
Review Article
Austin J Cerebrovasc Dis & Stroke. 2017; 4(5): 1073.
Advances in the Management of Coagulopathy in Traumatic Brain Injury
Cheng R* and Singh V*
University of Califrnia, San Francisco, USA
*Corresponding author: Vineeta Singh, Zuckerberg San Francisco General Hospital, University of California, San Francisco, USA
Roger Cheng, University of California, San Francisco, USA
Received: September 20, 2017; Accepted: October 23, 2017; Published: November 20, 2017
Abstract
Coagulopathy is a frequently observed complication of trauma and in the subset of the traumatic brain injury (TBI) population who present with intracerebral hemorrhage (ICH), coagulopathy leading to progression of hemorrhagic injury (PHI) has high morbidity. Hyperfibriolysis is thought to be a primary driver of coagulopathy in traumatic injury, while TBI has been additionally associated with coagulopathy from platelet dysfunction and disseminated intravascular coagulation. Iatrogenic coagulopathy from premorbid antiplatelet and anticoagulant use further complicates management in this sensitive population. Traditional tests of coagulopathy may not fully reflect these processes; viscoelastic testing and alternative markers such as fibrinogen and D-dimer may be useful clinically as adjuncts. Pro-thrombotic agents previously studied in polytrauma, as well as interventions and reversal agents studied in the spontaneous intracerebral hemorrhage population show promise in the treatment of traumatic ICH and may become useful tools for the management of this disabling condition.
Keywords: Neurotrauma; Intracerebral hemorrhage; Coagulopathy; TBI
Introduction
Coagulopathy in traumatic brain injury
Coagulopathy is a frequently seen complication associated with traumatic brain injury (TBI). Recent studies have shown that the prevalence of TBI-induced coagulopathy to be 32-35%, and its presence has been shown to be a poor prognostic factor [1-4]. In patients known to have traumatic ICH (tICH) with parenchymal injury, progression of hemorrhage injury (PHI) is one of the most feared complications, and the ability to recognize and treat coagulopathy in a timely manner is naturally an area of great interest. The aim of this article is to provide a concise review of the current knowledge regarding the mechanisms, diagnosis, and management of coagulopathy in the TBI population.
Mechanisms of traumatic coagulopathy
Many mechanisms have been proposed to explain the development of traumatic coagulopathy (Figure 1). Early on, it was theorized that loss and consumption of coagulation factors and platelets from trauma, compounded iatrogenic ally by dilution of remaining factors, acidosis, and hypothermia from large volume resuscitation fluids were primary drivers. However, many of these issues have been addressed with elements of modern trauma resuscitation protocols such as the use of balanced transfusions with matched ratios of packed cells, plasma, and platelets. When investigated systematically in the PROMMTT study for example, coagulopathy was observed despite limited use of crystalloid and absence of significant hypothermia [5]. Moreover, there is evidence that traumatic coagulopathy occurs prior to significant loss of coagulation factors, and is linked instead with degree of shock and severity of injury [5,6].
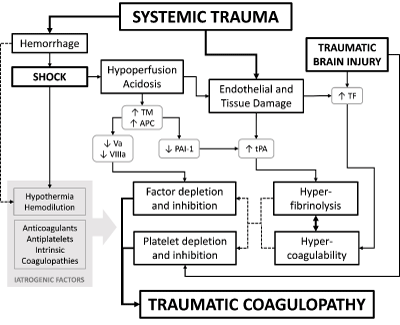
Figure 1: Theorized mechanisms of traumatic coagulopathy, and the contribution of traumatic brain injury. TF: tissue factor, TM: Thrombomodulin, APC: activated
Protein C, tPA: tissue plasminogen activator, PAI-1: plasminogen activator inhibitor-1.
