
Research Article
Austin J Cerebrovasc Dis & Stroke. 2018; 5(2): 1079.
A Comparison of Flat-Panel CT to Non-Contrast Enhanced CT in the Detection of Intracranial Hemorrhage
Larrabure LN¹*, Yu JF¹, Pung L², Amans MR¹, Cooke DL¹ and Hetts SW¹
¹Department of Radiology and Biomedical Imaging, University of California, San Francisco, USA
²Siemens Healthineers GmbH, Germany
*Corresponding author: Larrabure LN, Department of Radiology and Biomedical Imaging, University of California, San Francisco, USA
Received: April 20, 2018; Accepted: May 15, 2018; Published: June 12, 2018
Abstract
Purpose: Outcomes of acute ischemic strokes (AIS) are associated with length of time to reperfusion. Most AIS patients receive non-contrast enhanced CT (NECT) to detect intracranial hemorrhage and determine eligibility for intravenous tPA and/or mechanical embolectomy. We hypothesize that flatpanel CT (FPCT) images produced by modern x-ray angiography equipment are as sensitive to intracranial hemorrhage as standard NECT images.
Methods: 19 cases were collected through a retrospective chart review of endovascular cases conducted at UCSF Moffitt-Long Hospital between April 2015 and December 2015. Two neuroradiologists independently viewed in random sequence the de-identified images. Intra-rater and inter-rater agreements were assessed using overall percent agreement, positive percent agreement, and kappa statistic. FPCT’s sensitivity, specificity, and positive and negative predictive value were calculated for the detection of intracranial hemorrhage (ICH), subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), intraparenchymal hemorrhage (IPH) and subdural hemorrhage (SDH).
Results: Intra-rater and inter-rater agreements were sufficient for all categories of hemorrhage except SDH. Excluding SDH cases, FPCT has hemorrhage detection sensitivity of 0.89 (CI 0.75-0.97), specificity of 1 (CI 0.85- 1), PPV of 1 (CI 0.90-1), and NPV of 0.85 (0.65 - 0.96). In the identification of hemorrhage location, FPCT has a sensitivity for SAH, IVH, and IPH of 0.68 (CI 0.48-0.84), 0.79 (CI 0.59-0.92), and 0.58 (CI 0.28-0.85), respectively.
Conclusion: FPCT is similar to NECT in the detection of intracranial hemorrhage and has potential as a diagnostic test for intracranial hemorrhage during AIS imaging triage.
Keywords: Stroke; Flat-panel computed tomography; Subarachnoid hemorrhage; Intraventricular hemorrhage; Intraparenchymal hemorrhage; Subdural hemorrhage
Abbreviations
AIS: Acute Ischemic Stroke; FPCT: Flat-panel Computed Tomography; NECT: Non-contrast Enhanced Computed Tomography; CT: Computed Tomography; UCSF: University of California, San Francisco; tPA: Tissue Plasminogen Activator; ICH: Intracranial Hemorrhage; SAH: Subarachnoid Hemorrhage; IVH: Intraventricular Hemorrhage; IPH: Intraparenchymal Hemorrhage; SDH: Subdural Hemorrhage; CI: Confidence Interval; PPV: Positive Predictive Value; NPV: Negative Predictive Value; SD: Standard Deviation; KVP: Kilovoltage Peak; FOV: Field of View; HU: Hounsfield Unit; MRI: Magnetic Resonance Imaging; CTA: Computed Tomography Angiography; AVF: Arteriovenous Fistula; AVM: Arteriovenous Malformation
Introduction
In AIS, time is critically important: with 1.9 million neurons lost per minute, clinical outcomes after reperfusion correlate with the time spent ischemic [1-3]. However, the current algorithm of care for AIS patients is to first receive a NECT to identify possible contraindications for IV tPA such as intracranial hemorrhage or large completed brain infarction that would raise the risk for reperfusion hemorrhage in already dead brain tissue. If the patient is demonstrated by CT angiogram to have an intracranial large vessel occlusion, the patient is then transported to the angiography suite for endovascular mechanical embolectomy. Every minute counts in the treatment of AIS, with improved outcomes for less time between onset of the stroke and reperfusion [4-5]. Each step in the treatment protocol adds time until reperfusion. Angiography suites equipped with FPCT may be able to eliminate the need for imaging studies prior to the transport to the angiography suite. Previous studies have suggested that imaging in the angiography suite (FPCT) could be reliable and comparable to other forms of imaging in assessment of hemorrhage and blood volume [6-12]. If FPCT is determined to be as sensitive to ICH as standard NECT in this future prospective study, this would allow elimination of NECT imaging as a separate step in the AIS treatment protocol needed for IV tPA administration and embolectomy. If FPCT is comparable to NECT currently used in the early steps of triage of AIS, then it may be possible to decrease delay in treatment of patients and improve overall outcomes. In 2016, Leyhe, et al. performed a 102 patient retrospective study comparing FPCT to NECT and showed FPCT had comparable sensitivity and specificity to NECT in the detection of SAH, IVH, and IPH [13]. Building on this study, our group is preparing an international multicenter prospective study collaboration with the Leyhe group, comparing standard stroke management with NECT to one stop management with FPCT in the endovascular suite. In preparation for this collaboration, a study ensuring comparability in a tertiary cerebrovascular referral center in North America is warranted. We aim to assess the detectability of brain hemorrhages using FPCT images specifically in our tertiary hospital’s population.
