Figure 5: Effect of TM combination on brain Ang II peptide level in global
ischemic mice model. Data are expressed as mean±SEM. Superscript f,
g denotes p<0.01, p<0.001 vs. IR; y denotes p<0.01 vs. TM5 respectively
significance with one way ANOVA followed by Dunnett-t test. The
abbreviations indicate as IR- ischemic reperfused; TM5- telmisartan (5
mg/kg); NM5-nimodipine (5 mg/kg) and ASP100 – Aspirin (100 mg/kg)
respectively.
Effect of TM combination on NF-κB expression in global ischemic mice model
The NF-κB expression was found to be decreased with all the treatment groups in comparison to ischemic group. Administration of TM5 and NM5 (p<0.001) in BCCAo occluded mice has significantly reduced the brain NF-κB expression in comparison to TM5 alone treated mice (Figure 6A and B).
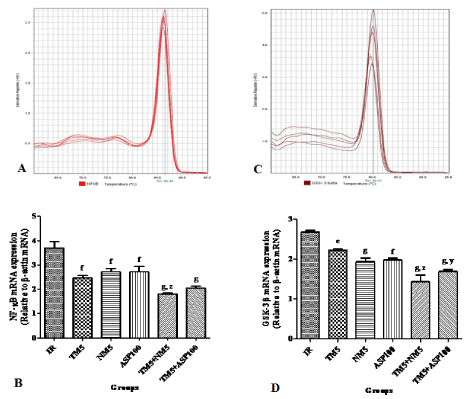
Figure 6: Effect of TM combination on NF-κB & GSK-3β expression in global ischemic mice model. [A] & [C] represents the melt curve of NF-κB & GSK-3β; [B] &
[D] indicates the relative mRNA expression of NF-κB & GSK-3β in brain. Data are expressed as mean±SEM. Superscript e, f, g denotes p<0.05, p<0.01, p<0.001
vs. IR; y, z denotes p<0.01, p<0.001 vs. TM5 respectively significance with one way ANOVA followed by Dunnett-t test. The abbreviations indicate as IR- ischemic
reperfused; TM5- telmisartan (5 mg/kg); NM5-nimodipine (5 mg/kg) and ASP100 – Aspirin (100 mg/kg) respectively.
Effect of TM combination on GSK-3β expression in global ischemic mice model
In comparison to ischemic mice, the GSK-3β expression was significantly reduced with all the treatment groups. The brain GSK- 3β expression was significantly reduced further with TM5+NM5 (p<0.001) and TM5+ASP100 (p<0.01) combinations when compared to the TM5 treated ischemic mice (Figure 6C and D).
Effect of TM combination on EAAT-2 expression in global ischemic mice model
Treatments with TM5 (p<0.05), NM5 (p<0.05), TM5+NM5 (p<0.001) and TM5+ASP100 (p<0.001) have significantly increased the EAAT-2 expression in ischemic mice. Combination of TM5 with NM5 (p<0.001) and TM5 with ASP100 (p<0.05) have significantly increased the brain EAAT-2 expression in ischemic mice in comparison to EAAT-2 expression level of TM5 group (Figure 7A and B).
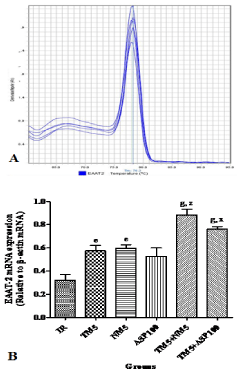
Figure 7: Effect of TM combination on EAAT-2 expression in global ischemic
mice model. [A] represents the melt curve of EAAT-2; [B] indicates the relative
mRNA expression of EAAT-2 in brain. Data are expressed as mean±SEM.
Superscript e, g denotes p<0.05, p<0.001 vs. IR; x, z denotes p<0.05,
p<0.001 vs. TM5 respectively significance with one way ANOVA followed by
Dunnett-t test. The abbreviations indicate as IR- ischemic reperfused; TM5-
telmisartan (5 mg/kg); NM5-nimodipine (5 mg/kg) and ASP100 – Aspirin (100
mg/kg) respectively.
Effect of TM combination on AT1 expression in global ischemic mice model
The AT1 receptor expression was significantly reduced with TM5 (p<0.05), TM5+NM5 (p<0.01) and TM5+ASP100 (p<0.05) treatment groups in comparison to ischemic group. In comparison to TM5 treatment group, no significant alteration of AT1 receptor expression in brain was observed with other treatments in global ischemic mice (Figure 8A and B).
