
Review Article
Austin J Cerebrovasc Dis & Stroke. 2022; 9(1): 1088.
Correlation between Atrial Fibrillation and Heart Failure with Preserved Ejection Fraction: A Review
Prasad RM1#*, Banga S2#, Salazar AM1#, Yavari M1#, Pandrangi P2#, Baloch ZQ2# and Ip J3#
1Department of Internal Medicine, Michigan State University, Sparrow Hospital, Lansing, Michigan, USA
2Department of Cardiology, Michigan State University, Sparrow Hospital, Lansing, Michigan, USA
3Department of Cardiology, Sparrow Hospital, Lansing, Michigan, USA
#These Authors Take Responsibility for All Aspects of the Reliability and Freedom from Bias of the Data Presented and Their Discussed Interpretation
*Corresponding author: Rohan Madhu Prasad, Sparrow Clinical Research Institute, 1200 E. Michigan Ave, Suite 550, Lansing, MI 48912, USA
Received: February 03, 2022; Accepted: March 02, 2022; Published: March 09, 2022
Abstract
Previous studies have demonstrated that patients with atrial fibrillation (AF) and heart failure with preserved ejection fraction (HFpEF) may develop the latter. The prevalence of 20-43% for AF in patients with HFpEF and prevalence of 50- 60% for HFpEF in AF patients. The pathophysiology indicates that AF usually precedes HFpEF, but each disease can promote the progression of the other one. Multiple mechanisms have been posited, such as left atrial (LA) fibrosis and myopathy as well as volume/pressure overload. Moreover, the combination of AF and HFpEF is associated with an increased rate of mortality as the presence of AF worsens the hemodynamics of HF. The diagnosis of HFpEF in patients with AF is underestimated, as the symptoms, laboratory values, and imaging techniques can be skewed by the presence of AF. Unfortunately, there are limited randomized controlled trials that recommend guideline-based treatments, such as choosing between rate and rhythm control. This narrative review aims to illustrate and summarize the pathophysiology, diagnosis, and treatment in the current literature for patients with AF and HFpEF.
Keywords: Atrial fibrillation; Heart failure with preserved ejection fraction; Catheter ablation
Abbreviations
AF: Atrial Fibrillation; ANP: Atrial Natriuretic Peptide; BNP: pro-B-type Natriuretic Peptide; EAT: Epicardial Adipose Tissue; EKG: Electrocardiogram; GSDMD: Gasdermin D; HF: Heart Failure; HFpEF: Heart Failure with Preserved Ejection Fraction; HFrEF: Heart Failure with Reduced Ejection Fraction; LA: Left Atrium; LV: Left Ventricle; NYHA: New York Heart Association; RAAS: Renin- Angiotension-Aldosterone-System; RCTs: Randomized Controlled Trials; RV: Right Ventricle; ST2: Suppression of Tumorigenicity 2 Receptor
Introduction
Two of the main cardiac diseases in the developed world are atrial fibrillation (AF) and heart failure (HF) [1,2]. The three types of AF are paroxysmal (episodes of arrhythmia that terminate spontaneously or with intervention within seven days of onset), persistent (episodes that continue for more than 7 days and are not self-terminating), longstanding persistent (continuous episodes for more than 12 months), and permanent (joint decision between patient and clinician to stop further attempts to restore or maintain sinus rhythm) [3]. The rapid and random atrial impulses during AF can create a highly irregular fluctuation of the ventricular response interval, which is known as ventricular irregularity [4]. HF is a clinical syndrome with typical symptoms of dyspnea, orthopnea, lower limb swelling, and signs of elevated jugular venous pressure and pulmonary congestion. HF can be graded based on the New York Heart Association (NYHA) functional classification. HF can also be separated into different categories based on the ejection fraction: HF with preserved ejection fraction (HFpEF), HF with mid-range ejection fraction (HFmrEF), and HF with reduced ejection fraction (HFrEF). Both AF and HFpEF have high healthcare burdens, with an annual cost per patient of US$8705 and US$10,832, respectively [5,6]. Moreover, they have similar risk factors, such as older age, hypertension, diastolic dysfunction, smoking, obesity, and obstructive sleep apnea [7-9]. Patients with AF or HFpEF have a relatively poor prognosis and those with both have even worse outcomes, including an 80% increased risk of mortality [10]. The Get With The Guidelines - Heart Failure (GWTG-HF) registry also evaluated this population of patients and revealed they have higher in-hospital mortality (odds ratio of 1.2), overall readmissions, and HF readmissions [11]. Moreover, the pathophysiology, diagnosis, and mortality outcomes are well discussed in HFrEF patients, but not HFpEF. There is a large knowledge gap in regards to the effective treatment options for HFpEF [9]. The purpose of this study is to review and discuss the various sections of diagnosing and managing a patient with both AF and HFpEF. These sections will discuss the incidence and prevalence, pathophysiology, clinical outcomes, diagnostic methods, and treatment regimens of patients with AF and HFpEF.
Prevalence of AF and HFpEF
Recent studies have demonstrated that the prevalence of AF was approximately 20-43% in patients with HFpEF [12-16]. Of patients with AF, the prevalence of HFpEF was reported at 50-60% [12,17]. Of note, the prevalence of AF depends on the stage of the HF, specifically 5-10% in the NYHA classes I and II and 50% in NYHA class IV [18,19]. On the other hand, the risk of developing AF at any point during the HF disease course is reported around 60% [10,20]. AF is the most common sustained arrhythmia in patients with HF [21]. HFpEF likely has a higher prevalence rate with the permanent form of AF, as seen in the RealiseAF survey and EURObservational Research Programme [22,23]. Previously, the clinical diagnosis of AF and HFpEF could be challenging, since the typical symptoms of both conditions can overlap [19]. Further well-designed studies are required to determine the prevalence and incidence for AF and HFpEF and we propose the idea of excluding patients with pseudo-HFpEF in this patient population to obtain an accurate number. However, now there are multiple different modalities - such as laboratory markers, electrocardiogram (EKG), echocardiogram, and cardiac magnetic resonance imaging - that can be used to make a correct diagnosis. Therefore, large-scale studies should still be conducted to confirm these prevalence rates.
Pathophysiology for AF Instigating HFpEF
AF is a common cardiovascular disease with complex pathophysiology that contributes to significant patient morbidity and mortality [24]. The prevailing hypothesis of AF genesis is that rapid triggering from multiple atrial locations initiates propagating reentrant waves in a vulnerable atrial substrate. The pulmonary veins (PV) have been identified as the primary site of premature atrial beats that initiate frequent paroxysms of AF [25]. The molecular basis for PV triggering has been primarily attributed to abnormal calcium handling. A diastolic leak of calcium from the sarcoplasmic reticulum activates an inward sodium current via sodium-calcium exchanger resulting in spontaneous myocyte depolarization, such as early or delayed after-depolarization [26]. It has been documented that the myocytes from the pulmonary vein sleeves have electrophysiological features that make them distinct from those in the atria. For example, canine pulmonary vein sleeves display both decreased Ik1 and ICa (L) but increased currents compared with the atria1 [27]. This can generate a shorter action potential duration and less negative resting membrane potential. These combined mechanisms facilitate calcium-dependent after depolarization and triggered activity, explaining why the PV sleeves are the main site for the emergence of arrhythmia [28]. The perpetuation of AF mostly depends on the stabilization of reentry; however, the mechanism is controversial with the two dominant hypotheses being reentrant rotors and multiple independent wavelets. However, recent data have supported a third hypothesis or the double layer hypothesis, which suggests that electric dissociation of epicardial and endocardial layers also may facilitate reentry [29].
AF and HFpEF not only have similar risk factors, but also pathophysiologic mechanisms. These include diastolic dysfunction, atrial fibrosis, left atrial (LA) enlargement, and inflammation [9,30]. Studies indicate that AF most likely precedes the onset of HF, especially in HFpEF with a prevalence of 32% versus HFrEF with 23% [10,31]. Furthermore, successful cardioversion was possibly associated with improved diastolic dysfunction, which helps the claim that AF causes HFpEF [31]. The progression of AF may contribute to the progression and exacerbation of HFpEF [32,33].
