
Review Article
Austin J Cerebrovasc Dis & Stroke.2018; 5(1): 1075.
Homoarginine, Cardiovascular Risk and Mortality
Kayacelebi AA* and Tsikas D
Institute of Toxicology, Core Unit Proteomics, Hannover Medical School, Hannover, Germany
*Corresponding author: Kayacelebi AA, Institute of Toxicology, Core Unit Proteomics, Hannover Medical School, Carl-Neuberg-Str-1, D-30625 Hannover, Germany
Received: November 15, 2017; Accepted: January 08, 2018; Published: January 25, 2018
Abstract
L-Homoarginine (hArg), originally considered a non-physiological compound in the human body, received special attention in recent years. hArg is synthesized from L-arginine and L-lysine by the catalytic action of arginine:glycine amidinotransferase (AGAT) which is also responsible for the synthesis of guanidinoacetate, the precursor of creatine. Low circulating hArg emerged as a novel cardiovascular and cerebrovascular risk factor. Animal experiments indicate that hArg supplementation has pleiotropic beneficial effects and improves cardiac and cerebrovascular function. Yet, the underlining mechanisms remain still unresolved. This article reviews the recent literature on the physiology, pathology and pharmacology of hArg in the cardio- and cerebrovascular systems.
Keywords: Arginine; Infarction; Nitric oxide; Risk factor; Stroke
Abbreviations
ADMA: Asymmetric Dimethylarginine; AGAT: Arginine: Glycine Amidinotransferase; DDAH: Dimethylarginine Dimethylaminohydrolase; GAA: Guanidinoacetate; hArg: L-Homoarginine; MMA: Monomethylarginine; NO: Nitric Oxide; NOS: Nitric Oxide Synthase; PRMT: Protein Arginine Methyltransferase; SDMA: Symmetric Dimethylarginine
Introduction
Origin and metabolism of biological homoarginine
For many decades, L-homoarginine (hArg) has been considered a non-physiological and non-proteinogenic amino acid and has been used at mM-concentrations as an experimental inhibitor of alkaline phosphatase activity. Today, we know that hArg occurs physiologically in the circulation and in urine in the lower μMrange of healthy humans. hArg is synthesized in the kidney by the mitochondrial arginine:glycine amidinotransferase (AGAT) from L-arginine and L-Lysine (Figure 1). AGAT also catalyzes the synthesis of guanidinoacetate (GAA) which is further converted to creatine by guanidinoacetate methyltransferase (Figure 1). Thereby, guanidinoacetate is by far the most abundant reaction product of AGAT [1]. At present, it is unknown whether AGAT requires cofactors/effectors for the synthesis of hArg and GAA. GAMT uses S-adenosylmethionine as a cofactor and methyl donor (Figure 1). The metabolism of hArg is little investigated. Alanine:glyoxylate aminotransferase 2 has been shown to oxidize the amino group of hArg [2]. Whether hArg is decarboxylated to form homoagmatine, analogous to the decarboxylation of L-arginine (Arg) to agmatine is unknown. hArg is considered a non-proteinogenic amino acid and no proteins are known to contain physiologically hArg. Yet, this demands further investigation. There is indication that human plasma proteins may contain substantial amounts of hArg residues [3]. Arg residues in proteins are NG-methylated by protein arginine methyltransferases (PRMT). Proteolysis of these proteins yields NG-monomethyl-L-arginine (MMA) and the NG-dimethyl-Larginines, asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). MMA, ADMA and SDMA are inhibitors of nitric oxide synthase (NOS) which converts Arg to nitric oxide (NO) and L-citrulline. The occurrence of biological NG-monomethyl- L-homoarginine and NG-dimethyl-L-homoarginines has not been reported thus far. Analogous to Arg, all NOS isoforms are known to convert hArg to NO [4]. However, the affinity of hArg to NOS is very low and it seems that hArg rather decreases NO synthesis from Arg when present at high concentrations (e.g., 100 μM) [4]. The pharmacokinetics of exogenous hArg has been investigated in healthy young volunteers [5] and in rats [6]. In rats, intraperitoneally injected hArg is distributed in main organs; kidney seems to be the preferred organ [6]. The elimination half-life of circulating hArg is of the order of 20 min in rats [6].