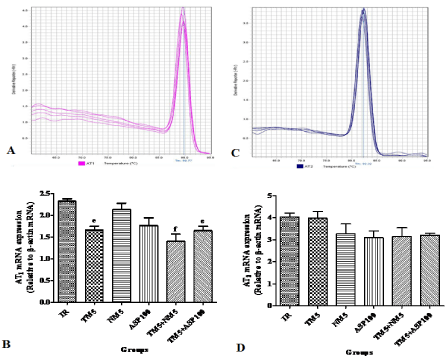
Figure 8: Effect of TM combination on AT1 & AT2 expression in global ischemic mice model. [A] & [C] represents the melt curve of AT1 & AT2; [B] & [D] indicates
the relative mRNA expression of AT1 & AT2 in brain. Data are expressed as mean±SEM. Superscript e, f denotes p<0.05, p<0.01 vs. IR respectively significance
with one way ANOVA followed by Dunnett-t test. The abbreviations indicate as IR- ischemic reperfused; TM5- telmisartan (5 mg/kg); NM5-nimodipine (5 mg/kg)
and ASP100 – Aspirin (100 mg/kg) respectively.
Effect of TM combination on AT2 expression in global ischemic mice model
Different treatments in ischemic mice have not shown any significant alteration of brain AT2 receptor expression in comparison to IR and TM5 treated mice (Figure 8C and D).
Correlation study
Correlation analysis between EAAT-2 expression vs. glutamate (p<0.04) vs. IL-1β (p<0.02) vs. IL-6 (p<0.008) in brain have shown positive correlation, which indicates that there is relationship between glutamate transport system with inflammatory cytokines in ischemic condition and treatment (Figure 9A, B and C).
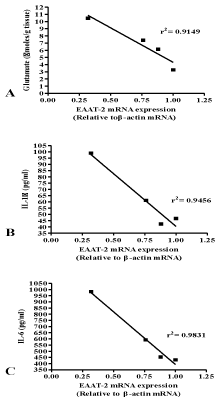
Figure 9: Correlation analysis of EAAT-2 expression with glutamate and/
or cytokines levels in brain. [A] represent the EAAT-2 mRNA expression
vs. glutamate; [B] indicates EAAT-2 mRNA expression vs. IL-1β level; [C]
denotes the EAAT-2 mRNA expression vs. IL-6 level in brain respectively.
Cresyl violet staining
BCCAo occlusion for 20 min and followed by reperfusion injury in mice (IR) has produced severe neuronal damage which is characterized by presence of dark stained neurons in the hippocampus regions of the brain. Treatment with TM5 has protected the neurons and restored the neuronal morphology in comparison to ischemic mice. The combination therapy of TM5+NM5 has shown well protected neuronal structure in hippocampus region of brain with less shrinkage of neurons in comparison to TM5 treatment. ASP100 have shown partial protection of neurons as observed by dark nuclear stained neurons (Figure 10 and 13).
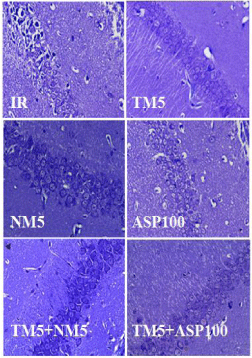
Figure 10: Represents the cresyl violet staining of CA1 region of hippocampus.
The abbreviations indicate as IR- ischemic reperfused; TM5- telmisartan
(5 mg/kg); NM5-nimodipine (5 mg/kg) and ASP100 – Aspirin (100 mg/kg)
respectively.
Synaptophysin immunohistochemistry (IHC) staining
Induction of ischemia in mice has shown loss of synaptophysin protein expression in the hippocampus region of the brain which was observed through loss of brown colour stained area in the hippocampus. TM5 treatment in ischemic mice has shown appearance of synaptophysin expressions in the hippocampus as evidenced by the restoration of synapse integrity. Combination therapy of TM5 with NM5 has shown more synaptophysin staining in the hippocampus in comparison to TM5 alone treated mice. TM5+ASP100 combination shows synaptophysin restoration but it was comparatively less than TM5+NM5 treatment. NM5 treatment also exhibited restoration of synaptophysin. ASP100 has not shown any significant synaptophysin expression in comparison to IR mice (Figure 11 and 13).