The current literature illustrates that AF may induce HFpEF through several etiologies. The mechanisms for the below pathophysiologies are depicted in Figure 1. The first mechanism is structurally where increased LA fibrosis and myopathy causes decreased LA function and compliance as well as LA dilation and enlargement. These changes in the LA results in the decreased ventricular filling, left ventricle (LV) myocardial fibrosis, and diastolic dysfunction, which can eventually lead to HFpEF [22,34]. Another mechanism is that AF creates a state of pressure/volume overload with altered subcellular calcium homeostasis that leads to heart failure and tachycardia-induced cardiomyopathy [35]. AF is known to cause cellular calcium loading and decreased calcium-transient amplitude that eventually leads to atrial remodeling, stunning, and fibrosis [36]. Patients with AF but without HFpEF have been shown to also develop increased LV filling pressures [37]. In patients with HFpEF and permanent AF, a study found that pericardial restraint caused statistically significant effects of impaired cardiac output at rest and during exertion [32]. Pericardial restraint develops when an elevated right heart pressure and volume cause’s increased LV filling pressures even with normal LV end-diastolic volume and diastolic compliance [34]. Additionally, atrioventricular annular remodeling can lead to progressive mitral and tricuspid regurgitation [24,34]. From there, mitral regurgitation can cause patients to develop dysfunctional mitral valve leaflets, increased intracardiac pressure, and finally HF [38].
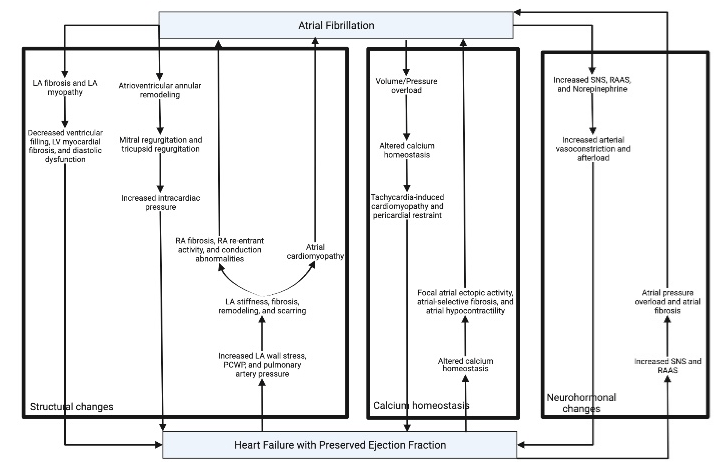
Figure 1: Vicious Cycle between AF and HFpEF - Part 1.
LA: Left Atrium; LV: Left Ventricle; PCWP: Pulmonary Wedge Pressure; RA: Right Atrium; RAAS: Renin-Angiotension-Aldosterone System; SNS: Sympathetic
Nervous System.
Another possible mechanism is the electrical concept of “AF begets AF”. Wijffels et al. evaluated goats and illustrated that continuous and rapid atrial pacing leads to progressive shortening of the atrial effective refractory period and increased duration of AF [39]. Some studies suggest that hyperactivity of the intrinsic cardiac autonomic nervous system, specifically the ganglionated plexi, may be a crucial element in the mechanism of acute atrial remodeling and “AF begets AF” [40]. Through its constant irregularity, AF creates an abnormal combination of the myocardium’s lusitropic and inotropic properties that leads to suboptimal electromechanical function and decreased cardiac output [19]. Moreover, AF can have detrimental effects on the LV function, such as the loss of atrial contractions, irregular ventricular filling, and irregular or high ventricular rate. However, most of these mechanisms usually occur during the acute onset of atrial tachyarrhythmia [41]. This was also demonstrated in a recent study that evaluated the relationship between longitudinal LV peak strain and preceding RR-interval in 10 patients with persistent AF. It showed that differences in preload of the current beat could explain the beat-to-beat variations in LV peak strain (particularly at a fast heart rate). This may play a role in the future for the measurement of LV peak strain in AF patients and impact in their management [42]. AF can lead to acute tachycardia, atrial systole loss, and shortened diastolic intervals, which can exaggerate both HFpEF and AF [9,43]. The strongest predictor of the development of HF was the presence of permanent AF. Other independent predictors were tachycardia at baseline and diffuse LV fibrosis [30,32]. Paroxysmal AF patients are likely to progress to long-standing persistent AF, especially those with severely decreased LA strain and compliance [32]. Permanent AF causes severe dysfunction of the atria, ventricles, and right ventricle (RV) - pulmonary vascular coupling [34]. The mechanism of AF-induced LV fibrosis is associated with tachycardia-mediated cardiomyopathy and chronic tachycardia like AF produces reversible systolic dysfunction. A similar effect with dysregulated excitationcontraction coupling and calcium handling is seen in patients with rapid ventricular pacing [31]. Additionally, aging-associated myocardial stiffness from long-standing AF can lead to ventricularvascular uncoupling and diastolic dysfunction [43]. Furthermore, AF may directly lead to RV dysfunction through decreased longitudinal performance [31].
Finally, AF increases the levels of sympathetic nervous system tone, renin-angiotensin-aldosterone-system (RAAS) activity, and plasma norepinephrine, which leads to increased arterial vasoconstriction, increased afterload, and eventually HFpEF. These neurohormones also cause further tachycardia, ventricular irregularity, and AF [9,19].
Pathophysiology of HFpEF Worsening AF
The LVEF range for HFpEF was not clearly defined for many years, with some studies suggesting an EF cut-off value between 40% to 55% [16]. However, recent data published by the European Society of Cardiology in 2016 established standardized definitions for the different types of heart failure. HFrEF is the term used to describe patients with an EF of <40% and HFpEF is defined by an EF >50%. Therefore, patients with an EF in the range of 40-49% represent a grey area, which is now defined as heart failure with mid-range ejection fraction (HFmrEF) [44-46]. Interestingly, the prevalence rates of AF, death, and HF hospitalization increase with EF, which is pertinent for patients with HFmrEF and HFpEF [45,46]. By definition, patients with HFpEF have a normal EF; however, tissue doppler imaging has shown that these patients can still have systolic dysfunction [37].
It is important to mention that HFpEF is usually preceded by other chronic medical conditions (like obesity, hypertension, diabetes or pulmonary hypertension), whereas HFrEF usually comes with acute/chronic ischemia and/or valvular abnormalities. These chronic conditions promote a systemic inflammatory state, giving rise to the pathophysiology of HFpEF with systolic and diastolic LV dysfunction that causes decreased diastolic relaxation and increased LV end-diastolic pressures. In a counterpart, in HFrEF there’s more predominance of a systemic neurohormonal activation rather than an inflammatory state [43]. HFpEF can cause major changes in other organs, including the kidneys and lungs. Patients that have chronic HFpEF and are diagnosed with incident AF have a higher risk for a worse prognosis [10]. In patients who were discharged after being admitted for acute decompensation of HFpEF, AF was found to have a modest increase of all-cause mortality after 30 days [46]. An enlarged LA appendage is a well-considered risk factor for AF [47]. In HF patients, LA sizes are 68% larger and are strong predictors of clinical outcomes [22,30]. This is likely because an effective atrial contraction is required to maintain normal filling of the LV, as it provides 25% of the cardiac output in a patient with HFpEF [9,48]. Restoration of an AF to sinus rhythm does not result in improvement of HF in patients with preexisting ventricular dilatation and elevated cardiac filling pressures [49]. Other explanations include uncontrolled heart rates at onset, inadequate upregulation of metabolic compensation, stroke, and adverse effects of antiarrhythmic and anticoagulation medications [19]. Additionally, a lead-time bias may play a role where the onset of AF is actually prior to HFpEF, but is not diagnosed until after HFpEF. In this scenario, as AF was allowed to progress uncontrolled it may lead to poorer outcomes [10].
The mechanisms for the pathophysiology of HFpEF patients developing AF are depicted in Figure 1 and 2. Firstly, HFpEF is associated with increased LA wall stress, pulmonary capillary wedge, and pulmonary artery pressures that create LA stiffness (different from HFrEF, in which there’s a greater eccentric LA remodeling), fibrosis, remodeling, and scarring. The locations of fibrosis have been demonstrated to create conduction heterogeneity and reentry pathways with a predilection to form an AF rhythm [9,10,31,47]. In a rat model, it was found that these changes may exacerbate right-sided heart disease, which can produce a substrate for AF maintenance due to RA fibrosis, RA re-entrant activity, and conduction abnormalities [50]. This mechanism might contribute to the previously mentioned pulmonary-induced atrial changes that may precipitate AF [31].
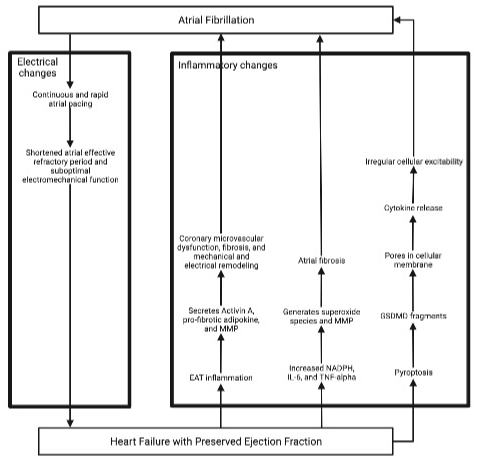
Figure 2: Vicious Cycle between AF and HFpEF - Part 2.