Hyperfibriolysis is hypothesized to be the driver of traumatic coagulopathy though the mechanism is unclear. Elevated levels of tPA have been observed in early traumatic injury [7,8], and increased release of tPA from endothelium secondary to ischemia or local injury/ stress may act as an initiator of systemic fibrinolysis. Activation of the protein C pathway has also been implicated. Tissue hypoperfusion (as measured in surrogate by base deficit) leads to increased formation of the thrombin-thrombomodulin complex, leading to increased activation of protein C and its subsequent anticoagulant effects via deactivation of factors Va and VIIIa, as well as consumption of PAI-1 and decreased inhibition of tPA.
TBI has also been linked to coagulopathy independent of systemic polytrauma. Brain tissue contains high levels of tissue factor, and it has been theorized that TBI affecting the brain parenchyma results in its release into systemic circulation, possibly as a result of transient disruption of the blood brain barrier, resulting in a disseminated intravascular coagulation (DIC) state [9-11]. Furthermore, TBI has been linked independently to platelet dysfunction, even without overt thrombocytopenia; studies on the mechanism of this dysfunction have implicated the cyclooxygenase pathway and inhibition of the platelet ADP and arachidonic acid receptors, however the underlying mechanism still remains unclear [12-14].
Several studies have attempted to characterize the time course of TBI associated coagulopathy. Prolongations in PT and aPTT, increase in D-dimer, and decrease in fibrinogen level have all been seen within minutes or hours of injury, with peak derangements occurring by around 6 hours before reversing [9,15-17] with the degree of coagulopathy correlating with the severity of injury. However, delayed or persistent coagulopathy has also been noted, developing 24 hours and beyond [15,18]. Hyperfibrinolysis and DIC may therefore be the drivers of early coagulopathy, while consumptive and iatrogenic processes may predominate with late or persistent coagulopathy, suggesting that there may be a potential window for intervention.
Diagnosis of coagulopathy in TBI
Standard laboratory studies of coagulation, including platelet count, prothrombin time (PT), partial thromboplastin time (PTT), and international normalized ratio (INR) are part of the panel routinely sent for trauma on admission to the emergency department (Figure 2). Elevated INR and decreased platelet count have been associated with expansion of hemorrhage [19-23] with most sources agreeing on a threshold of platelet count under 100 x10^3 and INR of either >1.2 or 1.5. However, the argument can be made that these tests may not provide a complete picture of the underlying pathophysiologic processes, for example actual platelet function, or hyperfibrinolysis [3]. In particular, prospective studies in the TBI population have linked decreased fibrinogen and elevated D-dimer to progressive hemorrhagic injury and as the most reliable predictor of poor outcomes [16,24].
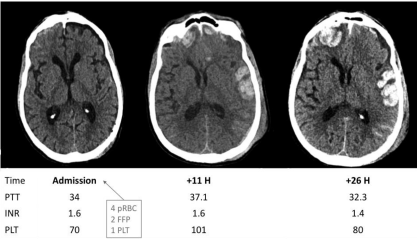
Figure 2: Example of hemorrhagic progression of contusion in a coagulopathic patient. Small bi-frontal and left temporal contusions have blossomed over the first
day of admission despite transfusion of clotting factors during initial resuscitation.
More recently, viscoelastic tests of coagulation function, namely thromboelastography (TEG) and rotational thromboelastometry (ROTEM) have been investigated as alternatives/adjuncts to conventional tests. In brief, these tests measure the mechanical properties of a blood clot formed upon introduction of an activation factor (for example, tissue factor or kaolin) to a sample of whole blood. This allows quantification of different phases of clot formation including initiation and propagation time, maximal clot strength, and lysis, the results of which can provide insight into specific components that drive hemostasis, including levels of clotting factors, fibrinogen, platelets, and fibrinolysis [25]. While a recent review found poor overall diagnostic accuracy of TEG/ROTEM in establishing coagulopathy in the trauma population compared against a standard based on PT/INR elevation [26]. It can be argued that the tests are not meant to be directly interchangeable. Recent meta-analysis has shown that it may be useful in guiding transfusion [27] and due to the faster turnaround time compared to conventional studies, there has been increased acceptance for its use in acute trauma [28]. Specific to ICH, a recent prospective study in the non-trauma population suggested that the kinetics of clot formation may be helpful in predicting hematoma expansion [29] although another recent study in the traumatic ICH population did not find results to be predictive of subsequent progression [30]. It is likely that viscoelastic testing may still play a role in the management of tICH in the correct clinical context (i.e., guiding transfusion of blood products) but its overall applicability as a standard of care test or prognostic tool remains to be established.