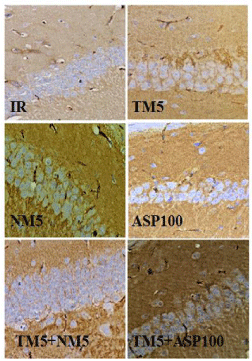
Figure 11: Represents the synaptophysin immunohistochemistry staining of
CA1 region of hippocampus.
The abbreviations indicate as IR- ischemic reperfused; TM5- telmisartan
(5 mg/kg); NM5-nimodipine (5 mg/kg) and ASP100 – Aspirin (100 mg/kg)
respectively.
Glial fibrillary acidic protein (GFAP) IHC staining
The expression of GFAP in the hippocampus region was found to be increased in IR mice. Treatment with TM5 in ischemic mice has reduced the appearance of GFAP staining in the hippocampus. Treatment of TM5+NM5 combination has shown more reduction of GFAP expression in hippocampus region in comparison to TM5 alone treated mice. NM5 and TM5+ASP100 administration in ischemic mice have shown reduction of GFAP expression but the effect was comparatively less when compared to TM5+NM5 treatment. ASP100 has shown less reduction of GFAP in comparison to IR mice (Figure 12 and 13).
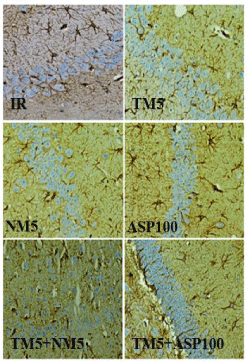
Figure 12: Represents the glial fibrillary acidic protein (GFAP)
immunohistochemistry staining of CA1 region of hippocampus.
The abbreviations indicate as IR- ischemic reperfused; TM5- telmisartan
(5 mg/kg); NM5-nimodipine (5 mg/kg) and ASP100 – Aspirin (100 mg/kg)
respectively.
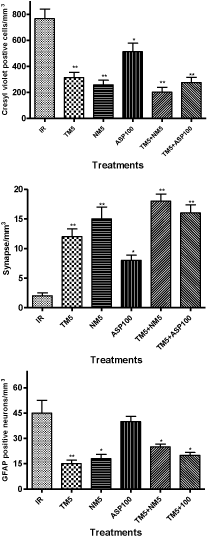
Figure 13: Represents the quantification of cresyl violet positive neurons,
synaptophysin stained synapse and GFAP positive neurons in the CA1
region of hippocampus of IR and different treatments in ischemic conditions.
The abbreviations indicate as IR- ischemic reperfused; TM5- telmisartan
(5 mg/kg); NM5-nimodipine (5 mg/kg) and ASP100 – Aspirin (100 mg/kg)
respectively. The statistical significance * and ** denotes p<0.05 and p<0.01
versus IR groups.
Discussion
During cerebral ischemia, neurons were exposed to hypoxia which leads to reduced mitochondrial function, increased intracellular cytosolic calcium level (Ca2+), excitatory amino acids (EAAs), and toxic free-radicals [2,22]. Occlusion of bilateral common carotid arteries in mice followed by reperfusion injury has reduced the motor and muscle co-ordination in animals which indicates the behavioral impairments mediated by ischemic injury [23,24]. The experimental findings have shown that the treatments with TM and NM have shown restoration of CBF, neurotransmitter alterations, inflammatory mediators and neurological dysfunctions in ischemic mice. Aspirin per se failed to alter many of the parameters studied except inflammatory markers. TM has shown synergistic effect with NM but with aspirin the synergistic effect was observed only with few of the parameters tested. Earlier our group has shown beneficial effect with TM and NM combination therapy in focal ischemia model [3]. Administration of mono sodium glutamate and release of excitatory amino acids produces anxiogenic behaviour in animals during ischemic conditions [25]. TM and NM controlled the glutamate, aspartate release which was evidenced through increased ambulatory behaviour and muscle grip strength in comparison to ischemic mice. Aspirin did not reverse the elevated excitatory amino acids hence it failed to show the improvement in behaviour. However, combination of TM with NM or ASP has shown synergistic response in behavioural parameter.
