
Review Article
Austin J Cerebrovasc Dis & Stroke. 2020; 7(1): 1084.
Stenosis of Carotid Arteries by Homocysteine: It’s Role in Neurological Events
Cacciapuoti F*
Department of Internal Medicine, “L. Vanvitelli” Campania University, Naples, Italy
*Corresponding author: Federico Cacciapuoti, Internal Medicine Department, “L. Vanvitelli” Campania University, Piazza L. Miraglia, 80131, Naples, Italy
Received: April 19, 2020; Accepted: June 23, 2020; Published: June 30, 2020
Abstract
Hyperhomocysteinemia (HHcy) was previously identified as a risk factor for atherosclerosis. In this study we evaluated the relationship between HHcy and carotid-artery stenosis and its role in inducing some neurological accidents. Several mechanisms exist, through increased homocysteine (Hcy) levels cause atherosclerosis of carotid arteries’ vessels. In turn, atherosclerosis frequently involves supra-aortic trunk (SAT), especially common carotid artery (CCA) and internal carotid artery (ICA), causing carotid plaques. Vulnerability of carotid plaques can yield intracerebral vascular events by thrombo-embolic mechanisms. That most frequently happens when HHcy is contemporary present in association with some common diseases, independently of atherosclerotic lesions of Willis circle vessels. Therapeutically for the primary prevention of plaques’ disruption and cerebral accidents, statin treatment and vitamins B supplementation have demonstrated to significantly reduce the frequency of both. On the contrary, these treatments for secondary prevention are uncertain and further studies are requested.
Keywords: Homocysteine; Carotid-artery atherosclerosis; TIA; Ischemic stroke
Introduction
High homocysteine (HHcy) serum levels are associated with different vascular disease [1], such as coronary artery disease, ischemic stroke and peripheral artery disease [2,3] as carotid-artery disease [4- 6]. Concerning that, data from several studies suggest that mild HHcy is a risk factor for stenosis and/or occlusion of carotid-arteries both in men and women, frequently yielding some intracerebral vascular accidents [7-11].
Anatomically, three branches arise from the superior border of aortic arch: brachiocephalic trunk (BCT), left common carotid artery (CCA) and left subclavian artery carotid (SCA). In turn, BTC later divides into right CCA and in right SCA. At bifurcation, Common carotid artery (CCA) of each side, at bifurcation divides in two parts: external (ECA) and intracranial carotid artery (ICA) (Figure 1). ECA provides blood flow supply to the scalp, face and neck, while ICA supplies blood to the brain. CCA presents an extracerebral share only. On the contrary, ICA divides in extracranial and intracranial tract. This last begins at the base of the skull and penetrates into the brain. Atherosclerotic process causes stenosis/occlusion of CCA or ICA through the plaques’ organization. The possible disruption of these could bring on disturbed intracranial arterial hemodynamics due to an artery-to-artery embolism or chronic hypoperfusion [12].
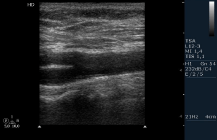
Figure 1: Echography of Supra-Aortic Trunk (SAT).
Legend: CCA: Common Carotid Artery; ECA: External Carotid Artery ICA:
Internal Carotid Artery.
According to Mannheim carotid intima-media thickness (IMT) consensus [13], IMT</=1.0mm was defined as IMP thickening (Figure 2). On the contrary, IMT>/=1.5mm or above the lumen was defined as a plaque (Figure 3). ICA stenosis was defined as greater than 50% luminal narrowing in at least one of ICAs, according to the criteria described by North American Symptomatic Carotid Endarterectomy Trial (NASCET) [14]. Finally, ICA occlusion was diagnosed if one or both ICAs were completely obstructed (Figure 4). It must be added that carotid stenosis or obstruction due to HHcy has been reported frequently associated with retinal vascular obstructive disease [15]. A previous study also reports that more than 30% of patients with ischemic stroke exhibited significant coronary artery stenosis, even without cardiac symptoms [16]. Finally, it must be added that both CCA and ICA stenosis or occlusion can be often associated with smoking, hypertension, diabetes and/or dyslipidemia in inducing cerebral accidents [10,11].
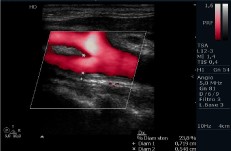
Figure 2: Color Echography of right SAT. Intima Media Thickness (IMT) (=1,4
mm) and the flow diameter measurements in extracranial ICA are pointed out.