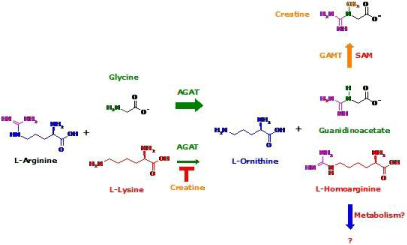
Figure 1: Biosynthesis of L-homoarginine and guanidinoacetate by arginine:glycine amidinotransferase (AGAT), and of creatine by guanidinoacetate
methyltransferase (GAMT) using S-adenosylmethionine (SAM) as the cofactor.
Biological activities of homoarginine
The biological activities of hArg are still almost entirely unknown. There is no convincing evidence that hArg is utilized as a substrate of NOS isoforms to form NO. Experiments with recombinant NOS isoforms suggest even that hArg competes with Arg, thus diminishing NO synthesis, admittedly at very high pathophysiologically irrelevant concentrations [4]. Oral hArg supplementation to healthy young subjects was found not to induce NO-related effects [5], suggesting that hArg is unlikely to act as a substrate of NOS to produce NO in humans. hArg has been reported to induce oxidative stress [7], but we did not confirm these results in vitro in mitochondria of HUVEC [8] and in vivo in the rat [6]. hArg supplementation has been reported to improve blood glucose in diet-induced obese mice [9], presumably in a manner independent of NO.
Vascular calcification is promoted by hyperphosphatemia and involves osteo-/chondrogenic transformation of vascular smooth muscle cells (VSMCs). hArg has been reported to augment osteo-/ chondrogenic transformation of VSMCs and vascular calcification [4]. hArg has also been reported to augment vascular calcification and aortic osteo-/chondrogenic signalling in mice after vitamin D3- overload [4].
hArg-caused decrease in NO synthesis from Arg and elevation of oxidative stress have been proposed, analogous to the synthetic NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME) [4]. Yet, given the low hArg concentration and its low affinity to NOS, other not yet elucidated mechanisms are more likely to contribute to the effects of hArg on phosphate-induced osteo-/chondrogenic transformation of VSMCs. The results of a recent study suggest that circulating hArg is not causally associated with various cardiometabolic risk factors, the systemic metabolic profile, type 2 diabetes mellitus (T2DM) in young adults [10]. In this study, circulating hArg (mean±SD, 1.79±0.68 μM in women, 1.93±0.61 μM in men) revealed to be an independent predictor for future hyperglycaemia and abdominal obesity in men and for T2DM in women [10]. Although very informative, this study did not provide information about underlying biochemical mechanisms.
Intravenous hArg infusion to rats (30 mg/kg/day) for 14 days resulted in almost 40-fold increase of serum hArg concentration and about 1.3-fold increase in serum L-ornithine concentration and reduced neo-intimal hyperplasia in balloon-injured rat carotids [11]. Although it cannot be excluded that hArg has contributed to NO, the lack of changes in expression levels of arginase I, endothelial NOS (eNOS) and inducible NOS (iNOS) upon hArg infusion and the lack of differences between the rats being infused hArg or Arg (30 mg/kg/day) with respect to nitrite, other NO-independent mechanisms are more likely to have contributed to the anti-proliferative effects observed in the study [11]. The drastically increased hArg concentrations upon hArg infusion are even likely to have “inhibited” the NOS-catalyzed formation of NO from Arg [4]. In vivo in the rat, we observed an increase in plasma nitrate concentration from 54 μM at baseline to 101 μM and 130 μM 15 min and 60 min after a single intraperitoneal injection of 440 mg hArg/kg [6]. Yet, again, it is not clear whether the increase in nitrate is due to NOS-catalyzed synthesis of NO from exogenous hArg which reached plasma concentrations of about 1330 μM, or from hArg-derived plasma Arg of which the concentration increased temporarily twofold [6].
Homoarginine in cardiovascular disease and stroke
Thus far, there is only a single condition in which higher circulating hArg concentrations were measured, that is pregnancy, both in normal and in abnormal pregnancy [12,13]. The serum hArg concentration was higher during the second trimester (4.8±1.7 μM) and the third trimester (5.3±1.5 μM) compared with those in nonpregnant women (2.7±1.0 μM) [12]. The serum hArg concentration was found to correlate with gestational age. In plasma of pregnant women who developed early preeclampsia (2.70 [2.30-3.31] μM) or late preeclampsia (3.53 [2.87-4.74] μM), the plasma hArg concentration did not differ from healthy pregnant women (3.31 [2.56-4.49] μM) in the first trimester [13].