Role of prothrombotic agents in traumatic ICH
The use of the anti-fibrinolytic agent tranexamic acid (TXA) in the setting of traumatic hemorrhage has become common, particularly after publication of the large, multinational randomized controlled CRASH-2 trial showing survival benefit to early administration [31]. Notably however, isolated TBI was exclusion to enrollment. A nested subgroup of 270 patients in the trial with concurrent TBI was subsequently analyzed and showed possible benefit though was unable to establish either benefit or harm [32]. Similarly, a recent 238-patient study double blind RCS likewise showed a trend towards benefit in reduction of hematoma expansion without statistical significance [33]; a smaller 80 patient single-blinded study showed benefit for hematoma expansion but did not comment on clinical outcome [34]. Two clinical trials investigating the use of TXA in TBI are currently in progress and slated for completion in 2017 [35,36].
Recombinant factor VIIa (rFVIIa) has also been used off label for the management of tICH. A recent retrospective case control study showed significantly higher mortality in patients that received rFVIIa (90 mcg/kg) for tICH not associated with anticoagulant use [37]. A review of the then-existing literature by the same authors showed by contrast mostly neutral or somewhat positive results, though trials were small, dosing was variable, and trials included a significant number of patients treated with warfarin. More recently, a nonrandomized prospective trial was performed to evaluate the use of low-dose rFVIIa (20 mcg/kg) in patients with tICH and concomitant coagulopathy not associated with anticoagulants or antiplatelet agents. Here, a benefit was seen in prevention of hemorrhage progression, but no significant survival benefit [38]. With lack of clear data on efficacy or even dosing, the role of rFVIIa in treatment of tICH outside of a research protocol is likely limited.
Management of medication associated coagulopathy
The use of antiplatelet and anticoagulant agents is widespread, and there will inevitably be an overlap between this population of patients and the tICH population. Rapid replacement of coagulation factors and/or reversal of these agents remain the primary intervention.
Antiplatelet agents: Antiplatelet agents such as aspirin and clopidogrel are in even more widespread use in the population than the anticoagulants, and appropriate management of coagulopathy in this setting has been a topic of long-standing debate. A recent multicenter trial evaluating empiric platelet transfusion for patients on antiplatelet therapy in the spontaneous ICH population found that platelet transfusion was inferior to standard care. In the TBI population, data has remained frustratingly inconclusive, with metaanalyses of older trial data [39,40] as well as a recent retrospective trial [41] showing no conclusive benefit. However, trials continue to investigate whether proper patient selection and timing may identify a population that may benefit [28]. Similarly, while there have been studies that show that administration of desmopressin (DDAVP) can measurably correct aspirin or clopidogrel induced platelet dysfunction, there have been none that conclusively demonstrate a clinical benefit.
Warfarin: With warfarin, degree of coagulopathy is readily quantified with PT and INR. Transfusion of fresh frozen plasma (FFP) was once the standard of care, however this treatment carries distinct disadvantages, in particular the time necessary to crossmatch and prepare units for transfusion, as well as the large volume (1-3 L) of FFP necessary to normalize INR. As such, the alternative use of prothrombin complex concentrate (PCC), which contains factors II, IX, X, +/- VII (3- vs. 4-factor) has become widespread in recent years. PCC is produced from human plasma which is concentrated to isolate these essential factors, and is distributed in a form which can be quickly reconstituted and administered in a small volume of infusion, and has been shown in studies to be effective in rapidly correcting warfarin associated coagulopathy [42]. Data specific to tICH is more limited, however use of PCC in a head trauma population has likewise been shown to dramatically reduce time to normalization of INR as well as to reduce incidence of hemorrhage expansion [43].