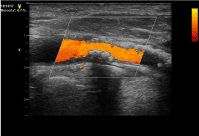
Figure 3: Color Echography of extracranial ICA previously subjected to
stenting. Evidence of a plaque reducing up to 50% the arterial lumen.
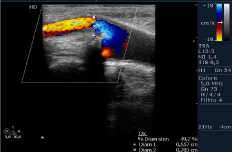
Figure 4: Color-Echography of right SAT. Reduction of flow in extracranial ECA and total obstruction of ICA.
Homocysteine
Hcy is an essential amino acid derived from the conversion of Methionine to Cysteine. It is metabolized via two pathwais: remethylation and trans-sulfuration. In the former, Hcy is re-converted to Methionine by a process requiring Methyltetrahydrofolate (derived from folic acid) and vitamin B12 as cofactors. On the contrary, when Methionine is in excess, Hcy metabolism is directed to the transsulfuration pathway, where it is sulfo-conjugated to cysteine by cystationine-B-synthase, having vitamin B6 as a cofactor [17].
Carotid plaques
As previously affirmed, elevated Hcy plasma levels have been indicated as an independent risk factor for atherosclerosis, responsible for carotid plaques. In HHcy-patients, approximately 20%-30% of stable ischemic strokes are due to stenosis of extracranial carotid branches, while intracranial carotid tracts account for 5%-10% of all vascular ischemic brain-events [18].
At morpho-histological examination, carotid plaques consist of lipid core with infiltration of inflammatory cells covered with fibrous cap. Referring to its consistency, a plaque can be stable or instable. This last is characterized by a thin cap with a large lipid core, active inflammation, accumulation of macrophages and platelets’ aggregation. From functional point of view, this can break, inducing a vessels-brain thrombo-embolization [18]. On the contrary, stable plaques are calcified and less inflamed. Obviously, the calcification is a marker of stability [15].
Plaques’ aetiology
Several mechanisms, such as endothelial dysfunction, oxidative stress, platelets’ adhesion and activation of coagulative cascade are responsible for atherosclerotic plaques in HHcy-patients [19-21]. But, HHcy can act also causing a reduction of HDL-lipoproteins for inhibition of Apo-lipoprotein-A (Apo-A) [22-25]. Apart these, some inflammatory events, as expression of adhesion molecules, recruitment of leukocytes, migration of monocyte and others are also present [26]. In these patients, all mechanisms previously described favor carotid stenosis that may be asymptomatic or symptomatic. Asymptomatic carotid artery atherosclerosis is evident by the presence of a significant amount of atherosclerotic buildup. Asymptomaticity refers to CCA or extracranial ICA stenosis without a history of recent ipsilateral acute events. On the contrary, symptomatic carotid artery atherosclerosis may result in intracerebral temporary or permanent accidents. These last are prone to rupture owing to their intrinsic composition, such as a large lipid score, thin fibrous cap, intraplaque hemorrhage and inflammatory cells infiltrates. Some of these (macrophages and T limphocytes) produce proteolytic enzymes and matrix metalloproteinases responsible for collagen breakdown and lesion instability [27-32].
Neurological sequences
The most frequent neurological findings derived from symptomatic carotid plaque rupture are: amaurosis fugax, TIA, minor stroke and stable, ischemic stroke.
Amaurosis fugax is a focal neurological deficit, characterized by a painless temporary loss of vision in one or both eyes. Obscured vision can be due to the papilledema and may last few seconds up to 1-5 minutes [33]. Minor stroke is defined as mild and non-disabling reduction/loss of some neurological functions with incomplete regression of these symptoms [34]. TIA is a sudden and temporary loss of blood flow to an area of the brain, usually lasting a few minutes to 1 hour. Symptoms disappear within 24 hours with complete recovery [35]. On the contrary, stable stroke is characterized from a persistence of the symptoms. The main symptoms that can characterize both cases (TIA and stable cerebral ischemia) are reported in table (Table 1). Ischemic stroke secondary to carotid plaques rupture represents about 20% of all ischemic strokes.
Main symptoms in TIA and ischemic stroke
*Sudden weakness of an arm/leg on one side of the body;
*Sudden paralysis of a limb (superior and/or inferior) on one site of the body;
*Loss of coordination;
*Confusion, headache, dizziness;
*Numbness of loss sensation in the face, or in a limb;
*Temporary loss of vision or blurred vision;
*Inability to speak clearly or slurred-spech.