TM and NM but not ASP decreased the release of glutamate, aspartate and GABA in all three regions studied. NM, a central Ca2+ channel blocker which has a good blood brain barrier (BBB) permeability and vasodilatation property used in the present study reduced the excessive glutamate/ aspartate levels and thus attenuated ischemic neuronal death [26,8]. Further NM also down regulated GSK-3β, which contributes to excitotoxic neuronal injury and studies have proven that the inhibition of GSK-3β protects neurons against excitotoxicity [27]. Recent studies have indicated that GSK-3β has role in inflammation by reducing the translocation of CREB (cAMP response element binding protein) into the nucleus which leads to increased expression of pro-inflammatory cytokines such as IL-1β and tumor necrosis factor- α (TNF-α) [28]. NM treatment improved the cognitive function in rat vascular dementia model by increased cerebral blood flow, attenuation of NF-κB, IL-1β and TNF-α levels in hippocampus region of brain [29]. In our study, NM down regulated the levels of pro-inflammatory mediators suggesting its anti inflammatory activity through inhibition of Ca2+ channel.
Many studies have proven that AT1 receptor mediated angiotensin II peptide response leads to induction of inflammation and apoptosis during ischemic condition [30]. The released Ang II peptide acts as vasoconstrictor and promotes neuronal injury. Recent study has shown that Ang II induced dysregulation in cytokines is mediated by GSK-3β mediated alterations in downstream transcriptional factors in neuronal cells [31]. TM mechanism may be attributed due to facilitation of glutamate uptake into astrocytes through EAAT-2 transporters. TM down regulated AT1 receptor and also the Ang II peptide thus reducing the inflammatory response. The brain Ang II peptide level was not altered with NM or ASP. This observation clearly denotes that the effect is dependent on AT receptors. In our study TM antagonism down regulated the GSK-3β and thus attenuating the pro-inflammatory response. This was further supported with the observation of positive correlation between EAAT-2 transporters vs. glutamate and brain cytokines (IL-1β, IL-6) with different treatments. The AT1 receptor expression was found to be down regulated with TM treatment and not AT2 expressions. AT1 receptor antagonism is required to attenuate the ischemia induced changes, down regulated AT1 receptor might be beneficial act by the biological system.
Our previous studies have shown that ASP attenuated LPSinduced sickness behaviour in rats [32]. In a recent study, Pre and post-ischemic treatment with ASP attenuated spatial memory impairment and neuronal death in the hippocampal CA1 region in rats subjected to repeated transient global cerebral ischemia [33]. Further, it has been reported that post-ischemic administration of ASP led to a significant reduction in infarct volume only in temporary but not in permanent cerebral ischemia [34]. In the present study, down regulation of pro-inflammatory mediators was observed with ASP. In a recent study, flurbiprofen, a non selective cyclooxygenase inhibitor, attenuated hepatic IR injury by inhibiting GSK-3β signaling [35]. The anti inflammatory mechanism of ASP may be due to COX mediated GSK-3β down regulation. Several lines of evidence suggest glutamate increases cytokines release and vice versa cytokines reinforce glutaminergic response [36]. ASP attenuated the inflammatory responses but failed to reduce the excitotoxicity. Although it down regulated both IL-1β and TNF-α, as both has been linked to the exacerbation and progression of several neurological symptoms through their action on the glutaminergic system [37] yet whether these cytokines contribute to modulate the glutaminergic component of neurotoxicants remains unclear [38].
The study can be concluded that Ang II/AT1 pathway mediated neuroprotection during ischemic reperfusion injury. TM with NM has shown better synergistic response than TM and ASP combination. Per se ASP could control only inflammatory mediators. NM by preventing calcium entry has shown neuroprotective activity. Cytokines and excitotoxicity are interlinked, clearance of glutamate attenuated the inflammatory mediator response and not vice versa. The possible mechanism of action of the drugs has been represented in Figure 14.
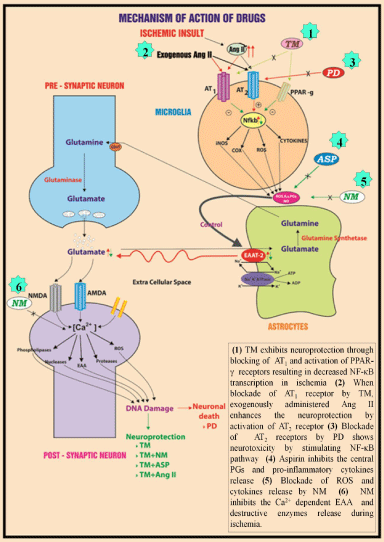
Figure 14: Proposed mechanism action of drugs in cerebral ischemia.