Non-vitamin K oral anticoagulants (NOACs): Since 2010, a number of non-vitamin K oral anticoagulants have entered the market, carrying FDA approvals for indications such as stroke prevention in non-valvular atrial fibrillation and treatment of deep vein thrombosis and pulmonary embolism, and have advantages over warfarin including no need for routine monitoring and comparatively fewer hemorrhagic complications in clinical trial data. Concurrent with increased availability in the market, the number of patients presenting with TBI and NOAC induced coagulopathy will undoubtedly increase. These medications primarily have one of two mechanisms - direct inhibition of thrombin (dabigatran), or factor Xa inhibition (apixaban, rivaroxaban, edoxaban). Unfortunately, these mechanisms also result in coagulopathy that is not straightforward to quantify, despite some measurable effects on conventional laboratory studies (PT and PTT). Reversal of the effects of NOACs has also been problematic, and treatment guidelines have generally involved either removal of the drug (activated charcoal if early ingestion, hemodialysis in the case of dabigatran) or treatment with PCC, despite lack of strong evidence of efficacy [44]. However, with FDA approval in 2015 of idarucizumab, a monoclonal antibody able to rapidly bind to and deactivate dabigatran, there is now an effective and specific reversal agent [45]. Likewise, andexanet alfa, a recombinant factor Xadecoy, is currently undergoing phase III clinical trials, and may soon be available as a reversal agent for the varied Xa inhibitors [46]. Also in development is ciraparantag (PER977), a small molecule that binds to heparin, low molecular weight heparin, dabigatran, and the factor Xa inhibitors [47]. It has shown to reverse the effects of edoxaban in human studies, and may prove to be an effective universal reversal agent for all NOACs in the future [48].
Conclusion
Coagulopathy is a frequently encountered and highly morbid complication of traumatic brain injury that has been consistently linked to poor outcomes. The processes underlying traumatic coagulopathy are different than those assayed by traditional laboratory tests, and therefore may be insufficiently sensitive to capture clinically relevant coagulopathies in trauma. Alternative markers such as fibrinogen, D-dimer, as well as viscoelastic testing may provide insights into the underlying processes and guide interventions. Antifibrinolytic agents used in polytrauma have shown some promise for use in isolated TBI and results from clinical trials are pending. While targeted therapies to reverse the effects of antiplatelet agents remain elusive, widespread availability and use of PCC has greatly improved the ability to reverse warfarin related coagulopathy, and reversal agents for the NOACs have started to enter the market after encouraging clinical trial results. Much research is still needed to completely define the pathogenesis of traumatic coagulopathy, and to eventually achieve the goal of early recognition and intervention upon this disabling condition.
References
- Epstein DS, Mitra B, O’Reilly G, Rosenfeld JV, Cameron PA. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: a systematic review and meta-analysis. Injury. 2014; 45: 819-824.
- Harhangi BS, Kompanje Ej, Leebeek FW, Maas AI. Coagulation disorders after traumatic brain injury. Acta Neurochir (Wien). 2008; 150: 165-175.
- Maegele M. Coagulopathy after traumatic brain injury: incidence, pathogenesis, and treatment options. Transfusion. 2013; 53: 28S-37S.
- Talving P, Benfield R, Hadjizacharia P, Inaba K, Chan LS, Demetriades D. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma. 2009; 66: 55-61.
- Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013; 75: S40-S47.
- Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway?. Ann Surg. 2007; 245: 812-818.
- Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014; 41: 514- 521.
- Chapman MP, Moore EE, Moore HB, Gonzalez E, Gamboni F, Chandler JG, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016; 80: 16-23.