Materials and Methods
Case selection
Cases (n=33) were collected through retrospective chart review of neuroendovascular cases conducted at UCSF Medical Center between April 2015 and December 2015. Inclusion eligibility required an axial FPCT conducted during endovascular treatment and a comparable NECT image. Specifically, if a FPCT was performed pre-treatment, then a comparable pre-treatment standard NECT imaging study was examined. For each case, gender, age, time between comparison images, and chart-recorded indications for endovascular treatment were collected.
Imaging protocol
FPCTs were performed using a biplane angiography system (Axiom Artis Zee or Q, Siemens Healthineers, Erlangen, Germany). Contrast medium was injected at a dilution ratio of 240 mgI/mL and at a rate of 1 ml/s for a total of 20s; 270 degree rotation was performed during a patient breath hold. A source voltage of either 70 kvp or 109 kvp was used with a FOV ranging from 42-48 cm and a slice matrix of 512x512. Images were reconstructed on the Siemens XWP workstation using a HU algorithm with a slice thickness of 0.3-1 mm.
NECT were performed using a GE Discovery CT750 HD multi detector scanner. A source voltage of 120 kvp and tube current of 300 was used and images were acquired with a FOV of 32 cm. Images were reconstructed at 3.75 mm per slice with a reconstruction FOV of 22 cm.
Image evaluation and statistical analysis
The FPCT and NECT images were de-identified, randomized, and presented retrospectively and independently to two interventional neuroradiologists blinded to case demographics and data. The neuroradiologists reported findings on the presence of hemorrhage, intracranial hemorrhage location subtype (e.g., SAH), and whether the image was of interpretable quality. To allow for assessment of intraobserver reliability, the images were presented to the neuroradiologists twice in random order. Intra-observer agreement and inter-observer agreement were assessed by calculating overall percent agreement, positive percent agreement, and unweighted kappa statistic for hemorrhage, SAH, IVH, IPH, and SDH subgroupings. Unweighted kappa statistic was calculated using VassarStats [14]. Subgroups with agreement kappa statistics less than 0.20 were determined to have no agreement and were removed from calculations.
FPCT’s sensitivity, specificity, PPV, and NPV were calculated with NECT as the comparison gold standard for intracranial hemorrhage, SAH, IVH, IPH and SDH using MedCalc [15]. The sensitivity, specificity, PPV, and NPV were calculated for all 17 cases that met eligibility and were interpretable, then calculated with the two SDH cases that failed to meet intra-/inter-observer reliability standards removed.
Results
Of all neuroendovascular cases conducted at UCSF Medical Center between April 2015 and December 2015, 33 cases with FPCT images were identified. Of the 33 cases, 19 cases met inclusion criteria, but two cases were determined to be not interpretable by the raters and were therefore not included, resulting in 17 eligible cases (Figure 1). Sixteen cases failed to meet criteria for the following reasons: no NECT comparison image (e.g, patient received MRI), treatment between comparison images, new stroke between comparison images, and FPCT image determined to be uninterpretable secondary to alternative processing algorithm or poor alignment of patient during imaging, and non-axial FPCT. The characteristics of the 17 cases are included in Table 1. The time between comparison images had a median of 26 hours and 45 minutes and a range of 2 hours and 39 minutes to 181 hours and 41 minutes. Of the cases, 8 cases had comparison times less than 24 hours, 7 cases had comparison times less than four days, and two cases had comparison times greater than four days.