Table 1: Leading symptoms can be present in TIA (temporarily) and in ischemic stroke (permanently).
It is caused by carotid plaques’ shattering, due to its vulnerability. In turn, plaque’ vulnerability depends by inflammation, extensive degradation of extracellular matrix, neovascularization and epigenetic changes. It must remember that lumen-narrowing lesions of arteriolar cerebral circulation (induced by the plaques-fragments) alter the local hemodynamic conditions by an arterio-arterial thrombo-embolism, giving both cerebral events [36]. Therefore, according to the Trial of Org.10172 in Acute Stroke Treatment (TOAST) [37], all cerebral accidents induced from carotid plaques disruption are included in third group (Table 2).
TOAST subtypes of ischemic stroke
1) Ischemic stroke by large-artery atherosclerosis;
2) Ischemic stroke by small-artery atherosclerosis;
3) Stroke of cardioembolic or embolic source;
4) Stroke of other determined etiology;
5) Stroke of undetermined etiology.
Table 2: Stroke subtypes of ischemic stroke in relation to etiology.
Treatments and future directions
Cerebral acute events derived from carotid plaques disruption are due to thrombo-embolic mechanism. These are characterized by clinical symptoms and/or focal signs of loss of different brain functions. Their appearance often happens when HHcy is contemporarily associated with other pathologies or some behavioral conditions, such as systemic hypertension, diabetes, cigarette smoking and/or dyslipidemia [38,39]. Some evidences suggest that Hcy-lowering treatment with folate and other B-vitamins obtains a positive effect for both primary and secondary prevention of these events, especially in subjects with a low intake or status of these vitamins [40]. Concordantly, the Framingham Study also suggested that the increased dietary and supplemental intake of vitamin B6 can decrease the stroke and other cerebral events-risk [41]. However, the large prospective Vitamin Stroke Prevention (VISP) trial did not show a benefit with treatment with B6-12 of folate in prevent recurrent stroke [42]. Neither VITATOPS (VITAmins TO Prevent Study) supports the vitamins B supplementation for secondary prevention of TIA or stroke or other events [43].
With reference to the plaques of carotid arteries, the North American Symptomatic Carotid Endarterectomy (NASCET) demonstrated that carotid endarterectomy significantly reduces the risk of neurological sequences of disruption of these [14]. The European Carotid Surgery Trial (ECST) also showed a significant benefit for high-grade of carotid stenosis [44].
With reference to carotid atherosclerosis, the results of NOMAS study suggest a relationship between elevated Hcy concentration and carotid plaques [45]. It must be added that statin drug therapy is believed to stabilize carotid plaques and has been shown to decrease the lipid content plaques [46]. Zhao et al. also found that lipidlowering therapy was not only significantly associated with lower lipid content of plaque but also with a tendency for increased calcium in treated human carotid arteries [47].
Finally, for secondary prevention of focal or global neurological deficits due to the disruption of carotid plaques, the antiplatelet drugs dypiridamole, aspirin, ticlopidine, and clopidogrel have been shown to be efficacious in the secondary prevention of these deficits [48].
Conclusion
In conclusion, the global uncertaines nowadays present on therapeutic treatments require further studies. These need to better define the possibilities to reduce the risk of carotid atherosclerotic plaques in HHcy-patients, the consequent risk of neurological acute events and the exact link existing among tHHcy, carotid plaques and cerebral accidents.
Acknowledgment
The Author read and approved the manuscript.
References
- Clarke R, Daly L, Robinson K. Hyperhomocysteinemia: an independent risk factor for vascular disease. NEJM. 1991; 324: 1149-1155.
- Stampfer MJ, Malinow MR, Willett WC. A prospective study of plasma homocysteine and risk of myocardial infarction in US physicians. JAMA. 1992; 268: 877-881.
- Kang SS, Wong PW, Malinow MR. Hyperhomocysteinemia as a risk factor for occlusive vascular disease. Ann. Rev. Nutr. 1992; 12: 297-298.
- Clarke R, Fitzgerard D, O’Brien C, O’Farrell C, Roche G, Parker G, et al. Hyperhomocystaenemia: a risk factor for extracranial carotid atherosclerosis. Irish. J. Med. Sci. 1992; 161: 61-65.