EAT: Epicardial Adipose Tissue; IL-6: Interleukin-6; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; MMP: Matrix Metalloproteinase; TNF-alpha: Tumor
Necrosis Factor-alpha.
Through the aforementioned structural changes, HFpEF can induce and contribute to atrial cardiomyopathy. The current definition for atrial cardiomyopathy is: ‘Any complex of alterations of the structure, architecture, contractility, and electrophysiology of the atrium that can produce relevant clinical manifestations’ [31]. Many studies have shown that the LA dimension could reflect AF burden, which has been reported to be associated with the risk of stroke in several device-detected AF trials [51]. Furthermore, a linear relationship has been demonstrated between the LA size and CHA2DS2-VASc score [52]. Several clinical factors in patients with atrial cardiomyopathies, such as those present in the CHA2DS2- VASc score, favor molecular alterations that predispose to oxidative stress at the level of myocytes and endothelial cells. Thus, these clinical factors increase the risk of prothrombotic factors and stroke [53].
Furthermore, patients with HFpEF have increased cellular calcium load due to phospholamban hyperphosphorylation and action potential prolongation as well as decreased calcium contractility sensitivity via reduced expression of total and phosphorylated myosinbinding protein C. This dysregulated calcium can lead to focal atrial ectopic activity, atrial-selective fibrosis, atrial hypocontractility, and eventually increased likelihood of thromboembolic events [31,54,55]. Moreover, altered calcium and potassium ion-channel expressions can lead to attenuated action potential duration, atrial contractility, and eventually AF [19].
The etiology of AF in patients with HFpEF is also linked to the inflammation of the epicardial adipose tissue (EAT) [49]. Through its proximity to the myocardium in both atriums and ventricles, the EAT may intensify the inflammatory process to produce coronary microvascular dysfunction, fibrosis, and mechanical and electrical remodeling [47]. A study obtained samples of EAT from patients undergoing coronary bypass surgery were analyzed in an organoculture model of rat atria. This study demonstrated that the EAT is a metabolically active tissue, which secretes Activin A, a pro-fibrotic adipokine, and matrix metalloproteinases [55]. Moreover, EAT and matrix metalloproteinases have been indicated in the pathogenesis of AF and HF [56-58]. Furthermore, HFpEF is known as a proinflammatory state, specifically with elevated levels of nicotinamide adenine dinucleotide phosphate oxidase, interleukin-6, and tumor necrosis factor-alpha. These three result in the generation of superoxide species, activation of matrix metalloproteinase enzymes, and eventually atrial fibrosis [9]. Once atrial fibrosis induces AF, the abnormal rhythm has methods to potentiate itself. Rapid atrial rate stimulates the enzyme nitric oxide Synthase, resulting in further oxidative species [9]. Furthermore, an increased level of inflammasome and pyroptosis in patients with HFpEF may lead to N-terminal Gasdermin D (GSDMD) fragments. The GSDMD forms pores in the cellular membrane and allows the cytokines to release, which could affect cellular excitability [59]. Finally, a pro-inflammatory state has been linked with triggering AF, as was demonstrated in patients who developed post-operative AF [60]. Unfortunately, this mechanism has not been clearly defined as currently it has only been studied in rats and the cytokines that are released are unknown.
In advanced HFpEF, elevated central venous pressure, such as left- and right-sided filling pressures, can cause a decrease in renal blood flow and renal perfusion pressure. These alterations activate the RAAS and the sympathetic nervous system, which leads to a reduction in glomerular filtration rate [61]. These renal changes may also contribute to the development of AF by promoting atrial pressure overload and fibrosis [61]. The precise etiology of atrial fibrosis remains ill-defined. However, it does appear that the atria are more susceptible to fibrosis than the ventricles with the involvement of three interrelated pathways - RAAS, TGF-B1, and oxidative stress [62]. HFpEF also promotes the development of increased pulmonary capillary wedge pressures due to the passive backward transmission of the elevated filling pressures into the pulmonary circulation [63].
Other Pathophysiology Pearls
Studies have demonstrated that patients with HFpEF and AF have elevated RA volumes along with decreased RA strain, compliance, and function. This is important to recognize as right atrium overload may be a direct cause of worsening AF. This can be explained by atrial dyssynchrony and systemic inflammation causing structural remodeling in the bilateral atria. Additionally, impaired LA compliance and function may allow the fluid accumulation in the left-sided vascular system and eventually trigger RV and right atrium remodeling [34]. It has also been demonstrated that not just an increased but an irregular pacing of cardiomyocytes contributes to atrial and ventricular remodeling. Irregular pacing increases diastolic calcium and activation of CAMKII and AMPK resulting in lipid accumulation reduced glucose uptake and increased glycogen synthesis. These metabolic changes are accompanied by an activation of pro-apoptotic signaling pathways, contributing to the mentioned structural remodeling [64].
As described above, obesity is a shared risk factor for the development of AF and HFpEF through its strong association with LV hypertrophy. However, HF patients with a body mass index greater than 25kg/m2 have a more favorable prognosis in terms of chronic and acute decompensated HF. This counterintuitive association is known as the obesity paradox. Possible etiologies for this are decreased systemic vascular resistance activity, renin levels, and pro-B-type natriuretic peptide (BNP) levels. The hypothesis is that patients with obesity and HF are more resistant to the harmful effects of AF [65]. It is important to mention that this paradoxical benefit of a medically unfavorable phenotype is particularly strong in the overweight and class I obesity, but less pronounced in the more severe or morbidly obese populations [66]. Moreover, a recent study with long-term follow-up demonstrated that patients with AF have a 35% reduction of all-cause mortality in the overweight and obese versus normal body mass index [67]. Most recent studies have also demonstrated that chronic inflammation plays a role in arrhythmogenesis in obese patients. An identified inflammatory marker is the NLP3 inflammasome, whose activity is enhanced in patients with increased BMI. This suggests that a selective inhibition of NLP3 would prevent the development of the reentry substrate and abnormal calcium release in these patients, thereby preventing obesity-related AF. This could be a novel pharmacological approach for the prevention and treatment of AF patients, however more studies still need to be done [68].
The pathophysiology from both AF and HFpEF increases the risk of blood clots via atrial fibrosis and decreased flow velocity of the LA. Importantly, fibrosis and abnormal diastolic filling pressures even without LA dilatation, AF, or HFpEF are associated with the formation of LA thrombi. Therefore, the degree of LA fibrosis may be the primary indicator of vascular brain injury [47]. In patients with AF, HF had a similar risk of stroke and systemic embolism, but was associated with a higher risk of all-cause death and vascular death [69]. Moreover, similar rates of stroke and systemic embolism are seen with HFpEF (1.3% and 3.9%) and HFrEF (1.6% and 2.7%), respectively [15,22].
Finally, women with AF are more likely to develop concentric LV hypertrophy and diastolic dysfunction. Females with HFpEF are intolerant to the AF effects and medications are ineffective; thus, they have an increased risk of mortality. Therefore, a rhythm control method should be considered in women with HFpEF [14].
Clinical Consequences
Patients with both HFpEF and AF have significant exertional intolerance [10,70]. In comparison to HFrEF, patients with HFpEF develop symptoms at rest at a statistically higher rate [71]. At peak exercise, these patients have decreased levels of peak oxygen consumption, peak oxygen pulse, peak circulatory power, and peak systolic blood pressure as well as increased ventilatory efficiency. Peak oxygen consumption is considered a surrogate for maximal aerobic capacity. The randomized controlled trial (RCT) ROCKET-AF study revealed that patients with AF have decreased peak systolic blood pressure and impaired contractile reserve during exercise, despite similar resting systolic blood pressure in patients with AF and non- AF. The increased ventilatory efficiency indicates that these patients have a higher amount of physiologic pulmonary dead space. These findings reveal that when AF and HFpEF coexist, the patients have impaired peak exercise capacity and functional submaximal exercise capacity, even with an adequate rate control regimen [72].
Using pooled data from I-Preserve (Irbesartan in Heart Failure with Preserved Systolic Function) and TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), patients with AF and HFpEF have a higher risk of allcause mortality, cardiovascular mortality, and HF hospitalization. Other studies have also demonstrated an increased rate of all-cause hospitalization and bleeding [30,73]. The higher adverse outcomes with HFpEF were also seen in other studies [10,13,22,46,74,75]. Two other studies illustrated that patients with AF on baseline EKG and HF have a 2.2-fold increased risk of cardiovascular mortality, HF hospitalization, and all-cause mortality versus the patients with a history of AF, but with sinus rhythm on baseline EKG [13,71]. One hypothesis for this effect is that since HFpEF relies heavily on LA function, the presence of LA dysfunction through AF significantly increases the mortality rates [73]. Furthermore, patients with any type of HF and actively in AF had considerably elevated rates of death, HF hospitalization, and stroke or transient ischemic attack [46].
The ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial compared the rates of stroke or systemic embolism in HFpEF (5.3 per 100 patientyears) versus HFrEF (8 per 100 patient-years) [73]. Although HFrEF had a higher rate, the study showed that patients with HFpEF possess elevated risk as well. Furthermore, a meta-analysis found that 46% of deaths in patients with AF were cardiac related, but only 5.7% were from non-hemorrhagic stroke or systemic embolism [76].
Diagnostic Work-up
A vast differential diagnosis should be considered in a patient with unexplained dyspnea, including HFpEF and AF. A depiction of how to work-up, diagnose, and treat patients with AF and HFpEF is illustrated in Figure 3. HFpEF can be diagnosed if the patient also has typical signs and symptoms, such as exercise intolerance, preserved LV ejection fraction, evidence of LV diastolic dysfunction like LA enlargement, elevated BNP, and response to therapy. This turns out to be more difficult when AF is also present, as it can also cause exercise intolerance, LA enlargement, and elevated BNP [22]. In the absence of any changes in heart rate or rhythm, symptomatic relief with diuretics is considered a strong indicator of the presence of HFpEF in AF patients; however, RCTs are needed to confirm this [22].
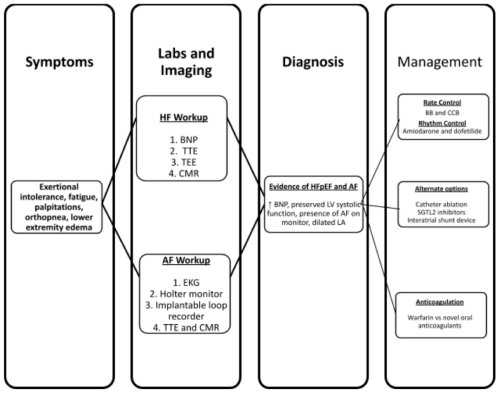
Figure 3: Pathway for Workup, Diagnosis, and Management in Patients with AF and HFpEF.
AF: Atrial Fibrillation; BB: Beta-Blockers; BNP: Pro-B-type Natriuretic Peptide; CCB: Calcium Channel Blockers; CMR: Cardiac Magnetic Resonance; EKG:
Electrocardiogram; LA: Left Atrium; LV: Left Ventricle; SGLT2: Sodium-Glucose Cotransporter-2; TEE: Transesophageal Echocardiogram; TTE: Transthoracic
Echocardiogram.
A useful marker in diagnosing acute exacerbations of HFpEF is BNP. However, cutoff values for BNP vary as the current RCTs have used different numbers, specifically greater than 600pg/mL, 900pg/ mL and 2000pg/mL [77,78]. A recent study showed that 60% of HFpEF patients without AF had a pro-BNP value of less than 400pg/ mL; whereas, only 9% of HFpEF patients with AF had a similar value. Moreover, AF patients with BNP levels greater than 400pg/mL had higher rates of HF hospitalizations, but incidentally lower mortality rates [79]. Other markers to be aware of are ST2 (suppression of tumorigenicity 2 receptor), spondin-1, platelet-derived growth factor subunit A, and insulin-like growth factor-binding protein-1. In response to volume or pressure overload, the myocardium and vascular endothelial cells secrete ST2. Spondin-1 is associated with decreased systolic function, hypertension, and a prothrombotic state. Both above cellular growth factors are non-cardiac specific, but were found to be linked with AF. Further studies are required to evaluate the true pathogenesis [78].
Additionally, a transthoracic echocardiogram can determine if a patient has HF and furthermore classify them as either HFpEF, HFmrEF, or HFrEF. However, when AF is present, the EF can be underestimated, and parameters of diastolic dysfunction are challenging to obtain and interpret. Therefore, patients with AF can be misdiagnosed as having HFrEF [80]. AF also interferes with the typical modalities of assessing diastolic dysfunction because of the changes in left atrial pressures and dimensions, changes in mitral inflow patterns, and desynchrony in atrial contraction [81]. Nonetheless, there are other echocardiographic parameters that can indicate HFpEF [22]. For example, the E/e’, a ratio of the early transmitral peak velocity to the early mitral annular velocity, was significantly associated with LV filling pressures, mortality, exercise capacity, prior ischemic stroke, quality of life. Other parameters that correlated with LV filling pressure were isovolumic relaxation time, mitral deceleration time, diastolic flow progression, and pulmonary venous flow measures [22]. Strain imaging can characterize aspects of abnormal LA mechanisms [34]. Tissue doppler imaging can show evidence of poor LA contraction through low A waves or tissue a’ velocities. [34] It can also evaluate LV systolic function and mitral annular velocity through cutoff values of s’ less than 5cm/s and e’ less than 7cm/s velocities, which have been associated with a 12-fold increase of adverse cardiac events. Moreover, even in patients with HFpEF and AF where the E/e’ ratio could not completely reflect systolic and diastolic function, the s’ and e’ velocities were still decreased [82]. Additionally, reduced LA reservoir or contractile strain from LA myopathy can be seen on speckle-tracking echocardiography [34].
An EKG should be performed on any patient with suspected AF or HFpEF. More than revealing any underlying arrhythmias, it can demonstrate evidence of LA enlargement and LV hypertrophy [34]. Furthermore, the presence of prolonged P waves on EKG typically indicates LA myopathy, but low-amplitude P waves have also been associated [34,83]. Furthermore, the interpretation of EKGs by artificial intelligence might be helpful in determining patients who are at high risk for developing AF [84]. In the event that a patient has a high pretest probability for AF, but was not found on EKG, they can undergo Holter monitor or implantable loop recorder for short-term and long-term monitoring [85].
Furthermore, there are echocardiographic measurements that can identify diastolic dysfunction in patients with AF, including peak diastolic mitral annulus velocity, E/e’ ratio in a single beat, and the time interval between the onset of early transmitral flow and the onset of early diastolic mitral annular movement [83]. In patients with AF and HFpEF, there can be evidence of RV systolic dysfunction, which is illustrated by decreased tricuspid annular plane systolic excursion [77]. LA dimension is considered to indicate the severity of diastolic dysfunction. Furthermore, the LA dimension has been shown to be an independent predictor of cardiac events, but there was no discernible difference between patients who had clinical events versus those who did not. The study proposes that LA volume could possibly be a value with a clinically significant cutoff [86]. However, the cutoff values for these parameters still need to be defined, preferably through cohorts with a large sample size of AF patients with and without HFpEF, where the latter is determined by invasive methods [76]. AF has been linked with decreased peak A wave velocity, which suggests that LA function is a more important marker of AF risk rather than LA dilatation in HFpEF [59].
Additionally, cardiac magnetic resonance imaging may reveal a LA remodeling-induced macroscopic scar [34]. LA strain was correlated to LA myopathy, symptoms, and outcomes in patients with HFpEF [47]. The TOCPAT study evaluated patients with HFpEF and demonstrated that LA function was a better marker of AF risk versus LA dilation [48]. Furthermore, patients with AF have significantly reduced global longitudinal strain, reduced RV fractional area change, and increased mitral regurgitation severity. These findings support the diagnosis of HFpEF in the above population (Table 1) [13]. Moreover, the HFpEF-Stress Trial was a prospective observational study that conducted real-time cardiac magnetic resonance exercise imaging in 75 patients with diastolic dysfunction on echocardiogram and dyspnea on exertion. The study illustrated that decompensated atrial dysfunction may act as a marker for left ventricular dysfunction and an early diagnostic sign of HFpEF [81]. Atrial dysfunction can be indicated by lower values of LA long axis strain, the distance from the mitral annulus to the most distal portion of the left atrial wall, as well as higher values of LA volume index [81].
Author
Publication Year
Trial Name
Study Design
Population
Total patients
Study arms
Conclusion
Oluleye et al. [16]
2014
None
RCT, multicenter
Older than 60 years with symptomatic HFpEF and at least one hospitalization for heart failure during the previous six months
4128
AF on baseline EKG versus history of AF but not on baseline EKG
History of AF was common and independently associated with an increased risk of fatal or nonfatal stroke. Patients with HFpEF and a history of AF should be considered at risk of stroke.
Cikes et al. [20]
2018
TOPCAT
RCT, multicenter
Older than 50 years with HFpEF and at least one sign or symptom of HF, had controlled systolic blood pressure, serum potassium level <5.0 mmol/L, and an estimated glomerular filtration rate of greater than 30 mL/min per 1.73 m2 of body surface area
1765
Received spironolactone versus placebo
In a well-defined HFpEF cohort, there is a significant association between AF confirmed by electrocardiogram at enrollment (but not a history of AF) and morbidity and mortality. There is also a markedly increased risk following the development of post-randomization AF during the course of the trial, in particular during the first 90 days after the episode of post-randomization AF. There was no effect of spironolactone on the risk of stroke in patients with any known AF at enrollment.