Over the last few years there appeared many articles that reported in unison that low circulating and low urinary hArg concentrations are associated with renal, cardiovascular and cerebrovascular diseases [14-33]. The effects of hArg on cardiovascular outcome and mortality were unknown until 2010. In the LURIC study, the hArg serum concentration was 2.6±1.1 μM in 3,305 patients referred for coronary angiography [14]. Patients with serum hArg concentration <1.85 μM were found to have a 4-fold higher rate of dying on cardiovascular disease than patients with serum hArg concentration > 3.1 μM. In the 4D study on 1244 patients with T2DM receiving maintenance hemodialysis the serum hArg was only 1.2±0.5 μM and had a 5-fold higher mortality rate compared to the patients of the LURIC study [14]. This study reported for the first time that low serum hArg concentration is independently associated with cardiovascular and all-cause mortality in patients with cardiovascular disease and T2DM. In the LURIC study, low serum hArg concentrations were found to be a novel risk factor for fatal strokes [15]. In the 4D study, low serum hArg concentration was found to be strongly associated with the presence of congestive heart failure and left ventricular hypertrophy, as well as with elevated brain natriuretic peptide concentration [16]. Low serum hArg concentration turned out to be a strong risk factor for sudden cardiac death and death due to heart failure in haemodialysis patients [16]. In the LURIC study, low serum hArg concentration was associated with myocardial dysfunction, poor energy metabolism and increased risk of fatal cardiovascular events [17]. Yet, neither the LURIC nor the 4D study delineated the underlying mechanisms and did not explore the significance of hArg in risk stratification and treatment of heart diseases.
In the Mild to Moderate Kidney Disease (MMKD) study, plasma hArg concentration (2.5±1.1 μM) was lower at lower GFR levels (2.9±1.0 μM, GFR>90 mL/min; 2.64±1.06 μM, GFR 60-90 mL/min; 2.52±1.24 μM, GFR 30-60 mL/min; 2.05±0.78 μM, GFR<30 mL/ min, respectively) [18]. In this study, plasma hArg concentration was significantly associated with the progression (i.e., doubling baseline serum creatinine and/or end-stage renal disease) of chronic kidney disease (CKD). The study suggested that low plasma hArg concentrations might be an early indicator of kidney failure and a potential target for the prevention of disease progression [18]. In elderly pre-dialysis CKD patients (eGFR, 34±18 mL/min/1.73 m2), circulating hArg concentration was positively associated with the eGFR, with patients being stronger compromised with renal function exhibiting lower hArg concentrations [19]. This study suggested that circulating hArg concentration decreases with advancing renal disease and is inversely related to progression to dialysis and mortality.
There is solid evidence from human and animal studies that AGAT is responsible for the synthesis of hArg [20,34]. In patients with ischemic stroke higher plasma hArg concentrations were found to be independently associated with a lower in all-cause mortality [20]. A genome-wide association study (n=2806) revealed that plasma hArg concentration was strongly associated with single nucleotide polymorphisms in the AGAT gene [20]. Cerebral damage and neurological deficits in experimental stroke were increased in AGAT-/- mice and attenuated by hArg supplementation (30 μg/kg/h via osmotic pump); in GAMT-/- mice the infarct size was decreased compared with control mice [20]. This study suggested that low circulating hArg concentration is associated with a poor outcome after ischemic stroke.
Subsequent studies demonstrated that hArg is of key importance for the renal, cardiovascular and cerebrovascular system. In 282 heart failure patients, plasma hArg concentration emerged as an independent marker of mortality [21]. Plasma hArg concentration was higher in patients who survived than in those who died during the follow-up (median (IQR) 1.89 (1.23) vs. 1.63 (0.99) μM) [21]. In this study, plasma hArg was found to correlate positively with plasma Arg concentration (r=0.45) and inversely with plasma SDMA concentration (r=-0.17). The population-based Dallas Heart Study (DHS) on 3514 participants evaluated the association of plasma hArg concentration with clinical and subclinical cardiovascular outcomes [22]. Median plasma hArg was 2.80 [2.14-3.54] μM. In multivariable models, higher plasma hArg concentrations were associated with lower rates of major adverse cardiovascular events and lower allcause mortality [22]. In this study, plasma hArg concentration was inversely and independently associated with aortic wall thickness, yet not with aortic plaque burden and coronary artery Ca2+.