Demographics
Number of Cases
17
Sex
Male
9
Female
8
Age
Average
56
Median
57
SD
11.7
Range
Lower limit
40
Upper limit
83
Time between
Average
53 hours
NECT and FPCT
0 minutes
45 seconds
Median
26 hours
45 minutes
0 seconds
SD
46 hours
7 minutes
18 seconds
Range
Lower limit
2 hours
39 minutes
0 seconds
Upper limit
181 hours
41 minutes
9 seconds
Medical Chart Listed Indications for Hospitalization
Stroke
Ischemic
1
SAH
7
IVH
5
IPH
1
SDH
2
Total
12
Brain Tumor
1
Aneurysm
5
AVF
1
AVM
1
Table 1: Demographics. SD: Standard Deviation; SAH: Subarachnoid Hemorrhage; IVH: Intraventricular Hemorrhage; IPH: Intraparenchymal Hemorrhage; SDH: Subdural Hemorrhage; AVF: Arteriovenous Fistula; AVM: Arteriovenous Malformation.
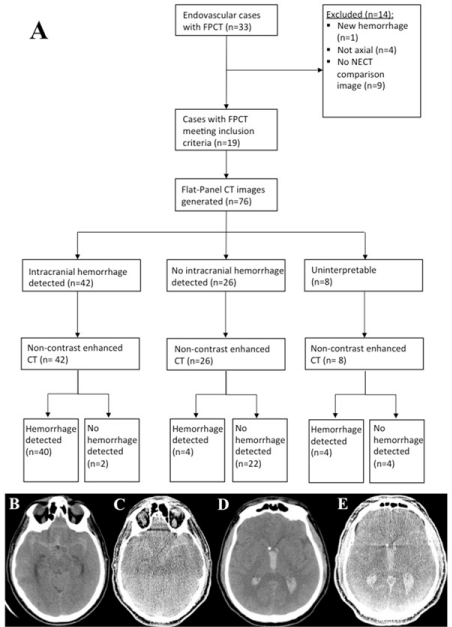
Figure 1: (A) Study design flowchart. A case of a SAH as seen on NECT
(B) and FPCT (C). A case of an IVH as seen on NECT (D) and FPCT (E).
FPCT: Flat-Panel Computed Tomography. NECT: Non-contrast Enhanced
Computed Tomography. CT: Computed Tomography.
The intra-rater agreement was assessed for both raters in each category (i.e., hemorrhage, SAH, IVH, IPH, and SDH) and the overall percent agreements, positive percent agreements, and kappa statistics are presented in Table 2. In the detection of intracranial hemorrhage, raters had 100 percent positive and overall intra-rater agreements with kappa statistics of 1, indicating perfect agreement. Inter-rater agreement for the category of hemorrhage was 0.81 (CI 0.67-0.96), which indicates almost perfect agreement. SAH and IVH detection had kappa statistics indicating substantial intra-rater agreement and inter-rater agreement. However, SDH and IPH had variable intra-rater agreement. IPH intra-rater agreement kappa statistics ranged from substantial to almost perfect agreement. SDH was not computable for one of the raters, resulting in a kappa statistic and positive percent agreement of 0%.
The sensitivity, specificity, and positive and negative predictive values of the FPCT in the detection of intracranial hemorrhage, SAH, IVH, IPH and SDH are listed in Table 3. Given the lack of agreement seen in the detection of SDH, all values were calculated with and without the two cases of SDH for comparison. One of the SDH cases removed also showed IPH and SAH; as a result, when the SDH cases were removed, the number of computed cases for those categories was affected. With the SDH cases removed, the sensitivity in the detection of intracranial hemorrhage decreased from 0.91 (0.78-0.97) to 0.89 (CI 0.75-0.97) and the specificity and PPV increased to 1 with confidence intervals of (0.85-1) and (0.9-1) respectively.