Shantsila et al. [115]
2020
IMPRESS-AF
RCT, single-center, abstract
Ambulatory patients
251
Received spironolactone versus placebo
Spironolactone therapy does not improve exercise capacity, cardiac function, or quality of life in patients with atrial fibrillation and preserved ejection fraction.
AF: Atrial Fibrillation; EKG: Electrocardiogram; HF: Heart Failure; HFpEF: Heart Failure with Preserved Ejection Fraction; IMPRESS-AF: Improved Exercise Tolerance in Participants with Preserved Ejection Fraction by Spironolactone on Myocardial Fibrosis in Atrial Fibrillation; RCT: Randomized Controlled Trial; TOPCAT: Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist.
Table 1: Randomized controlled trials evaluating patients with atrial fibrillation and heart failure with a preserved ejection fraction.
Rate Control versus Rhythm Control
An important decision in treating AF is determining whether to proceed with rate control, through medications to block the atrioventricular node, or rhythm control, through antiarrhythmics, cardioversion, or catheter ablation [3,87]. Rate control improves passive ventricular filling and systolic function; whereas, rhythm control reintroduces sinus rhythm and normal heart rates that benefit both passive and active ventricular filling [88]. The 2013 and 2014 guidelines recommend rhythm control for patients in AF with rapid ventricular rate and newly diagnosed HF [3,87]. This is because HF from tachycardia-mediated cardiomyopathy is reversible. Moreover, rhythm control is recommended for those who are symptomatic despite rate control [3,89].
Although there are no published RCTs evaluating what treatments are effective for AF in patients with HFpEF exclusively, the current literature does have studies that included patients with all types of HF. A prespecified subanalysis of the RCT of EASTAFNET4 compared early rhythm control versus rhythm control based on symptomatic control in patients with HF. Of the included 798 patients, 55.4% (n=442) of them had HFpEF, 26.4% (n=211) had HFmrEF, and 16.5% (n=132) had HFrEF. This subanalysis showed that early rhythm control is associated with a decreased risk of a composite of cardiovascular death, stroke, or hospitalization for worsening heart failure or acute coronary syndrome [90]. Moreover, RAFT-AF (A Randomized Ablation-based Atrial Fibrillation Rhythm Control Versus Rate Control Trial in Patients with Heart Failure and High Burden Atrial Fibrillation, NCT01420393) is an ongoing RCT evaluating whether catheter ablation with or without antiarrhythmics versus rate control with or without atrioventricular node ablation and pacemaker implantation is beneficial in patients with AF with HFpEF as well as HFrEF.
There is non-RCT that compares the two treatment strategies exclusively in patients with HFpEF. A prospective single-arm study evaluated 74 patients with HFpEF and concomitant AF who underwent catheter ablation and found there was a reasonable success of freedom from AF at 34 ± 16 months of follow-up. This study also showed that maintenance of sinus rhythm was associated with echocardiographic improvement. However, many patients required multiple procedures as well as rhythm control medications after the procedure [65]. A retrospective observational study of 283 patients compared rhythm and rate control in patients with AF and concomitant HFpEF. Of these, 107 patients were in the rhythm-control group with catheter ablation and/or antiarrhythmics; whereas, 176 were in the rate control group with beta-blockers, calcium channel blockers, or digoxin. After propensity matching, 79 patients were selectively matched in each arm. During the median follow-up period of 24 months, the maintenance of sinus rhythm was significantly associated with a lower incidence of a composite of CV death or hospitalization for HF [88]. Furthermore, no clinical difference between rate and rhythm control method was found in the retrospective AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial [89]. Three other retrospective trials revealed that patients who are in sinus rhythm are less likely to experience cardiovascular death or HFrelated hospitalization; however, decreased all-cause mortality was not found. This suggests that rhythm control may be beneficial, but does not actually provide definitive evidence [91-93]. Maintenance of SR was associated with improved parameters of E/e’ ratio, LA volume, and peak tricuspid regurgitant pressure gradient [91]. In contrast, the AF-CHF trial demonstrated that the patient’s rhythm was linked with an improved NYHA classification, but not a mortality difference [93,94]. In patients with AF and HFpEF who are 65 years or older, rhythm control was associated with a 6.7% lower one-year all-cause mortality rate, as compared to rate control [95]. Therefore, AF may indicate that the patient’s disease is more advanced, but it might not be specifically linked with worse outcomes.
It is difficult to maintain an adequate rhythm control method in elderly patients with multiple comorbidities, such as HFpEF [22]. Most antiarrhythmics are contraindicated in HF patients, except amiodarone and dofetilide, due to their safety profiles and narrow therapeutic indices [96]. A pooled study of patients in the AFFIRM and AF-CHF (Atrial Fibrillation and Congestive Heart Failure) trials determined that amiodarone had similar outcomes of maintaining sinus rhythm and decreasing the overall burden of AF between HFpEF and HFrEF patients [97]. Dofetilide, another rhythm control medication, has comparable efficacy to amiodarone. It is important to know that dofetilide has limited availability outside the United States, requires careful dose adjustment in patients with kidney disease, and has a risk of torsades de pointes. Sotalol is less beneficial, can cause torsades de pointes, and should be avoided in patients with severe LV dysfunction. Due to greater than a 20% increase in mortality over three years, digoxin should be carefully used in patients with AF and HF [98]. Contraindications to dronedarone include unstable or advanced HF, severe systolic dysfunction, permanent AF, and polypharmacy with digoxin. Dronedarone can be considered in patients with stable class I or II HF and EF greater than 35% [19]. Propafenone and flecainide are harmful in patients with HFrEF and by proxy HFpEF [95]. Moreover, catheter ablation was required in patients for longterm maintenance of sinus rhythm, and it also allowed patients to either stop taking or decrease the dosage of their antiarrhythmic medications [91]. Furthermore, cardioversion is another option for rhythm control, but prospective and RCTs investigating this method without the usage of antiarrhythmics are needed to determine its true efficacy.
By blocking the atrioventricular node, beta-blockers are commonly the first-line medications for a rate control method. Other options are non-dihydropyridine calcium channel blockers and digoxin. A post hoc analysis of the RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation II) study demonstrated that the stringency of controlling the heart rate did not have an effect on cardiovascular morbidity and mortality, symptoms, and quality of life. However, it is important to note that in the lenient rate group the mean heart rate was 85 bpm, which was significantly less than the maximum value of 110bpm [99]. Moreover, a higher mortality rate was demonstrated in the Swedish HF Registry when the rate was greater than 100bpm. Beta-blocker usage was also linked with a more favorable prognosis [100]. The hypothesis behind these studies is that by lowering the heart rate, there is enough time for the diastole to adequately fill. In advanced HFpEF, patients have restricted LV filling, decreased stroke volume during exercise, and chronotropic incompetence. Therefore, patients depend on the ability to increase their heart rate during exercise [9].
Catheter Ablation
Another treatment option for recurrent AF is catheter ablation. A prospective study evaluated the effectiveness of catheter ablation versus antiarrhythmics and/or beta-blockers in patients with AF and HFpEF. The study found that sinus rhythm was maintained in 70% of patients in the catheter ablation group (24/35). Moreover, the Kaplan-Meier curve and multivariate analysis revealed that performing pulmonary vein antrum isolation by catheter ablation was a statistically significant and the only predictive factor for HF rehospitalization [101]. Despite this, the patients who did receive catheter ablation do require rehospitalizations for further ablations and cardioversions to maintain sinus rhythm [101].
In a retrospective study, radiofrequency ablation was found to have short-term advantages with 75% having AF-free rates. However, 40% of patients experienced an atrial arrhythmia within five years [102]. A hybrid epicardial and endocardial radiofrequency ablation was seen to be retrospectively effective in patients with long-standing persistent AF. Moreover, the hybrid radiofrequency ablation was associated with significantly improved results of sinus rhythm conversion, LA remodeling reversal, and improved LV function [103]. A retrospective study showed that patients with AF and diastolic dysfunction who received catheter ablation had a higher rate of maintaining sinus rhythm without antiarrhythmics versus those with systolic dysfunction [102]. Another retrospective study reported that catheter ablation’s efficacy is similar in patients with HFpEF and HFrEF [104]. A similar trial found no significant differences in recurrence of atrial arrhythmia, all-cause hospitalizations, and mortality [105]. These findings are pertinent because many studies have theorized that with the altered pathophysiology and higher mortality rate of HFpEF versus HFrEF, there would also be a difference in efficacy after catheter ablation. However, these studies demonstrate that prognosis is favorable in both HFrEF and HFpEF.