The DIAST-CHF study evaluated whether serum ADMA, SDMA and hArg concentrations are associated with diastolic dysfunction in primary care patients at cardiovascular risk with preserved left ventricular ejection fraction [23]. Lower serum hArg concentrations were associated with diastolic dysfunction, whereas higher serum ADMA and SDMA concentrations were associated with the severity of diastolic dysfunction [23]. Evaluation of this and other studies suggests that hArg may counter act the biological actions of ADMA and SDMA] [24]. In female takotsubo (stress-induced) cardiomyopathy patients, plasma hArg concentration was found to be lower than in healthy age-matched women [25].
Given the particular importance of the kidney as the main organ of hArg synthesis, the significance of the kidney function for hArg and cardiovascular outcome was evaluated in the LURIC study [26]. This study revealed that low serum hArg concentration is strongly related to decreased kidney function, adverse cardiovascular events and death due to heart failure. High urinary hArg excretion [27], high plasma hArg concentration [28,29], but lower ADMA concentration [30] are associated with low rates of cerebrovascular events, graft loss, progression of kidney failure and all-cause mortality in renal transplant recipients [31].
Consistently with the studies discussed above, low plasma hArg concentration was identified as a risk marker for incident major adverse cardiovascular events in patients with acute chest pain [32]. In this study, plasma hArg concentration was predictive for patients with high-sensitivity assayed troponin I (>27 pg/mL). Plasma hArg concentration was also associated with a trial fibrillation [32]. Reported plasma hArg concentrations were 2.37 [1.85-2.95] μM in acute coronary syndrome, 2.34 [1.80-2.92] μM in acute myocardial infarct and 2.40 [1.95-3.05] μM in unstable angina pectoris [32].
In elderly newly diagnosed stroke patients, we found much lower plasma hArg concentrations in women (n=24; 1.02 [0.83-1.63] μM) than in men (n=54; 1.64 [1.09-2.02] μM) (hArg molar ratio women: men=0.62) [33]. In the female patients, we found only a single correlation of age with aortic intima-media thickness (IMT, r=0.533, P=0.007) and aortic distensibility (r=-0.432, P=0.034). In the male patients not only age correlated with aortic IMT (r=0.672, P=0.0001) and aortic distensibility (r=-0.635, P<0.0001), but also BMI (r=-0.273, P=0.046), HDL (r=0.292, P=0.032), with the plasma concentration of the major NO metabolites nitrite (r=0.329, P=0.015) and nitrate (r=0.364, P=0.007), as well as with creatinine (r=0.289, P=0.034) [33]. In healthy young men, circulating hArg concentration was found to be positively associated with high-risk carotid-intima-media thickness (IMT) and carotid distensibility [10].
In patients with primary hyperparathyroism (PHPT), considerable lower serum hArg concentrations were measured than in matched patients without PHPT (1.16 [0.95-1.66] μM vs. 1.62 [1.33- 2.04] μM) [35]. Interestingly, serum parathyroid hormone (PTH) concentration did not correlate with serum hArg concentration in PHPT (PHPT; r=0.03, P=0.8), unlike in the patients without PHPT (r=-0.23, P=0.02). In renal transplant patients, we found a weak inverse correlation between plasma hArg and PHT concentration, yet with no significant effect modification by PTH in the association for mortality or graft failure [28].
hArg and creatine supplementation
While low circulating hArg concentration is consistently associated with renal, cardiovascular and cerebrovascular disease, as outlined above, only very recently has been demonstrated that orally supplemented hArg may indeed improve cardiac function in the setting of a murine model of post-myocardial infarction heart failure [36]. Oral supplementation of adult female C57BL/6J mice with 14 mg/L hArg as hydrochloride salt in the drinking water for four weeks increased almost 3-fold the hArg concentration in plasma (0.29±0.03 vs. 0.89±0.07 μM) and myocardial tissue (17.6±2.7 vs. 48.8±6.8 nmol/g protein), without altering cardiac haemodynamic parameters. Six weeks after myocardial infarction, there were no differences between untreated and hArg-treated mice with respect to infarct size, heart rat, ejection fraction, end-diastolic volume, endsystolic volume, stroke volume or cardiac output [36]. However, there were significant differences in some haemodynamic parameter, especially after β-adrenergic stimulation, including LV contractility (dP/dtmax), contractile reserve (ΔdP/dtmax), relaxation (dP/dtmin) and tau. The equivalent human daily dose is estimated to be 250 mg [36]. Given the known pharmacokinetics and pharmacodynamics of hArg in healthy subjects [5], the effects of hArg supplementation studies in humans suffering from renal, cardiovascular and cerebrovascular diseases remain to be performed.