Acknowledgement
The authors express their special to Dr. S. Shanthakumari, Professor, Department of Pathology, PSGIMS&R, Coimbatore, for immunohistochemistry studies, and Dr. Sivaram Hariharan, Professor, PSG College of Pharmacy, Coimbatore, for the English proof reading of this article.
References
- O'Collins VE, Macleod MR, Cox SF, Van Raay L, Aleksoska E, Donnan GA, et al. Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J Cereb Blood Flow Metab. 2011; 31: 962-975.
- Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab. 2004; 24: 133-150.
- Justin A, Sathishkumar M, Sudheer A, Shanthakumari S, Ramanathan M. Non-hypotensive dose of telmisartan and nimodipine produced synergistic neuroprotective effect in cerebral ischemic model by attenuating brain cytokine levels. Pharmacol Biochem Behav. 2014; 122: 61-73.
- Noda, A, Fushiki, H, Murakami, Y, Sasaki, H, Miyoshi, S, Kakuta, H, et al. Brain penetration of telmisartan, a unique centrally acting angiotensin II type 1 receptor blocker, studied by PET in conscious rhesus macaques. Nucl Med Biol. 2012; 39: 1232-1235.
- Kasahara Y, Taguchi A, Uno H, Nakano A, Nakagomi T, Hirose H, et al. Telmisartan suppresses cerebral injury in a murine model of transient focal ischemia. Brain Res. 2010; 1340: 70-80.
- Sato K, Yamashita T, Kurata T, Lukic V, Fukui Y, Hishikawa N, et al. Telmisartan reduces progressive oxidative stress and phosphorylated a-synuclein accumulation in stroke-resistant spontaneously hypertensive rats after transient middle cerebral artery occlusion. J Stroke Cerebrovasc Dis. 2014; 23: 1554-1563.
- Kakarieka A, Schakel EH, Fritze J. Clinical experiences with nimodipine in cerebral ischemia. J Neural Transm Suppl. 1994; 43: 13-21.
- Babu CS, Ramanathan M. Post-ischemic administration of nimodipine following focal cerebral ischemic–reperfusion injury in rats alleviated excitotoxicity, neurobehavioural alterations and partially the bioenergetics. Int J Dev Neurosci. 2011; 29: 93-105.
- Scriabine A, van den Kerckhoff W. Pharmacology of nimodipine. A review. Ann N Y Acad Sci. 1988; 522: 698-706.
- Li Y, Hu X, Liu Y, Bao Y, An L. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2009; 56: 580-589.
- Sanz JM, Chiozzi P, Colaianna M, Zotti M, Ferrari D, Trabace L, et al. Nimodipine inhibits IL-1β release stimulated by amyloid β from microglia. Br J Pharmacol. 2012; 167: 1702-1711.
- Castillo J, Leira R, Moro MA, Lizasoain I, Serena J, Dávalos A. Neuroprotective effects of aspirin in patients with acute cerebral infarction. Neurosci Lett. 2003; 339: 248-250.
- Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, et al. Indications for early aspirin use in acute ischemic stroke : A combined analysis of 40 000 randomized patients from the chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000; 31: 1240-1249.
- Pulsinelli WA, Jacewicz M, Levy DE, Petito CK, Plum F. Ischemic brain injury and the therapeutic window. Ann N Y Acad Sci. 1997; 835: 187-193.
- Schellinger PD, Orberk E, Hacke W. Antithrombotic therapy after cerebral ischemia. Fortschr Neurol Psychiatr. 1997; 65: 425-434.
- Tsuchiya D, Hong S, Suh SW, Kayama T, Panter SS, Weinstein PR. Mild hypothermia reduces zinc translocation, neuronal cell death, and mortality after transient global ischemia in mice. J Cereb Blood Flow Metab. 2002; 22: 1231-1238.
- Kumaran D, Udayabanu M, Nair RU, Katyal A. Benzamide protects delayed neuronal death and behavioural impairment in a mouse model of global cerebral ischemia. Behav Brain Res. 2008; 192: 178-184.
- Boissier JR, Simon P. Action of caffeine on the spontaneous motility of the mouse. Arch Int Pharmacodyn Ther. 1965; 158: 212-221.
- Balkaya M, Kröber JM, Rex A, Endres M. Assessing post-stroke behavior in mouse models of focal ischemia. J Cereb Blood Flow Metab. 2013; 33: 330-338.