- Selhub J, Jacques PF, Bostom AG, D’Agostino R, Wilson PWF, Belanger AJ, et al. Association between plasma homocysteine concentration and extracranial carotid-artery stenosis. NEJM. 1995; 332: 286-291.
- Malinow MR, Nieto FJ, Szko M, Bond G. Carotid artery intimal-medial wall thickening and plasma homocysteine in asymptomatic adults: the Atherosclerosis Risk in Communities Study. Circulation. 1993; 87: 1107- 1113.
- Eikelboom JW, Hankey GJ, Anand SS, Lofthouse E, Staples N, Baker RI. Association between high homocysteine and ischemic stroke due to largeand small-artery disease but not other etiologic subtypes of ischemic stroke. Stroke. 2000; 31: 1069-1075.
- Tan NC, Venketasubramanian N, Saw SM, Tjia TL. Hyperhomocysteinemia and risk of ischemic stroke among young Asian adults. Stroke. 2002; 33: 1956-1962.
- Sasaki T, Watanabe M. Nagai Y, Hoshi T, Takasawa M, Nukata M, et al. Association of plasma homocysteine concentration with atherosclerotic plaques and lacunar infarction. Stroke. 2002; 33: 1493-1496.
- Jeong SK, Seo JY, Cho YI. Homocysteine and internal carotid artery occlusion in ischemic stroke. J. Atheroscl. Thromb. 2010; 17: 963-969.
- Rahman A, Quraishi FA, Miah NA, Hakim M, Saha V, Akteruzzman MS, et al. Relationship between homocysteine and carotid artery stenosis in ischemic stroke. Bangladesh J. Neurosci. 2012; 28: 1-9.
- Sacco RL. Clinical practice. Extracranial carotid stenosis. NEJM. 2001; 345: 1113-1118.
- Yamazaki M, Uchiyama S. Pathophysiology of carotid stenosis. Brain Nerve. 2010; 62: 1269-1275.
- Wahlgen CM, Zheng W, Shaalon W, Tang J, Bassiony HS. Relationship between degree of plaque calcification, fibrous cap, inflammatory gene expression and symptomatology. Cerebr. Dis. 2009; 27: 193-200.
- Nadalur KR, Baskurt E, Hagspice HD, Phillips CD, Kramer CM. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque with MDCT. Am. J. Roentgen. 2005; 184: 295- 298.
- Naghavi M, Libby P, Falk E, Casscells SW, Litovski S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies. Part I Circulation. 2003; 108: 1664-1672.
- Selhub J. Homocysteine metabolism. Annu. Rev. Nutr. 1999; 19: 7-46.
- Abdusalam M, Feng J. Distinguish the stable and unstable plaques based on arterial waveform analysis. Proc. Struct. Integrity. 2019; 15: 2-7.
- Welch GN, Upchurch GRJ, Loscalzo J. Homocysteine, oxidative stress, and vascular disease. Hosp. Pract (Minneap). 1997; 32: 81-92.
- Cacciapuoti F. Homocysteine and vascular disease: doubts and certainties. Ann. Vasc. Med. Res. 2017; 4: 1059.
- Jin Z, Liu Y. DNA methylation in human disease. Gen. Dis. 2018; 5: 1-8.
- Barther PJ, Ry KH. Homocysteine and cardiovascular disease. Is HDL the link? Circ. Res. 2006; 99: 565-566.
- Liao D, Tan H, Hui R, Li Z, Jiang X, Gaubatz J, et al. Hyperhomocysteinemia decreases circulating HDL by inhibiting Apo-A protein synthesis and enhancing HDL-C clearance. Cir. Res. 2006; 99: 598-606.
- Mikael LG, Genest J jr., Rozen R. Elevated homocysteine reduces Apolipoproein-A-1 expression in hyperhomocysteinemic mice and animals with coronary artery disease. Circ. Res. 2006; 98: 564-571.
- Gordon T, Castelli WP, Hjortland MC, Kannel EB, Dawber TR. High density lipoprotein as a predictive factor against coronary heart disease. Am. J. Med. 1997; 62: 707-714.
- Castelli WP, Garrison PJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels; the Framingham Study. JAMA. 1986; 256: 2835-2838.
- Mugha MM, Khan MK, DeMarco JK, Majid A Shamoun F, Abela GS. Symptomatic and asymptomatic carotid artery plaque. Expert. Rev. Cardiovasc. Ther. 2011; 9: 1315-1330.