In heart failure patients, myocardial AGAT mRNA expression and AGAT activity were measured to be elevated and to decrease during recovery [37]. This finding was interpreted as an elevated creatine synthesis in the myocardium. In this study, AGAT activity was measured as the formation rate of GAA, the precursor of creatine. Oral high-dose creatine supplementation to healthy subjects (20 g/d for 1 week and 5 g/d for 19 subsequent weeks) has been shown to decrease circulating GAA concentration (by -25%) and to simultaneously increase circulating hArg concentration (by +35%) [38]. In haemodialysis patients treated with creatine (2 g/d for 4 weeks), circulating GAA concentration decreased (by -15%) [39]. Although this study did not report circulating hArg concentrations, circulating hArg concentration is likely to increased considerably upon creatine supplementation. A shift of the double-faced AGAT activity from GAA to hArg formation rate in the myocardium seems to be beneficial. However, the creatine supplementation human studies [38,39] have not provided evidence that such a shift occurred in the myocardium, as circulating GAA and hArg concentrations mainly reflect the contribution of the kidney [37].
Conclusion
hArg is a natural non-proteinogenic amino acid. In humans, hArg is biosynthesized in the kidneys from Arg and Lys by mitochondrial AGAT. hArg circulates in blood and is excreted unchanged in the urine. Yet, the metabolism of hArg is incompletely investigated. Subjects suffering from renal, cardiovascular or cerebrovascular diseases have lower circulating and lower excretory hArg concentrations compared to healthy subjects. In normal and abnormal pregnancy, circulating hArg concentration is higher than in non-pregnant women.
The biological functions of hArg are little understood. Although hArg can be converted by NOS to NO, the almost 50-fold lower concentration of hArg and the 5-fold lower affinity of hArg to NOS in comparison to Arg suggest that other not yet recognized biological activities are more likely to explain the recently revealed significance of hArg in renal, cardiovascular and cerebrovascular diseases. Whether hArg is a risk marker or risk factor warrants further investigations. AGAT also catalyzes the synthesis of GAA which is subsequently converted by GAMT to creatine which in turn inhibits AGAT activity.
hArg may antagonize the biological effects of ADMA and SDMA, presumably independent of NO. Therefore, investigations on hArg need to consider measurement of GAA, ADMA and SDMA in addition to hArg in blood and urine for a comprehensive elucidation of the contribution of AGAT, GAMT and PRMT to renal, cardiovascular and cerebrovascular diseases. The reported pharmacokinetics of hArg in healthy young subjects provides a solid basis for clinical studies addressing effects of supplemented hArg in cerebrovascular disease and stroke.
References
- Tsikas D, Wu G. Homoarginine, arginine, and relatives: analysis, metabolism, transport, physiology, and pathology. Amino Acids. 2015; 47: 1697-1702.
- Rodionov RN, Oppici E, Martens-Lobenhoffer J, Jarzebska N, Brilloff S, Burdin D, et al. A novel pathway for metabolism of the cardiovascular risk factor homoarginine by alanine:glyoxylate aminotransferase 2. Sci Rep. 2016; 6: 35277.
- Kayacelebi AA, Beckmann B, Gutzki FM, Jordan J, Tsikas D. GC-MS and GC-MS/MS measurement of the cardiovascular risk factor homoarginine in biological samples. Amino Acids. 2014; 46: 2205-2217.
- Alesutan I, Feger M, Tuffaha R, Castor T, Musculus K, Buehling SS, et al. Augmentation of phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovasc Res. 2016; 110: 408-418.
- Atzler D, Schönhoff M, Cordts K, Ortland I, Hoppe J, Hummel FC, et al. Oral supplementation with L-homoarginine in young volunteers. Br J ClinPharmacol. 2016; 82: 1477-1485.