Intra-rater Agreement
Inter-rater Agreement
Rater 1
Rater 2
Hemorrhage
Overall Percent Agreement
100%
100%
91%
Positive Percent Agreement
100%
100%
87%
Kappa Statistic
1 (CI 1 – 1)
1 (CI 1 – 1)
0.81 (CI 0.67 – 0.96)
SAH
Overall Percent Agreement
88%
88%
85%
Positive Percent Agreement
76%
78%
72%
Kappa Statistic
0.76 (CI 0.54 – 0.98)
0.76 (CI 0.55 – 0.98)
0.70 (CI 0.53 – 0.87)
IVH
Overall Percent Agreement
94%
91%
84%
Positive Percent Agreement
85%
81%
66%
Kappa Statistic
0.87 (CI 0.70 – 1)
0.82 (CI 0.63 – 1)
0.66 (CI 0.48 – 0.84)
IPH
Overall Percent Agreement
88%
97%
90%
Positive Percent Agreement
50%
89%
61%
Kappa Statistic
0.60 (CI 0.24 – 0.97)
0.92 (CI 0.77 – 1)
0.70 (CI 0.48 – 0.91)
SDH
Overall Percent Agreement
94%
100%
94%
Positive Percent Agreement
33%
Cannot be calculated
0%
Kappa Statistic
0.47 (CI 0 – 1)
Cannot be calculated
0 (CI 0 – 0.95)
Table 2: Intra-rater and inter-rater agreements. CI: Confidence Interval; SAH: Subarachnoid Hemorrhage; IVH: Intraventricular Hemorrhage; IPH: Intraparenchymal Hemorrhage; SDH: Subdural Hemorrhage.
Hemorrhage
SAH
IVH
IPH
SDH
Value
95% Confidence Interval
Value
95% Confidence Interval
Value
95% Confidence Interval
Value
95% Confidence Interval
Value
95% Confidence Interval
Lower Limit
Upper Limit
Lower Limit
Upper Limit
Lower Limit
Upper Limit
Lower Limit
Upper Limit
Lower Limit
Upper Limit
nsitivity
0.91
0.78
0.97
0.66
0.47
0.81
0.88
0.69
0.97
0.69
0.41
0.89
0.33
0.84
0.91
Specificity
0.92
0.73
0.99
0.75
0.58
0.88
0.86
0.72
0.95
0.96
0.87
1
1
0.95
1
True Positive (PPV)
0.95
0.84
0.99
0.7
0.51
0.85
0.79
0.59
0.92
0.85
0.55
0.98
1
0.025
1
True Negative (NPV)
0.85
0.65
0.96
0.71
0.54
0.85
0.93
0.8
0.98
0.91
0.8
0.97
0.97
0.9
1
Without SDH cases:
Sensitivity
0.89
0.75
0.97
0.68
0.48
0.84
0.79
0.59
0.92
0.58
0.28
0.85
Specificity
1
0.85
1
0.84
0.67
0.95
0.91
0.75
0.98
0.96
0.86
0.99
True Positive (PPV)
1
0.9
1
0.79
0.58
0.93
0.88
0.69
0.97
0.78
0.4
0.97
True Negative (NPV)
0.85
0.65
0.96
0.75
0.58
0.88
0.83
0.66
0.93
0.9
0.79
0.97
Table 3: The sensitivity, specificity, and positive and negative predictive values of the FPCT in the detection of intracranial hemorrhage, SAH, IVH, IPH and SDH. PPV: Positive Predictive Value; NPV: Negative Predictive Value; SAH: Subarachnoid Hemorrhage; IVH: Intraventricular Hemorrhage; IPH: Intraparenchymal Hemorrhage; SDH: Subdural Hemorrhage.
Discussion
The role of flat-panel CT in AIS triage is contingent on its comparability to the current gold standard in initial AIS assessment: NECT. This study focused on one of the main decision points in AIS management: the detection of intracranial hemorrhage. If intracranial hemorrhage is noted, the patient is no longer eligible for IV tPA. Currently, NECT is used in the determination of IV tPA and/or embolectomy eligibility, thus this project sought to compare FPCT’s intracranial hemorrhage detection against gold standard NECT. Given that a failure to identify a hemorrhage prior to lytic medication administration can have devastating outcomes, FPCT’s sensitivity to intracranial hemorrhage detection is critical.