- Andrieu T, Bertolini R, Nichols SE, Setoud R, Frey FJ, Baker ME, et al. A novel steroidal antiandrogen targeting wild type and mutant androgen receptors. Biochem Pharmacol. 2011; 82: 1651-1662.
- Schmidt-Kastner R, Wietasch K, Weigel H, Eysel UT. Immunohistochemical staining for glial fibrillary acidic protein (GFAP) after deafferentation or ischemic infarction in rat visual system: features of reactive and damaged astrocytes. Int J Dev Neurosci. 1993; 11: 157-174.
- Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007; 54: 34-66.
- Zhang LQ, Xu JN, Wang ZZ, Zeng LJ, Ye YL, Zhang WP, et al. Application of locomotor activity test to evaluate functional injury after global cerebral ischemia in C57BL/6 mice. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2014; 43: 339-345.
- Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010; 2: 13.
- Ramanathan M, Sivakumar S, Anandvijayakumar PR, Saravanababu C, Pandian PR. Neuroprotective evaluation of standardized extract of Centella asciatica in monosodium glutamate treated rats. Indian J Exp Biol. 2007; 45: 425-431.
- Schwartz-Bloom RD, Sah R. gamma-Aminobutyric acid (A) neurotransmission and cerebral ischemia. J Neurochem. 2001; 77: 353-371.
- French RL., Heberlein U. Glycogen synthase kinase-3/Shaggy mediates ethanol-induced excitotoxic cell death of Drosophila olfactory neurons. Proc. Natl. Acad. Sci. U.S.A. 2009; 106: 20924–20929.
- Maixner DW, Weng HR. The Role of Glycogen Synthase Kinase 3 Beta in Neuroinflammation and Pain. J Pharm Pharmacol (Los Angel). 2013; 1: 001.
- Zhang XL, Zheng SL, Dong FR, Wang ZM. Nimodipine improves regional cerebral blood flow and suppresses inflammatory factors in the hippocampus of rats with vascular dementia. J Int Me Res. 2012; 40: 1036-1045.
- Wilms H, Rosenstiel P, Unger T, Deuschl G, Lucius R. Neuroprotection with angiotensin receptor antagonists: a review of the evidence and potential mechanisms. Am J Cardiovasc Drugs. 2005; 5: 245-253.
- Agarwal D, Dange RB, Raizada MK, Francis J. Angiotensin II causes imbalance between pro- and anti-inflammatory cytokines by modulating GSK-3β in neuronal culture. Br J Pharmacol. 2013; 169: 860-874.
- Prakash R, Ramanathan M. Effect of COX-Inhibitors Attentuated LPS Induced Behavioural Alterations in Male Wistar Rats. J Pharm Sci & Res. 2013; 5: 226-230.
- Pu F, Motohashi K, Kaneko T, Tanaka Y, Manome N, Irie K, et al. Neuroprotective effects of Kangen-karyu on spatial memory impairment in an 8-arm radial maze and neuronal death in the hippocampal CA1 region induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2009; 109: 424-430.
- Yan BC, Park JH, Lee CH, Yoo KY, Choi JH et al. Increases of antioxidants are related to more delayed neuronal death in the hippocampal CA1 region of the young gerbil induced by transient cerebral ischemia. Brain Res. 2011; 1425: 142-154.
- Fu H, Chen H, Wang C, Xu H, Liu F, Guo M, et al. Flurbiprofen, a cyclooxygenase inhibitor, protects mice from hepatic ischemia/reperfusion injury by inhibiting GSK-3ß signaling and mitochondrial permeability transition. Mol Med. 2012; 18: 1128-1135.
- Galic MA, Riazi K, Pittman QJ. Cytokines and brain excitability. Front Neuroendocrinol. 2012; 33: 116-125.
- Santello M, Volterra A. TNFα in synaptic function: switching gears. Trends Neurosci. 2012; 35: 638-647.
- Viviani B, Boraso M, Marchetti N, Marinovich M. Perspectives on neuroinflammation and excitotoxicity: a neurotoxic conspiracy? Neurotoxicology. 2014; 43: 10-20.
Citation: Justin A, Divakar S, Ramamoorthy V and Ramanathan M. Therapeutic Efficacy and Mechanism of Action Assessment of AT1 Blocker Telmisartan with Calcium Channel Blocker Nimodipine and Cox Inhibitor Aspirin in Global Ischemic Mice Model. Austin J Cerebrovasc Dis & Stroke. 2016; 3(2): 1045. ISSN : 2381-9103