- Günes DN, Kayacelebi AA, Hanff E, Lundgren J, Redfors B, Tsikas D. Metabolism and distribution of pharmacological homoarginine in plasma and main organs of the anesthetized rat. Amino Acids. 2017; 49: 2033-2044.
- Sasso S, Dalmedico L, Magro DD, Pereira EM, Wyse AT, de Lima DD. Differential in vitro effects of homoarginine on oxidative stress in plasma, erythrocytes, kidney and liver of rats in the absence and in the presence α-tocopherol, ascorbic acid or L-NAME. Amino Acids. 2015; 47: 1931-1939.
- Hanff E, Kayacelebi AA, Herrmann C, Obermann M, Das AM, Tsikas D. Unaltered l-arginine/NO pathway in a MELAS patient: Is mitochondrial NO synthase involved in the MELAS syndrome? Int J Cardiol. 2016; 223: 479-481.
- Stockebrand M, Hornig S, Neu A, Atzler D, Cordts K, Boger RH, et al. Homoarginine supplementation improves blood glucose in diet-induced obese mice. Amino Acids. 2015; 47: 1921-1929.
- Seppala I, Oksala N, Jula A, Kangas AJ, Soininen P, Hutri-Kahonen N, et al. The biomarker and causal roles of homoarginine in the development of cardiometabolic diseases: an observational and Mendelian randomization analysis. Sci Rep. 2017; 7: 1130.
- Dellera F, Ganzetti GS, Froio A, Manzini S, Busnelli M, Meinitzer A, et al. L-homoarginine administration reduces neointimal hyperplasia in balloon-injured rat carotids. Thromb Haemost. 2016; 116: 400-402.
- Valtonen P, Laitinen T, Lyyra-Laitinen T, Raitakari OT, Juonala M, Viikari JS, et al. Serum L-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ J. 2008; 72: 1879-1884.
- Khalil AA, Tsikas D, Akolekar R, Jordan J, Nicolaides KH. Asymmetric dimethylarginine, arginine and homoarginine at 11-13 weeks' gestation and preeclampsia: a case-control study. J Hum Hypertens. 2013; 27: 38-43.
- Marz W, Meinitzer A, Drechsler C, Pilz S, Krane V, Kleber ME, et al. Homoarginine, cardiovascularrisk, and mortality. Circulation. 2010; 122: 967-975.
- Pilz S, Tomaschitz A, Meinitzer A, Drechsler C, Ritz E, Krane V, et al. Low serum homoarginine is a novel risk factor for fatal strokes in patients undergoing coronary angiography. Stroke. 2011; 42: 1132-1134.
- Drechsler C, Meinitzer A, Pilz S, Krane V, Tomaschitz A, Ritz E, et al. Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur J Heart Fail. 2011; 13: 852-859.
- Pilz S, Meinitzer A, Tomaschitz A, Drechsler C, Ritz E, Krane V, et al. Low homoarginine concentration is a novel risk factor for heart disease. Heart. 2011; 97: 1222-1227.
- Drechsler C, Kollerits B, Meinitzer A, Marz W, Ritz E, Konig P, et al. Homoarginine and progression of chronic kidney disease: results from the Mild to Moderate Kidney Disease Study. PLoS One. 2013; 8: e63560.
- Ravani P, Maas R, Malberti F, Pecchini P, Mieth M, Quinn R, et al. Homoarginine and mortality in pre-dialysis chronic kidney disease (CKD) patients. PLoS One. 2013; 8: e72694.
- Choe CU, Atzler D, Wild PS, Carter AM, Boger RH, Ojeda F, et al. Homoarginine levels are regulated by L-arginine:glycine amidinotransferase and affect stroke outcome: results from human and murine studies. Circulation. 2013; 128: 1451-1461.
- Atzler D, Rosenberg M, Anderssohn M, Choe CU, Lutz M, Zugck C, et al. Homoarginine--an independent marker of mortality in heart failure. Int J Cardiol. 2013; 168: 4907-4909.
- Atzler D, Gore MO, Ayers CR, Choe CU, Böger RH, de Lemos JA, et al. Homoarginine and cardiovascular outcome in the population-based Dallas Heart Study. Arterioscler Thromb Vasc Biol. 2014; 34: 2501-2507.