In our study, the two interventional neuroradiologists had almost perfect agreement, 0.81 (CI 0.67-0.96), in the detection of any intracranial hemorrhage and at least substantial agreement in subgroups of SAH, IVH, and IPH. The low observer agreement in the detection of SDH could be secondary to the low number of cases available that met criteria. Further studies, with a larger number of SDH cases, would be warranted to properly assess SDH identification and detection using FPCT imaging.
To correct for the poor SDH agreement, the sensitivity, specificity, PPV, and NPV were calculated both with and without the SDH cases included for comparison. With the SDH cases removed, the sensitivity of intracranial hemorrhage detection was 0.89 (CI 0.75-0.97), which is important in assessing the role that FPCT could play in initial stroke assessment and workup. The 0.89 (CI 0.75-0.97) sensitivity is high and promising for a future role of FPCT in stroke management. However, any agreement less than perfect suggests that intracranial hemorrhages may be missed on FPCT. We found the PPV of FPCT hemorrhage detection to be 1 (CI 0.90 - 1). The sensitivity and PPV combined indicate that should a patient have an intracranial hemorrhage, 89% of the time the hemorrhage will be detected and if a hemorrhage is detected a clinician can be 100% certain that the hemorrhage is truly present in the cohort of patients examined herein. FPCT has potential as a diagnostic test for intracranial hemorrhage, particularly in light of the possible decrease in time-to-reperfusion and consequent mortality and outcomes benefit.
To interpret the quality of FPCT as a diagnostic test in hemorrhage detection, it is important to consider its specificity and NPV in addition to its sensitivity and PPV. Our study found FPCT’s detection of intracranial hemorrhage to have a specificity of 1 (CI 0.85-1) and a NPV of 0.85 (0.65-0.96), which indicates a clinician can feel confident that a patient without an intracranial hemorrhage will not be falsely positive on FPCT imaging and consequently miss the opportunity of IV tPA administration.
Beyond simply detecting intracranial hemorrhages of any type, we also assessed the ability to localize intracranial hemorrhages (i.e., SAH, IVH, IPH, and SDH) using FPCT imaging. One limitation of this study was the low number of cases in each category of hemorrhage. Despite this limitation, the sensitivities ranged from 0.58 (CI 0.28-0.85) in IPH to 0.79 (CI 0.59-0.92) in IVH and positive predictive values ranged from 0.78 (CI 0.40-0.97) in IPH to 0.88 (CI 0.69-0.97) in IVH. The values are likely heavily affected by the low number of cases; however, these results are promising.
Limitations in this study and interpretation of the results are natural consequences of the retrospective nature of the study design and limited number of cases available. Of the 33 identified FPCT cases, sixteen failed criteria for reasons including lack of CT comparison images and incomplete image data with poor processing in the record. With only seventeen cases, there was a limitation in the number of available cases in each category of hemorrhage, making some variations in the images secondary to things such as time delay between comparison images a larger factor. In our study, the median time between comparison images was approximately 26 hours and 45 minutes; the impact of this time delay on the amount of blood present to be detected is unclear. Another concern is the impact that the presence of contrast in some FPCT images, a consequence of being acquired during an angiography procedure or following a pre-procedure CTA, could have on the results.
Leyhe, et al. observed differing results: SAH, IVH, and IPH were detected with sensitivities of 0.95, 0.97, and 1 and with specificities of 0.97, 1, and 0.99 compared to this study’s findings of 0.68 (CI 0.48-0.84), 0.79 (CI 0.59-0.92), 0.58 (CI 0.28-0.85), respectively. The differences could potentially be explained by the diversity of machine generations employed in this study, differences in patient population, an increased median time between FPCT and NECT acquisitions, and the inclusion of pre-interventional imaging studies compared to Leyhe, et al. peri-interventional to post-interventional comparisons. Our study shows that even with diverse technology and pre-interventional imaging, FPCT has good sensitivity and specificity indicating a prospective study is warranted. In collaboration with the Leyhe group, we are preparing an international multicenter clinical trial with randomized management allocation, open-label treatment and blinded endpoint evaluation. The trial will compare standard stroke management with NECT to one stop management with FPCT in the endovascular suite for patients presenting with stroke at four hospitals in Germany, one hospital in Japan, and UCSF associated hospitals in California. This paper demonstrates that future prospective studies can be successful given FPCT’s similar sensitivity and PPV to NECT in the setting of diverse technologies and patient populations.
