
Special Article - Hemorrhagic Stroke
Austin J Cerebrovasc Dis & Stroke. 2017; 4(4): 1069.
Spontaneous Regression of a Large High-Flow Intracranial Dural Arteriovenous Fistula
Yen AJ¹*, Hetts SW², Stout C³, Antoniette L4 and Halbach VV²
¹School of Medicine, University of California San Francisco, USA
²Departments of Radiology and Biomedical Imaging, University of California San Francisco, USA
³Departments of Neuroscience, UC Riverside, USA
4Department of Neuroradiology, Sutter Health – Sacramento, USA
*Corresponding author: Yen AJ, School of Medicine, University of California San Francisco, 505 Parnassus Ave. San Francisco, CA 94143, USA
Received: June 29, 2017; Accepted: July 25, 2017; Published: August 08, 2017
Abstract
Dural arteriovenous fistulas (DAVFs) are vascular anomalies that form a connection between meningeal vessels and dural venous sinuses or cortical veins. A 47-year-old female with a history of rheumatoid arthritis and hypercoagulable state treated with warfarin was found to have a large transverse sinus DAVF with high-flow fast arteriovenous shunting. Her initial diagnostic angiogram performed at an outside institution clearly showed the large DAVF. Eight months later, a second diagnostic angiogram demonstrated that her DAVF had spontaneously resolved. Spontaneous regression of a DAVF is rare, and this is the largest DAVF that our institution has seen to resolve on its own. DAVFs have previously been known to spontaneously resolve, but few cases have been reported since it was first described in 1976. The mechanism for spontaneous closure is unknown, but several different mechanisms have been proposed. We postulate that spontaneous regression in this patient is related to her underlying hypercoagulable state and the high-flow nature of the DAVF. High-flow DAVFs can resolve spontaneously, and cautious modification of anticoagulation in select patients in order to induce fistula thrombosis may provide an avenue of management to consider.
Keywords: Dural arteriovenous fistula; DAVF; Spontaneous regression; Resolution; Hypercoagulability; High flow
Case Presentation
Intracranial dural arteriovenous fistulas (DAVFs) are rare vascular malformations that connect meningeal arteries to dural venous sinuses or cortical veins. They were originally believed to be benign lesions compared to pial arteriovenous malformations (AVMs) until intracranial hemorrhage from a DAVF was observed [1]. Although hemorrhage from ruptured cortical venous varices is the most serious and worrisome complication, DAVFs can present with a variety of symptoms, including headaches, seizures, pulsatile tinnitus, and vision disturbances. Few DAVFs have been reported to resolve spontaneously since the phenomenon was first described in 1976 [2]. The reason for spontaneous closure is largely unknown, although several mechanisms have been proposed. In this report, we describe a case of spontaneous resolution of a large DAVF in a patient with a hypercoagulable state.
A 47-year-old female was seen at an outside institution and found to have a large transverse sinus DAVF with high-flow fast arteriovenous shunting. One year prior to discovery of the DAVF, she underwent work-up at an outside institution for a seven-year history of daily occipital headaches and was found on MRI to have leftsided transverse and sigmoid sinus thromboses (Figure 1). She was clinically deemed to have a hypercoagulable state and was prescribed warfarin. After continued headaches and the development of pulsatile tinnitus, a catheter angiogram at the outside institution showed a large transverse sinus DAVF, supplied by the left occipital artery and the left middle meningeal artery and drained by the right-sided transverse sinus, sigmoid sinus, and internal jugular vein (Figure 2-4). She was referred to our institution for angiography and possible embolization of her DAVF.
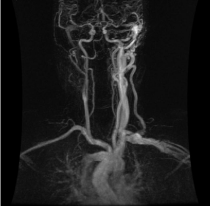
Figure 1: Anteroposterior magnetic resonance angiogram obtained from the
patient’s outside institution demonstrating a high-flow vascular lesion with
arteriovenous shunting at the left transverse sigmoid sinus junction.
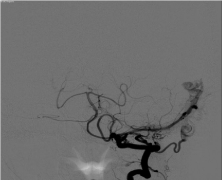
Figure 2: Anteroposterior digital subtraction angiography image obtained
from the patient’s outside institution of a left vertebral artery injection
demonstrating collateral flow to the occipital artery and filling of the dural
branch of the vertebral artery.
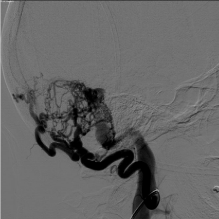
Figure 3: Lateral digital subtraction angiography image obtained from the
patient’s outside institution of a left occipital artery injection demonstrating
arteriovenous shunting from the occipital artery to the transverse sigmoid
sinus junction.