- Pilz S, Edelmann F, Meinitzer A, Gelbrich G, Doner U, Dungen HD, et al. Associations of methylarginines and homoarginine with diastolic dysfunction and cardiovascular risk factors in patients with preserved left ventricular ejection fraction. J Card Fail. 2014; 20: 923-930.
- Tsikas D, Kayacelebi AA. Do homoarginine and asymmetric dimethylarginine act antagonistically in the cardiovascular system?. Circ J. 2014; 78: 2094-2095.
- Kayacelebi AA, Nguyen TH, Neil C, Horowitz JD, Jordan J, Tsikas D. Homoarginine and 3-nitrotyrosine in patients with takotsubo cardiomyopathy. Int J Cardiol. 2014; 173: 546-547.
- Tomaschitz A, Meinitzer A, Pilz S, Rus-Machan J, Genser B, Drechsler C, et al. Homoarginine, kidney function and cardiovascular mortality risk. Nephrol Dial Transplant. 2014; 29: 663-671.
- Frenay AR, Kayacelebi AA, Beckmann B, Soedamah SS, de Borst MH, van den Berg E, et al. High urinary homoarginine excretion is associated with low rates of all-cause mortality in renal transplant recipients. Amino Acids. 2015; 47: 1827-1836.
- Kayacelebi AA, Minovic I, Hanff E, Frenay AS, de Borst MH, Feelisch M, et al. Low plasma homoarginine concentration is associated with high rates of all-cause mortality in renal transplant recipients. Amino Acids. 2017; 49: 1193-1202.
- Drechsler C, Pihlstrom H, Meinitzer A, Pilz S, Tomaschitz A, Abedini S, et al. Homoarginine and clinical outcomes in renal transplant recipients: Results from the assessment of Lescol in Renal Transplantation Study. Transplantation. 2015; 99: 1470-1476.
- Frenay ARS, van den Berg E, de Borst MH, Beckmann B, Tsikas D, Feelisch M, et al. Plasma ADMA associates with all-cause mortality in renal transplant recipients. Amino Acids. 2015; 47: 1941-1949.
- Pilz S, Meinitzer A, Gaksch M, Grübler M, Verheyen N, Drechsler C, et al. Homoarginine in the renal and cardiovascular systems. Amino Acids. 2015; 47: 1703-1713.
- Atzler D, Baum C, Ojeda F, Keller T, Cordts K, Schnabel RB, et al. Low Homoarginine Levels in the Prognosis of Patients With Acute Chest Pain. J Am Heart Assoc. 2016; 5: e002565.
- Haghikia A, Yanchev GR, Kayacelebi AA, Hanff E, Bledau N, Widera C, et al. The role of L-arginine/L-homoarginine/nitric oxide pathway for aortic distensibility and intima-media thickness in stroke patients. Amino Acids. 2017; 49: 1111-1121.
- Davids M, Ndika JD, Salomons GS, Blom HJ, Teerlink T. Promiscuous activity of arginine:glycine amidinotransferase is responsible for the synthesis of the novel cardiovascular risk factor homoarginine. FEBS Lett. 2012; 586: 3653-3657.
- Tomaschitz A, Verheyen N, Gaksch M, Meinitzer A, Pieske B, Kraigher-Krainer E, et al. Homoarginine in patients with primary hyperparathyroidism. Am J Med Sci. 2015; 349: 306-311.
- Cullen ME, Yuen, AH, Felkin LE, Smolenski RT, Hall JL, Grindle S, et al. Myocardial expression of the arginine:glycine amidinotransferase gene is elevated in heart failure and normalized after recovery: potential implications for local creatine synthesis. Circulation. 2006; 114: I16-I20.
- Atzler D, McAndrew DJ, Cordts K, Schneider JE, Zervou S, Edzard Schwedhelm, et al. Dietary supplementation with homoarginine preserves cardiac function in a murine model of post-myocardial infarction heart failure. Circulation. 2017; 135: 400-402.
- Derave W, Marescau B, VandenEede E, Eijnde BO, De Deyn PP, Hespel P. Plasma guanidino compounds are altered by oral creatine supplementation in healthy humans. J Appl Physiol (1985). 2014; 97: 852-857.
- Taes YE, Marescau B, De Vriese A, De Deyn PP, Schepers E, Vanholder R, et al. Guanidino compounds after creatine supplementation in renal failure patients and their relation to inflammatory status. Nephrol Dial Transplant. 2008; 23: 1330-1335.