Catheter ablation, when effective, was associated with the decreased LA diameter, size, and volume as well as reversal of LA remodeling and improved LV EF. This was especially in patients who converted to sinus rhythm, but was also seen in patients who had future recurrences of AF. The proposed etiology was that extensive catheter ablation decreased the AF burden [106-109]. As with any procedure, there are complications to catheter ablation. Some of these include the formation of LA scars (which can further decrease LA compliance and distensibility), myocardial injury (which can exaggerate LA fibrosis), decreased LA systolic function, and impaired ability of LA to transport pulmonary venous blood [32,47]. Therefore, these complications would induce and exaggerate AF as described above.
In conclusion, this method is potentially helpful for patients with comorbid AF and HFpEF. However, further studies, specifically RCTs, are required to evaluate and confirm this theory. RAFT-AF (Catheter Ablation With or Without Antiarrhythmic Drug Control of Maintaining Sinus Rhythm Versus Rate Control with Medical Therapy and/or Atrio-ventricular Junction Ablation and Pacemaker Treatment for Atrial Fibrillation, NCT01420393) is an ongoing RCT that is comparing catheter ablation versus medical rate control.
Other Medications and Procedural Options
Besides the aforementioned treatments, there are still options that can be used in patients with AF and HFpEF. As described above, the patients with AF and HF have an increased risk of stroke and systemic embolic events; however, this risk can be greatly reduced with guideline-based anticoagulation therapy [76,110]. Moreover, the AFFIRM trial showed that therapeutic anticoagulation was associated with significantly decreased long-term mortality [87]. Currently, the recommendations for which patients with AF need to be anticoagulated are based on the CHADS-VASc score, with males requiring 2 points and females requiring 3. As HF - either HFpEF or HFrEF - count as one point, all patients with AF who do not have any contraindications should be treated appropriately [9,87]. In patients with AF and HF, the novel oral anticoagulants were demonstrated to have a better efficacy and safety profile, as compared to warfarin [111]. However, further trials need to be designed specifically in HFpEF before recommendations can be designed.
Furthermore, the high rate of cardiovascular mortality in AF demonstrated that the treatment regimen to control the cardiac comorbidities needs to be altered and more well defined [76]. As in most patients with HF, the core of the treatment regimen is based on optimizing the patient’s fluid status. When the overall volume is normal, however, the LA pressure is typically elevated due to LA myopathy. Therefore, further diuresis generates symptoms, is counter-productive, and contraindicated [9]. Additionally, simvastatin, pirfenidone, and poly-unsaturated omega-3-fatty acids have been illustrated to attenuate atrial fibrosis and AF development in HF patients [34].
The sodium-glucose cotransporter 2 inhibitors (SGLT2i), namely dapagliflozin and empagliflozin, are showing promising results in patients with HF. The DECLARE-TIMI 58 (Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes) trial included a total of 17,160 patients and 1,316 of them had HFpEF [112]. In a RCT, the HFpEF had a 12% reduction in HF hospitalizations and cardiovascular deaths, as compared to 38% reduction in HFrEF [113]. The EMPEROR-Preserved (Empagliflozin in Heart Failure with a Preserved Ejection Fraction) RCT showed that empagliflozin had a 21% lower relative risk in the primary composite outcome of cardiovascular death or hospitalization for HF compared to placebo and was mainly due to a 29% lower risk of hospitalization for HF. A subgroup of patients with AF revealed that empagliflozin was associated with a 22% lower relative risk of the primary outcome compared to the placebo. These results support the fact that SGLT2i can be another therapeutic option to consider in these patients to decrease morbidity and mortality, regardless of the presence or absence of diabetes [114].
Although mineral corticoid receptor antagonists are strongly indicated for patients with HFrEF, they are not recommended for HFpEF. Spironolactone has been recommended by certain studies [93] and shown to be not useful in others (Table 1) [9,115-117]. A hypothesis is that HFpEF’s less LA fibrosis and more LA stiffness and inflammation renders spironolactone less effective [73,118]. Candesartan, an angiotensin receptor blocker, was associated with reducing the risk of new-onset AF, especially in HFrEF and somewhat in HFpEF, in the CHARM (Candesartan in Heart failure - Assessment of Reduction in Mortality and Morbidity) study [119]. In dogs, the angiotensin II inhibitor enalapril was associated with preventing the comorbid sequelae of CHF and AF [120,121]. Moreover, the PARAMOUNT (Prospective comparison of ARNI with ARB on Management Of heart failure with preserved ejection fraction) phase II trial evaluated LCZ696, the angiotensin receptor neurolysin inhibitor, which was associated with decreased LA volume in HFpEF patients [122].
Additionally, the short-term safety and patency outcome of an interatrial shunt device was evaluated in a small sample of patients with HFpEF and elevated left atrial pressure by the RCT named REDUCE LAP-HF I (A Study to Evaluate the Corvia Medical, Inc. IASD System to Reduce Elevated Left Atrial Pressure in Patients With Heart Failure). At a one-month follow-up, the IASD appeared to be safe, reduced pulmonary capillary wedge pressure during exercise, and none of the participants developed new-onset AF or atrial flutter [123]. Furthermore, a one-year follow-up of this study demonstrated 100% device patency and no major adverse cardiac, cerebrovascular, or renal events [124]. Therefore, a large-scale RCT, namely REDUCE LAP-HF II (National Clinical Trial 03088033), is currently ongoing with an estimated completion date of September 2026.
Conclusion
AF and HFpEF are strongly associated in multiple facets of their disease course, traversing all of incidence, pathophysiology, and mortality outcomes. With the development of echocardiogram and cardiac magnetic resonance imaging, a diagnosis of HFpEF can be made in patients with AF. However, further RCTs are required to confirm that rhythm control is more efficacious, specifically with catheter ablation. Anticoagulation and SGLT2i have been recommended for usage in patients with AF and HFpEF. Other methods also require confirmation of efficacy, including implantable defibrillators, interatrial shunt device, and LA shunt.
References
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114: 119-125.
- Kim MH, Johnston SS, Chu BC, et al. Estimation of Total Incremental Health Care Costs in Patients with Atrial Fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011; 4: 313-320.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014; 130: 2071-2104.
- Camm AJ, Malik M, Bigger JT Jr, et al. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996; 93: 1043-1065.
- Liao L, Allen LA, Whellan DJ. Economic burden of heart failure in the elderly. Pharmacoeconomics. 2008; 26: 447-462.
- Vermond RA, Geelhoed B, Verweij N, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from the Netherlands. J Am Coll Cardiol. 2015; 66: 1000-1007.
- https://pubmed.ncbi.nlm.nih.gov/16203909/
- Patel RB, Vaduganathan M, Shah SJ, Butler J. Atrial Fibrillation in heart failure with preserved ejection fraction: Insights into mechanisms and therapeutics. Pharmacology & Therapeutics. 2017; 176: 32-39.
- Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355: 251-259.
- Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, et al. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of Get with the Guidelines-Heart Failure. Circ Heart Fail. 2012; 5: 191-201.
- Khazanie P, Liang L, Qualls LG, et al. Outcomes of Medicare beneficiaries with heart failure and atrial fibrillation. JACC Heart Fail. 2014; 2: 41-48.
- Santhanakrishnan R, Wang N, Larson MG, et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Ejection Fraction. Circulation. 2016; 133: 484-492.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016; 133: 447-454.
- Goyal P, Almarzooq ZI, Cheung J, et al. Atrial Fibrillation and heart failure with preserved ejection fraction: Insights on a unique clinical phenotype from a nationally-representative United States cohort. Int J Cardiol Heart Vasc. 2018; 266: 112-118.
- Zafrir B, Lund LH, Laroche C, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14,964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. European Heart Journal. 2018; 39: 4277-4284.
- Oluleye OW, Rector TS, Win S, et al. History of Atrial Fibrillation as a Risk Factor With Heart Failure and Preserved Ejection Fraction. Circ Heart Fail. 2014; 7: 960-966.
- Pandey A, Kim S, Moore C, et al. Predictors and Prognostic Implications of Incident Heart Failure in Patients with Prevalent Atrial Fibrillation. JACC Heart Fail. 2017; 5: 44-52.
- Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016; 13: 131-147.
- Kotecha D, Lam CSP, Veldhuisen DJV, et al. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J Am Coll Cardiol. 2016; 68: 2217-2228.
- Cikes M, Claggett B, Shah AM, et al. Atrial Fibrillation in Heart Failure with Preserved Ejection Fraction: The TOPCAT Trial. JACC Heart Fail. 2018; 6: 689-697.
- Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2010; 12: 1076-1084.