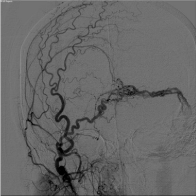
Figure 4: Anteroposterior digital subtraction angiography image obtained
from the patient’s outside institution demonstrating contralateral supply to the
dural arteriovenous fistula from branches of the right external carotid artery.
Her past medical history is significant for rheumatoid arthritis and the above-mentioned hypercoagulable state, as well as a remote history of several DVTs. She has no history of smoking, other tobacco use, alcohol use, or illicit drug use. Her brother is also noted to have a hypercoagulable state.
Although the patient was prescribed a low molecular weight heparin bridge between her usual warfarin and the time of angiography at our institution, that prescription was not filled. Thus, the patient’s warfarin was stopped four days prior to her procedure with no anticoagulant bridging. Upon presentation to our institution, her international normalized ratio (INR) was 1.5, despite a goal INR of 2.0-3.0. 8 months after the initial angiogram from an outside hospital, diagnostic angiogram at our institution demonstrated that her DAVF had spontaneously resolved with no residual supply and no other accompanied angiographic findings (Figure 5-7). As a result, no embolization was performed. The patient reported no changed in symptoms related to spontaneous occlusion of her DAVF. No treatment was given thereafter.
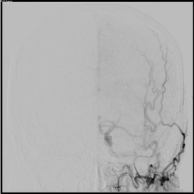
Figure 5: Anteroposterior digital subtraction angiography image of a left
external carotid artery injection demonstrating resolution of the previously
visualized dural arteriovenous fistula with no residual supply.
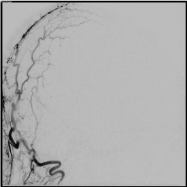
Figure 6: Lateral digital subtraction angiography image of a left occipital
artery injection demonstrating resolution of the previously visualized dural
arteriovenous fistula with no residual supply.
Discussion
In this report, we described a case of a large DAVF that spontaneously resolved in a patient with a known hypercoagulable state. Despite our institution’s extensive familiarity with dural arteriovenous fistulas, this is the largest DAVF that we have seen to resolve on its own. The mechanism of this closure is likely related to the patient’s underlying hypercoagulable state and the high-flow nature of the DAVF. The high flow through this patient’s draining veins may have damaged venous endothelium more rapidly than low flow, since veins typically are exposed to much lower flow rates than arteries. This continuous endothelial damage, combined with the patient’s hypercoagulable state, may explain why her DAVF spontaneously thrombosed while she was not optimally anticoagulated.
The first report of spontaneous resolution of a DAVF was in 1976 [2], and to our knowledge, there have been only 22 other cases published since then. The mechanism of spontaneous resolution of DAVFs has not yet been elucidated, although some authors believe that occlusion and subsequent reopening of the draining sinus can trigger spontaneous closure of the DAVF [2,3]. If hematoma occurs and causes mass effect, the resulting compression on the feeding arteries could also explain the spontaneous resolution [4]. Recently, the gradual closure of a DAVF after a large intracerebral hemorrhage was documented by serial angiograms [5]. Contrast media given during digital subtraction angiography (DSA) can affect the endothelium of the feeding arteries and has been proposed as another possible explanation [6,7], though in high-flow lesions, the first-pass dwell time of contrast on the endothelium is minimal. It has been suggested that a distinction for spontaneous closure be made between spontaneous DAVFs and post-traumatic DAVFs [8], with spontaneous DAVFs being more likely to close without treatment. With long-term follow-up, up to 12% of untreated patients may experience spontaneous resolution - most commonly in the transverse and cavernous sinuses [9] - although this timeframe is across many years, and the flow rate of these occluded DAVFs is unclear.
In this case, the patient’s hypercoagulable state likely contributed to spontaneous regression of her DAVF, yet it has also been suggested that DAVFs may be acquired in the setting of hypercoagulability [10]. This patient had a Cognard type I DAVF, and although type I DAVFs is believed to be more likely to undergo spontaneous closure or thrombosis [7], spontaneous closure has been reported for all Cognard types. Despite reports that contrast agents given during an angiogram can cause spontaneous closure; this is an unlikely explanation for our patient due to the high flow rate of her DAVF.