- Wu JT, Wu LL. Linking inflammation and atherogenesis: soluble markers identified for the detection of risk factors and early risk assessment. Chem. Clin. Acta. 2006; 366: 74-80.
- Dickson BC, Gotlieb HI. Towards understanding acute destabilization of vulnerable atherosclerotic plaques. Cardiovasc. Pathol. 2003; 12: 237-248.
- Carr SC, Farb A, Pearce WH, Virmani R, Yao JS. Activated inflammatory cells are associated with plaque rupture in carotid stenosis. Surgery. 1997; 122: 757-763.
- Jander S, Sitzer M, Schmann R, Schroeter M, Sieber M, Steinmetz H, et al. Inflammation in high-grade carotid stenosis: a possible role for macrophages and T cells in plaque destabilization. Stroke. 1998; 29: 1625-1630.
- Galis ZS, Suchova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 1994; 94: 2493-2503.
- Burde RM. Amaurosis fugax: an overwiew. J. Cli. Neurophalmol. 1989; 185- 189.
- Crespi V, Braga M, Beretta S, Carolei A, Bignamini A, Sacco S. A pratical definition of minor stroke. Neurol. Sci. 2013; 34: 1083-1086.
- Amort M, Fluri F, Schafer J, Weisskoft F, Katan M, Burow A, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc. Dis. 2011; 32: 57-64.
- Pelisek J, Eckstein HH, Zemeke A. Pathophysiological mechanisms of carotid plaque vulnerability: impact on ischemic stroke. Arch. Immunol. Ther. Exp. 2012; 60: 431-444.
- Adams HP, Bendixen BH, Kappelle J, Biller J, Love BB, Gordon DL, et al. Classification subtypes of acute ischemic stroke-Definition for use in a multicenter clinical trial. Stroke. 1993; 24: 35-41.
- Okura T, Miyashi K, Irita J, Emoto D, Nagao T, Kukida M. et al. Hyperhomocysteinemia is one of the risk factors associated with cerebrovascular stiffness in hypertensive patients, especially elderly males. Sci. Rep. 2014; 4: 53-63.
- Evers S, Koch HG, Grotemeyer KH, Lange B, Deufel T, Ringelstein EB. Features, symptoms and neurophysiological findings on stroke associated with hyperhomocysteinemia. Arch. Neurol. 1997; 54: 1276-1282.
- Hermann W, Lorenzi S, Obeid R. Review of the role of hyperhomocysteinemia and B-vitamins deficiency in neurological and psychiatric disorders-current evidence and preliminary recommentations. Fortsch. Neurol. Psychiatry. 2007; 75: 515-527.
- Jacobs b, Sacco R, Lui R. Abala B, Benson R, Pablos-Mendez A. Low dietary intake of vitamin B6 is associated with an increased risk of ischemic stroke. Stroke. 1999; 30: 252.
- Pettigrew LC, Bang H, Chambless LE, Howard VJ, Toole JF, and VISP Investigators. Assessment of pre and post-methionine load homocysteine for prediction of recurrent stroke and coronary artery disease in the Vitamin Intervention for Stroke Prevention (VISP) trial. Atherosclerosis. 2008; 200: 345-349.
- VITATOPS Trial Study Group. B vitamins in patients with recent transient ischemic attack or stroke in the VITAmins To Prevent Stroke (VITATOPS) trial: a randomized, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010; 9: 855-865.
- ECST Investigators. Interim results for symptomatic patients with severe (70- 90%) or with mild (0-29%) carotid stenosis. Lancet. 1991; 337: 1235-1243.
- Gardener H, Della Morte D, Elkind MS, Sacco RL, Gundek T. Lipids and carotid plaque in the Northern Manhattan Study (NOMAS). BMC Cardiovasc. Disord. 2009; 9: 55.
- Crisby M, Nordin-Fredriksson G, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decrease lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implication for plaque stabilization. Circulation. 2001; 103: 926-933.
- Zhao X, Yuan C, Hatsukami TS, Frechette EH, Kang XS, Maravilla KR, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo bi MRI: a case control study. Arterioscl. Thromb. Vasc. Biol. 2001; 21: 1623-1629.
- Diener H, Cunha L, Forbes C, Sivenius S, Smets P, Loventhal A. European Stroke Prevention. Study 2: Dypiridamole and acetylsalicylic acid in the secondary prevention of stroke. J. Neurol. Sci. 1996; 143: 1-13.