- Silva-Cardoso J, Zharinov OJ, Ponikowski P, et al. Heart failure in patients with atrial fibrillation is associated with a high symptom and hospitalization burden: the RealiseAF survey. Clin Cardiol. 2013; 36: 766-774.
- Lip GY, Laroche C, Popescu MI, et al. Heart failure in patients with atrial fibrillation in Europe: a report from the EURObservational Research Programme Pilot survey on Atrial Fibrillation. Eur J Heart Fail. 2015; 17: 570-582.
- Chung MK, Refaat M, Shen WK, et al. Atrial Fibrillation: JACC Council Perspectives. J Am Coll Cardiol. 2020; 75: 1689-1713.
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339: 659-666.
- Vest JA, Wehrens XH, Reiken SR, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005; 111: 2025-2032.
- Ehrlich JR, Cha TJ, Zhang L, et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol. 2003; 551: 801-813.
- Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006; 47: 1196-1206.
- Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, Pathophysiology, and Clinical Outcomes. Circ Res. 2017; 120: 1501-1517.
- Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003; 91: 2D-8D.
- Patel RB, Shah SJ. Therapeutic Targeting of Left Atrial Myopathy in Atrial Fibrillation and Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2020; 5.
- Packer M. What have we learned from randomized controlled trials of catheter ablation for atrial fibrillation in patients with chronic heart failure? Circ Arrhythm Electrophysiol. 2019; 12: e007222.
- Slee A, Saad M, Saksena S. Heart failure progression and mortality in atrial fibrillation patients with preserved or reduced left ventricular ejection fraction. Journal of Interventional Cardiac Electrophysiology. 2019; 55: 325-331.
- Reddy YNV, Obokata M, Verbrugge FH, et al. Atrial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J Am Coll Cardiol. 2020; 76: 1051-1064.
- Hohendanner F, Messroghli D, Bode D, et al. Atrial remodeling in heart failure: recent developments and relevance for heart failure with preserved ejection fraction. ESC Heart Fail. 2018; 5: 211-221.
- Goette A, Kalman JM, Aguinaga L, et al. EHRA/HS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016; 18: 1455-1490.
- Brouwers FP, de Boer RA, van der Harst P, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of Prevend. Eur Heart J. 2013; 34: 1424-1431.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129: e521-e643.
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995; 92: 1954-1968.
- Lu Z, Scherlag BJ, Lin J, et al. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol. 2008; 1: 184-192.
- Naji F, Pagliaruzzi M, Penko M, Kanic V, Vokac D. Changes in left ventricular filling in patients with persistent atrial fibrillation. Int J Med Sci. 2013; 10: 1876-1879.
- Lyon A, van Mourik M, Cruts L, et al. Both beat-to-beat changes in RRinterval and left ventricular filling time determine ventricular function during atrial fibrillation. Europace. 2021; 23: i21-i28.
- Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardio. 2014; 11: 507-515.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. Eur Heart J. 2016; 37: 2129-2200.
- Sartipy U, Dahlstrom U, Fu M, Lund LH. Atrial Fibrillation in Heart Failure with Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Failure. 2017; 5: 565-574.
- Packer M, Lam CSP, Lund LH, Redfield MM. Interdependence of Atrial Fibrillation and Heart Failure with a Preserved Ejection Fraction Reflects a Common Underlying Atrial and Ventricular Myopathy. Circulation. 2020; 141: 4-6.
- Mesquita TRR, Zhang R, de Couto G, et al. Mechanisms of atrial fibrillation in aged rats with heart failure with preserved ejection fraction. Heart Rhythm. 2020; 17: 1025-1033.
- Packer M. Risks of Intensive Treatment of Long-Standing Atrial Fibrillation in Patients with Chronic Heart Failure with a Reduced or Preserved Ejection Fraction. Circ Cardiovasc Qual Outcomes. 2019; 12: e005747.
- Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014; 7: 31-38.
- Hiram R, Naud P, Xiong F, et al. Right Atrial Mechanisms of Atrial Fibrillation in a Rat Model of Right Heart Disease. J Am Coll Cardiol. 2019; 74: 1332- 1347.
- Sanfilippo AJ, Abascal VM, Sheehan M, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. 1990; 82: 792-797.
- Tsai CF, Huang PS, Chen JJ, et al. Correlation Between CHA2DS2-VASc Score and Left Atrial Size in Patients With Atrial Fibrillation: A More Than 15- Year Prospective Follow-Up Study. Front Cardiovasc Med. 2021; 8: 653405.
- Boccanelli A. Beyond CHA2DS2-VASc in atrial fibrillation: the atrium and the risk of stroke. Eur Heart J Suppl. 2020; 22: E30-E33.
- Yeh YH, Wakili R, Qi XY, et al. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. 2008; 1: 93-102.
- Venteclef N, Guglielmi V, Balse E, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipofibrokines. Eur Heart J. 2015; 36: 795-805.
- Nagashima K, Okumura Y, Watanabe I, et al. Association between epicardial adipose tissue volumes on 3-dimensional reconstructed CT images and recurrence of atrial fibrillation after catheter ablation. Circ J. 2011; 75: 2559- 2565.
- Tsao HM, Hu WC, Wu MH, et al. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am J Cardiol. 2011; 107: 1498-1503.
- Polyakova V, Loeffler I, Hein S, et al. Fibrosis in end stage human heart failure: severe changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol. 2011; 151: 18-33.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5837728/
- Heijman J, Muna AP, Veleva T, et al. Atrial Myocyte NLRP3/CaMKII Nexus Forms a Substrate for Postoperative Atrial Fibrillation. Circulation Research. 2020; 127: 1036-1055.
- Ding WY, Gupta D, Wong CF, Lip GYH. Pathophysiology of atrial fibrillation and chronic kidney disease. Cardiovasc Res. 2021; 117: 1046-1059.
- Everett TH 4th, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007; 4: S24-S27.
- Rosenkranz S, Kramer T, Gerhardt F, Opitz C, Olsson KM, Hoeper MM. Pulmonary hypertension in HFpEF and HFrEF: Pathophysiology, diagnosis, treatment approaches. Pulmonale Hypertonie bei HFpEF und HFrEF: Pathophysiologie, Diagnose, Behandlungsansätze. Herz. 2019; 44: 483- 490.
- Lenski M, Schleider G, Kohlhaas M, et al. Arrhythmia causes lipid accumulation and reduced glucose uptake. Basic Res Cardiol. 2015; 110: 40.
- Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013; 62: 1857-1865.
- Elagizi A, Kachur S, Lavie CJ, et al. An Overview and Update on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog Cardiovasc Dis. 2018; 61: 142-150.
- Pandey A, Gersh BJ, McGuire DK, et al. Association of Body Mass Index with Care and Outcomes in Patients with Atrial Fibrillation: Results from the ORBIT-AF Registry. JACC Clin Electrophysiol. 2016; 2: 355-363.
- Scott L Jr., Fender AC, Saljic A, et al. NLRP3 inflammasome is a key driver of obesity-induced atrial arrhythmias. Cardiovasc Res. 2021; 117: 1746-1759.
- Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. New Eng J Med. 2008; 358: 2667-2677.
- Elshazly MB, Senn T, Wu Y, et al. Impact of Atrial Fibrillation on Exercise Capacity and Mortality in Heart Failure With Preserved Ejection Fraction: Insights From Cardiopulmonary Stress Testing. J Am Heart Assoc. 2017; 6: e006662.
- Kristensen SL, Mogensen UM, Jhund PS, et al. N-Terminal Pro-B-Type Natriuretic Peptide Levels for Risk Prediction in Patients with Heart Failure and Preserved Ejection Fraction According to Atrial Fibrillation Status. Circ Heart Fail. 2019; 12: e005766.
- van Diepen S, Helkamp AS, Patel MR, et al. Efficacy and Safety of Rivaroxaban in Patients With Heart Failure and Nonvalvular Atrial Fibrillation: Insights from ROCKET AF. Circ Heart Fail. 2013; 6: 740-747.
- Lam CSP, Rienstra M, Tay WT, et al. Atrial Fibrillation in Heart Failure with Preserved Ejection Fraction: Association with Exercise Capacity, Left Ventricular Filling Pressures, Natriuretic Peptides, and Left Atrial Volume. JACC Heart Fail. 2017; 5: 92-98.
- Jobs A, Schwind J, Katalinic A, et al. Prognostic significance of atrial fibrillation in acute decompensated heart failure with reduced versus preserved ejection fraction. Clinical Research in Cardiology. 2019; 108: 74- 82.
- Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015; 8: 295-303.
- Xiong Q, Lau YC, Senoo K, Lane DA, Hong K, Lip GYH. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: a systematic review and meta-analysis of randomized trials. Eur J Heart Fail. 2015; 17: 1192-200.