- Halpern CH, Reilly PM, Turtz AR, Stein SC. Traumatic coagulopathy: the effect of brain injury. J Neurotrauma. 2008; 25: 997-1001.
- Keimowitz RM, Annis BL. Disseminated intravascular coagulation associated with massive brain injury. J Neurosurg. 1973; 39: 178-180.
- Goodnight SH, Kenoyer G, Rapaport SI, Patch MJ, Lee JA, Kurze T. Defibrination after Brain-Tissue Destruction: A Serious Complication of Head Injury. N Engl J Med. 1974; 290: 1043-1047.
- Davis PK, Musunuru H, Walsh M, Cassady R, Yount R, Losiniecki A, et al. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit care. 2013; 18: 201-208.
- Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012; 73: 13-19.
- Nekludov M, Bellander BM, Blombäck M, Wallen HN. Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma. 2007; 24: 1699- 1706.
- Lustenberger T, Talving P, Kobayashi L, Inaba K, Lam L, Plurad D, et al. Time course of coagulopathy in isolated severe traumatic brain injury. Injury. 2010; 41: 924-928.
- Nakae R, Takayama Y, Kuwamoto K, Naoe Y, Sato H, Yokota H. Time Course of Coagulation and Fibrinolytic Parameters in Patients with Traumatic Brain Injury. J Neurotrauma. 2016; 33: 688-695.
- Stein SC, Smith DH. Coagulopathy in traumatic brain injury. Neurocrit Care. 2004; 1: 479-488.
- Greuters S, van den Berg A, Franschman G, Viersen VA, Beishuizen A, Peerdeman SM, et al. Acute and delayed mild coagulopathy are related to outcome in patients with isolated traumatic brain injury. Crit Care. 2011; 15: R2.
- Folkerson LE, Sloan D, Cotton BA, Holcomb JB, Tomasek JS, Wade CE. Predicting progressive hemorrhagic injury from isolated traumatic brain injury and coagulation. Surgery. 2015; 158: 655-661.
- Joseph B, Pandit V, Meyer D, Butvidas L, Kulvatunyou N, Khalil M, et al. The significance of platelet count in traumatic brain injury patients on antiplatelet therapy. J Trauma Acute Care Surg. 2014; 77: 417-421.
- Van Beek JG, Mushkudiani NA, Steyerberg EW, Butcher I, McHugh GS, Lu J, et al. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007; 24: 315-328.
- Yuan Q, Sun YR, Wu X, Yu J, Li ZQ, Du ZY, et al. Coagulopathy in Traumatic Brain Injury and Its Correlation with Progressive Hemorrhagic Injury: A Systematic Review and Meta-Analysis. J Neurotrauma. 2016; 33: 1279-1291.
- Zhang D, Gong S, Jin H, Wang J, Sheng P, Zou W, et al. Coagulation Parameters and Risk of Progressive Hemorrhagic Injury after Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Biomed Res Int. 2015; 261825.
- Juratli TA, Zang B, Litz RJ, Sitoci KH, Aschenbrenner U, Gottschlich, B, et al. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. J Neurotrauma. 2014; 31: 1521-1527.
- Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012; 26: 1-13.
- Hunt H, Stanworth S, Curry N, Woolley T, Cooper C, Ukoumunne O, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015; 16.
- Wikkelso A, Wetterslev J, Moller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016; CD007871.
- Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández- Mondéjar E, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Critical care. 2016; 20: 1364-8535.
- Kawano-Castillo J, Ward E, Elliott A, Wetzel J, Hassler A, McDonald M, et al. Thrombelastography detects possible coagulation disturbance in patients with intracerebral hemorrhage with hematoma enlargement. Stroke. 2014; 45: 683-688.
- Rao AJ, Laurie A, Hilliard C, Fu R, Lennox T, Barbosa R, et al. 185 The Utility of Thromboelastography for Predicting the Risk of Progression of Intracranial Hemorrhage in Traumatic Brain Injury Patients. Neurosurgery. 2016; 6: 173- 174.
- Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients Health Technol Assess. 2013; 17: 1-79.
- Perel P, Al-Shahi Salman R, Kawahara T, Morris Z, Prieto-Merino D, Roberts I, et al. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: the effect of tranexamic acid in traumatic brain injury--a nested randomised, placebo-controlled trial. Health Technol Assess. 2012; 16: 1-54.
- Yutthakasemsunt S, Kittiwatanagul W, Piyavechvirat P, Thinkamrop B, Phuenpathom N, Lumbiganon P. Tranexamic acid for patients with traumatic brain injury: a randomized, double-blinded, placebo-controlled trial. BMC Emerg Med. 2013; 13: 20.
- Jokar A, Ahmadi K, Salehi T, Sharif-Alhoseini M, Rahimi-Movaghar V. The effect of tranexamic acid in traumatic brain injury: A randomized controlled trial. Chin J Traumatol. 2017; 20: 49-51.
- Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013; 17: 1-79.
- Stephens SW, Williams C, Gray R, Kerby JD, Wang HE, Bosarge PL. Using social media for community consultation and public disclosure in exception from informed consent trials. J Trauma Acute Care Surg. 2016; 80: 1005- 1009.
- DeLoughery EP, Lenfesty B, DeLoughery TG. A retrospective case control study of recombinant factor VIIa in patients with intracranial haemorrhage caused by trauma. Br J Haematol. 2011; 152: 667-669.
- Yuan Q, Wu X, Du ZY, Sun YR, Yu J, Li ZQ, et al. Low-dose recombinant factor VIIa for reversing coagulopathy in patients with isolated traumatic brain injury. J Crit Care. 2015; 30: 116-120.
- Leong LB, David TK. Is Platelet Transfusion Effective in Patients Taking Antiplatelet Agents Who Suffer an Intracranial Hemorrhage?. J Emerg Med. 2015; 49: 561-572.
- Nishijima DK, Zehtabchi S, Berrong J. Legome E. Utility of platelet transfusion in adult patients with traumatic intracranial hemorrhage and preinjury antiplatelet use: a systematic review. J Trauma Acute Care Surg. 2012; 72: 1658-1663.
- Kim DY, O’Leary M, Nguyen A, Kaji A, Bricker S, Neville A, et al. The Effect of Platelet and Desmopressin Administration on Early Radiographic Progression of Traumatic Intracranial Hemorrhage. J Neurotrauma. 2015; 32: 1815-1821.
- Bershad EM, Suarez JI. Prothrombin complex concentrates for oral anticoagulant therapy-related intracranial hemorrhage: a review of the literature. Neurocrit care. 2010; 12: 403-413.
- Edavettal M, Rogers A, Rogers F, Horst M, Leng W. Prothrombin complex concentrate accelerates international normalized ratio reversal and diminishes the extension of intracranial hemorrhage in geriatric trauma patients. Am Surg. 2014; 80: 372-376.
- Raval AN, Cigarroa JE, Chung MK, Diaz-Sandoval LJ, Diercks D, Piccini JP, et al. Management of Patients on Non-Vitamin K Antagonist Oral Anticoagulants in the Acute Care and Periprocedural Setting: A Scientific Statement From the American Heart Association. Circulation. 2017; 135: e604-e633.
- Pollack CV, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015; 373: 511-520.
- Connolly SJ, Gibson CM, Crowther M. Andexanet Alfa for Factor Xa Inhibitor Reversal. N Engl J Med. 2016; 375: 2499-2500.
- Ansell JE, Bakhru SH, Laulicht BE, Steiner SS, Grosso M, Brown K, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014; 371: 2141-2142.
- Ansell JE, Bakhru SH, Laulicht BE, Steiner SS, Grosso MA, Brown K, et al. Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb Haemost. 2017; 117: 238-245.