DAVFs, along with AVMs, remain a leading cause of both hemorrhagic and ischemic stroke [11]. Hemorrhage is a common presentation of DAVFs [12], especially in males and in lesions with pial artery supply [13,14]. The current Borden-Shucart and Cognard grading scales [15,16] of DAVFs focus on venous drainage patterns as a predictor for hemorrhage risk; higher grade DAVFs – especially those with cortical venous reflux - are more prone to rupture [11, 17] and up to 18% of DAVFs may present with hemorrhage [13,16,18]. Given that this patient’s hypercoagulability likely contributed to resolution of her DAVF, cautious modification of anticoagulation regimens with close observation may be a plausible approach to management of DAVFs in order to induce thrombosis of the fistula while still maintaining adequate anticoagulation.
Conclusion
Spontaneous regression of dural arteriovenous fistulas (DAVF) is rare, although longer follow-up may reveal it to be less infrequent than previously believed. Here we report a case of spontaneous resolution of a large high-flow DAVF in a patient with a hypercoagulable state. Further research is needed to elucidate the true mechanisms of spontaneous closure, but this patient offers additional evidence that hypercoagulability may be an inciting cause. This case also demonstrates that even high-flow DAVFs can resolve spontaneously, and careful adjustment to anticoagulation in select patients may provide an avenue of management to consider.
References
- Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology. 1969; 93: 1071-1078.
- Magidson MA, Weinberg PE. Spontaneous closure of a dural arteriovenous malformation. Surg Neurol. 1976; 6: 107-110.
- Rohr J, Gauthier G. Spontaneous regressions of a dura mater arteriovenous fistula of the posterior fossa. Rev Neurol. 1985; 141: 240-244.
- Olutola PS, Eliam M, Molot M, Talalla A. Spontaneous regression of a dural arteriovenous malformation. Neurosurgery. 1983; 12: 687-690.
- Al-Afif S, Nakamura M, Gotz F, Krauss JK. Spontaneous closure of a dural arteriovenous fistula. J Neurointerv Surg. 2015; 7: e28.
- Voormolen V, Geens K, Van Den Hauwe L, Parizel PM. Spontaneous closure of cerebral dural arteriovenous fistulas with direct cortical venous drainage: a case report. Interv Neuroradiol. 2009; 15: 359-362.
- Tsuji K, Nakagawa N, Fukawa N, Kato A. Spontaneous closure of a dural arteriovenous fistula immediately after cerebral angiography using a gadolinium contrast agent. J Stroke Cerebrovasc Dis. 2014; 23: e449-e452.
- Luciani A, Houdart E, Mounayer C, Saint Maurice JP, Merland JJ. Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR Am J Neuroradiol. 2001; 22: 992-996.
- Kim DJ, terBrugge K, Krings T, Willinsky R, Wallace C. Spontaneous angiographic conversion of intracranial dural arteriovenous shunt: long-term follow-up in nontreated patients. Stroke. 2010; 41: 1489-1494.
- Ghandi D, Chen J, Pearl M, Huang J, Gemmete JJ, Kathuria S. Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. AJNR Am J Neuroradiol. 2012; 33: 1007-1013.
- Yeh SJ, Tsai LK, Liu HM, Yip PK, Jeng JS. Ischemic stroke in patients with intracranial dural arteriovenous fistulas. J Formos Med Assoc. 2011; 110: 299-305.
- Clarencon F, Biondi A, Sourour NA, Di Maria F, Iosif C, Nouet A, et al. Spontaneous closure of intracranial dural arteriovenous fistulas: a report of 3 cases. Clin Neurol Neurosurg. 2013; 115: 971-975.
- Singh V, Smith WS, Lawton MT, Halbach VV, Young WL. Risk factors for hemorrhagic presentation in patients with dural arteriovenous fistulae. Neurosurgery. 2008; 62: 628-635.
- Li C, Wang Y, Li Y, Jiang C, Yang X, Wu Z. Clinical and Angioarchitectural Risk Factors Associated with Intracranial Hemorrhage in Dural Arteriovenous Fistulas: A Single-Center Retrospective Study. PLoS One. 2015; 10: e0131235.
- Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995; 82: 166-179.
- Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Neuroradiology. 1995; 194: 671-680.
- Soderman M, Pavic L, Edner G, Holmin S, Andersson T. Natural history of dural arteriovenous shunts. Stroke. 2008; 39: 1735-1739.
- Hacein-Bay L, Konstas AA, Pile-Spellman J. Natural history, current concepts, classification, factors impacting endovascular therapy, and pathophysiology of cerebral and spinal dural arteriovenous fistulas. Clin Neurol Neurosurg. 2014; 121: 64-75.