- Shin HW, Kim H, Son J, et al. Tissue Doppler Imaging as a Prognostic Marker for Cardiovascular Events in Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J Am Soc Echocardiogr. 2010; 23: 755-761.
- Wyse DG, Waldo AL, DiMarco JP, et al. Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators: A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002; 347: 1825.
- Linssen GC, Rienstra M, Jaarsma T, et al. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2011; 13: 1111-1120.
- Gorter TM, Melle JPV, Rienstra M, et al. Right Heart Dysfunction in Heart Failure with Preserved Ejection Fraction: The Impact of Atrial Fibrillation. J Cardiac Fail. 2018; 24: 177-185.
- Aldaas OM, Malladi CL, Hsu JC. Atrial fibrillation in patients with heart failure with preserved ejection fraction. Curr Opin Cardiol. 2020; 35: 260-270.
- Eapen ZJ, Greiner MA, Fonarow GC, et al. Associations between atrial fibrillation and early outcomes of patients with heart failure and reduced or preserved ejection fraction. Am Heart J. 2014; 167: 369-375.e2.
- Jadidi A, Muller-Edenborn B, Chen J, Keyl C, Weber R, Allgeier J, et al. The duration of the amplified sinus-P-wave identifies presence of left atrial low voltage substrate and predicts outcome after pulmonary vein isolation in patients with persistent atrial fibrillation. JACC Clin Electrophysiol. 2018; 4: 531-543.
- Attia ZI, Noseworthy PA, Lopez-Jimenez F, et al. An artificial intelligenceenabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019; 394: 861-867.
- Jamal S, Prasad RM, Vipparla NS, Srivastava S, Baloch ZQ, Banga S, et al. Implantable cardiac monitors: State of the art. Interv Cardiol. 2021; 13: 127-133.
- Santema BT, Kloosterman M, Gelder ICV, et al. Comparing biomarker profiles of patients with heart failure: atrial fibrillation vs. sinus rhythm and reduced vs. preserved ejection fraction. Eur Heart J. 2018; 39: 3867-3875.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128: e240-e327.
- Eapen ZJ, Greiner MA, Fonarow GC, et al. Associations between atrial fibrillation and early outcomes of patients with heart failure and reduced or preserved ejection fraction. Am Heart J. 2014; 167: 369-375.e2.
- The AFFIRM investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. New Eng J Med. 2002; 347: 1825-1833.
- Rillig A, Magnussen C, Ozga AK, et al. Early Rhythm Control Therapy in Patients With Atrial Fibrillation and Heart Failure. Circulation. 2021; 144: 845-858.
- The AFFIRM Investigators. Relationships between sinus rhythm, treatment, and survival in the atrial fibrillation follow-up investigation of rhythm management (AFFIRM) study. Circulation. 2004; 109: 1509-1513.
- Saksena S, Slee A, Waldo AL, et al. Cardiovascular outcomes in the AFFIRM trial (atrial fibrillation follow-up investigation of rhythm management): an assessment of individual antiarrhythmic drug therapies com- pared with rate control with propensity score-matched analyses. J Am Coll Cardiol. 2011; 58: 1975-1985.
- Suman-Horduna I, Roy D, Frasure-Smith N, et al. Quality of life and functional capacity in patients with atrial fibrillation and congestive heart failure. J Am Coll Cardiol. 2013; 61: 455-460.
- Talajic M, Khairy P, Levesque S, et al. Maintenance of sinus rhythm and survival in patients with heart failure and atrial fibrillation. J Am Coll Cardiol. 2010; 55: 1796-1802.
- Cadrin-Tourigny J, Wyse DG, Roy D, et al. Efficacy of amiodarone in patients with atrial fibrillation with and without left ventricular dysfunction: a pooled analysis of AFFIRM and AF-CHF trials. J Cardiovasc Electrophysiol. 2014; 25: 1306-1313.
- Kelly JP, DeVore AD, Wu J, et al. Rhythm Control Versus Rate Control in Patients with Atrial Fibrillation and Heart Failure With Preserved Ejection Fraction: Insights From Get with The Guidelines-Heart Failure. J Am Heart Assoc. 2019; 8: e011560.
- Turakhia MP, Santangeli P, Winkelmayer WC, et al. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol. 2014; 64: 660-668.
- Mulder BA, Veldhuisen DJV, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation and heart failure: a post-hoc analysis of the RACE II study. Eur J Heart Fail. 2013; 15: 1311-1318.
- Li SJ, Sartipy U, Lund LH, et al. Prognostic significance of resting heart rate and use of β-blockers in atrial fibrillation and sinus rhythm in patients with heart failure and reduced ejection fraction: findings from the Swedish Heart Failure Registry. Circ Heart Fail. 2015; 8: 871-879.
- Cha YM, Wokhlu A, Asirvatham SJ, et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol. 2011; 4: 4724-4732.
- Toplisek J, Pernat A, Ruzic N, et al. Improvement of Atrial and Ventricular Remodeling with Low Atrial Fibrillation Burden after Hybrid Ablation of Persistent Atrial Fibrillation. PACE. 2016; 39: 216-224.
- Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2019; 31: 682-688.
- Black-Maier E, Ren X, Steinberg BA, et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. 2018; 15: 651-657.
- Aldaas OM, Malladi CL, Mylavarapu PS, et al. Comparison of Outcomes After Ablation of Atrial Fibrillation in Patients With Heart Failure With Preserved Versus Reduced Ejection Fraction. Am J Cardiol. 2020; 136: 62-70.
- Pump A, Di Biase L, Price J, et al. Efficacy of catheter ablation in nonparoxysmal atrial fibrillation patients with severe enlarged left atrium and its impact on left atrial structural remodeling. J Cardiovasc Electrophysiol. 2013; 24: 1224-1231.
- Beukema WP, Elvan A, Sie HT, Misier AR, Wellens HJ. Successful radiofrequency ablation in patients with previous atrial fibrillation results in a significant decrease in left atrial size. Circulation. 2005; 112: 2089-2095.
- Tops LF, Bax JJ, Zeppenfeld K, Jongbloed MR, van der Wall EE, Schalij MJ. Effect of radiofrequency catheter ablation for atrial fibrillation on left atrial cavity size. Am J Cardiol. 2006; 97: 1220-1222.
- Reant P, Lafitte S, Ja¨ıs P, et al. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation. 2005; 112: 2896-2903.
- McMurray JJ, Ezekowitz JA, Lewis BS, et al. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail. 2013; 6: 451-460.
- Gomez-Outes A, Lagunar-Ruız J, Terleira-Fernandez AI, Calvo-Rojas G, Suarez-Gea ML, Vargas-Castrillon E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016; 68: 2508-2521.
- Yang SS, Han W, Zhou HY, et al. Effects of spironolactone on electrical and structural remodeling of atrium in congestive heart failure dogs. Chin Med J (Engl). 2008; 121: 38-42.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019; 380: 347-357.
- Kato ET, Silverman MG, Mosenzon O, et al. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation. 2019; 139: 2528-2536.
- Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021; 385: 1451-1461.
- Shantsila E, Shahid F, Sun Y, et al. Spironolactone in atrial fibrillation with preserved cardiac fraction: the IMPRESS-AF trial. Journal of the American Heart Association. 2020; 9: e016239.
- Neefs J, van den Berg NWE, Krul SPJ, Boekholdt SM, de Groot JR. Effect of Spironolactone on Atrial Fibrillation in Patients with Heart Failure with Preserved Ejection Fraction: Post-Hoc Analysis of the Randomized, Placebo-Controlled TOPCAT Trial. American Journal of Cardiovascular Drugs. 2020; 20: 73-80.
- Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dys-function and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013; 62: 263-271.
- Ducharme A, Swedberg K, Pfeffer MA, et al. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J. 2006; 152: 86-92.
- Li D, Shinagawa K, Pang L, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001; 104: 2608-2614.
- Shi Y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002; 54: 456-461.
- Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomized controlled trial. Lancet. 2012; 380: 1387-1395.
- Jhund PS, Claggett B, Packer M, et al. Independence of the blood pressure lowering effect and efficacy of the angiotensin receptor neprilysin inhibitor, LCZ696, in patients with heart failure with preserved ejection fraction: an analysis of the PARAMOUNT trial. Eur J Heart Fail. 2014; 16: 671-677.
- Feldman T, Mauri L, Kahwash R, et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized, Sham-Controlled Trial. Circulation. 2018; 137: 364-375.
- Shah SJ, Feldman T, Ricciardi MJ, et al. One-Year Safety and Clinical Outcomes of a Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction in the Reduce Elevated Left Atrial Pressure in Patients With Heart Failure (REDUCE LAP-HF I) Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018; 3: 968-977.