Conclusion
FPCT’s sensitivity and PPV are similar to non-contrast enhanced CT in the detection of hemorrhage and could potentially be used as part of the AIS protocol. The potential decrease in time-to-reperfusion associated with FPCT’s use over NECT could have significant impact on clinical outcomes in AIS. Further research is needed to assess the role of FPCT in the localization of hemorrhage.
References
- Hung SC, Lin CJ, Guo WY, Chang FC, Luo CB, Teng MMH, et al. Toward the era of a one-stop imaging service using an angiography suite for neurovascular disorders. BioMed research international. 2013.
- Khatri P, Yeatts SD, Mazighi M, Broderick JP4, Liebeskind DS5, Demchuk AM, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014; 13: 567-574.
- Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372: 1019-1030.
- Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009; 73: 1066-1072.
- Mazighi M, Chaudhry SA, Ribo M, Skoloudik D, Mokin M, Labreuche J, et al. Impact of Onset-to-Reperfusion Time on Stroke Mortality Clinical Perspective. Circulation. 2013; 127: 1980-1985.
- Khan A, Payabvash S, Qureshi M, Saeed O, Suri M and Qureshi A. Correlation between Flat Panel Computed Tomography and Conventional Computed tomography for Detection of Post Endovascular Treatment Hemorrhages. Neurology. 2015; 84: P4-300.
- Struffert T, Deuerling-Zheng Y, Engelhorn T, Kloska S, Gölitz P, Köhrmann M, et al. Feasibility of cerebral blood volume mapping by flat panel detector CT in the angiography suite: first experience in patients with acute middle cerebral artery occlusions. AJNR Am J Neuroradiol. 2012; 33: 618-625.
- Kau T, Hauser M, Obmann SM, Niedermayer M2, Weber JR3, Hausegger KA. Flat Detector Angio-CT following Intra-Arterial Therapy of Acute Ischemic Stroke: Identification of Hemorrhage and Distinction from Contrast Accumulation due to Blood-Brain Barrier Disruption. AJNR Am J Neuroradiol. 2014; 35: 1759-1764.
- Frölich AM, Buhk J-H, Fiehler J, Kemmling A. Voxel-Based Sensitivity of Flat-Panel CT for the Detection of Intracranial Hemorrhage: Comparison to Multi-Detector CT. Plos One. 2016.
- Payabvash S, Khan AA, Qureshi MH, Saeed O, Suri MF, Qureshi AI. Detection of Intraparenchymal Hemorrhage After Endovascular Therapy in Patients with Acute Ischemic Stroke Using Immediate Postprocedural Flat-Panel Computed Tomography Scan. Journal of Neuroimaging; 2016; 26: 213-218.
- Psychogios M- N, Buhk JH, Schramm P, Xyda A, Mohr A, Knauth M. Feasibility of Angiographic CT in Peri-Interventional Diagnostic Imaging: A Comparative Study with Multidetector CT. American Journal of Neuroradiology. 2010; 31: 1226-1231.
- Struffert T, Eyupoglu IY, Huttner HB, Engelhorn T, Doelken M, Saake M, et al. Clinical evaluation of flat-panel detector compared with multislice computed tomography in 65 patients with acute intracranial hemorrhage: initial results. Journal of Neurosurgery. 2010; 113: 901-907.
- Leyhe JR, Tsogkas I, Hesse AC, Behme D, Schregel K, Papageorgiou I, et al. Latest generation of flat detector CT as a peri-interventional diagnostic tool: a comparative study with multidetector CT. Journal of NeuroInterventional Surgery. 2016.
- Lowry R. Kappa as a Measure of Concordance in Categorical Sorting [Internet]. VassarStats.net. 2017.
- Diagnostic test evaluation calculator [Internet]. MedCalc Software. 2017.