
Research Article
Austin J Cerebrovasc Dis & Stroke. 2022; 9(1): 1089.
Determinants of Exclusion from Treatment with Alteplase in Acute Ischemic Stroke at the Largest Tertiary Center in Lebanon
Ibrikji S, Ismail H and El Ayoubi N*
Department of Neurology, American University of Beirut Medical Center, Beirut, Lebanon
*Corresponding author: El Ayoubi N, Department of Neurology, American University of Beirut Medical Center Cairo Street, Riad El Solh 1107 2020, Beirut, Lebanon
Received: September 01, 2022; Accepted: October 06, 2022; Published: October 13, 2022
Abstract
Objectives: Thrombolysis remains underutilized for Acute Ischemic Stroke (AIS) in healthcare systems in low- and middle-income countries. We aimed to investigate the factors associated with utilization of intravenous thrombolysis in an academic medical center in Lebanon.
Materials and Methods: This is a retrospective, cross-sectional, singlecentered, chart review-based study of AIS patients presenting to the American University of Beirut Medical Center (AUBMC) between January 2015 and October 2019. Included patients were older than 18 years and presented to the Emergency Department (ED) within 48 hours from symptom onset. Patient, disease, and health system response characteristics were collected and those eligible for and those who received thrombolysis within 4.5 hours onset by guidelines criteria were identified by chart review. Descriptive statistics, bivariate and multivariate analyses were performed for association with thrombolysis administration.
Results: Out of the 373 AIS patients, 17.4% were candidate for rTPA and of those, 38.5% did not receive treatment. Patients who were candidate for IV alteplase had a higher NIHSS (OR 1.22 [1.14-1.31], p<0.0001), total or partial anterior circulation stroke (OR 3.58 [1.49-8.6, p=0.004) as compared to posterior or lacunar strokes and a shorter duration since symptom onset (OR 0.14 [0.07- 0.27, p<0.0001). The multivariate analysis showed that thrombolysis among eligible patients associated with younger age (OR 1.05 [1.01-1.10], p=0.029), a higher NIHSS (OR 1.12 [1.01-1.25], p=0.041), and stroke code activation (OR 2.81 [1.16-6.81], P=0.022).
Conclusion: A good proportion of eligible AIS patients did not receive thrombolysis. To increase thrombolysis use in low- and middle-income countries, more consistent stroke code activation and education on age and stroke severity in eligibility are needed.
Keywords: Acute ischemic stroke; Intravenous alteplase; Third-world country; tPA
Abbreviations
Afib: Atrial Fibrillation; AIS: Acute Ischemic Stroke; AUBMC: American University of Beirut Medical Center; CAD: Coronary Artery Disease; CI: Contra-indications; CT: Computed Tomography; DWI: Diffusion-weighted Imaging; ED: Emergency Department; FDA: Federal Drug Administration; FLAIR: Fluid-attenuated Inversion Recovery; IV: Intravenous; IST: International Stroke Trial; LACI: Lacunar Cerebral Infarct; rTPA: recombinant Tissue Plasminogen Activator; MRI: Magnetic Resonance Imaging; NIHSS: National Institute of Health Stroke Scale; NTP: Non-target Patients; OR: Odds Ratio; PACI: Partial Anterior Circulation Infarct; PC: Posterior Circulation; POCI: Posterior Circulation Infarct; sICH: symptomatic Intracerebral Hemorrhage; SP: Stroke Patients; TIA: Transient Ischemic Attack; TP: Target Patients;; TPN: Target Population No; TPY: Target Population Yes.
Introduction
Intravenous (IV) recombinant Tissue Plasminogen Activator (rTPA) has been proven to be effective in reducing post-stroke morbidity, with a 16% absolute increase in survival over placebo [1]. Despite its approval by the Food and Drug Administration (FDA), the rate of IV rTPA administration remains infrequent, at around 2-10% of all patients with Acute Ischemic Stroke (AIS) [2]. Time to hospital presentation is the main obstacle; where only a quarter of patients arrive within the allowed timeframe for rTPA administration [3]. The second most common associated factor is a low National Institute of Health Stroke Scale (NIHSS) score, reflecting a minor stroke [4]. Besides those ineligibility criteria, around 30% of patients with AIS remain entitled to receive rTPA, but do not [3]. Factors associated with exclusion from rTPA administration include brain Computed Tomography (CT) findings of early ischemic changes, white matter disease, old stroke, and suspected tumor, seizures with confirmed stroke, hypertension (despite it being less than 185/100 mmHg), and aspirin prophylaxis. This might reflect the clinicians’ conservative approach towards rTPA, possibly secondary to the fear of inducing severe hemorrhagic complications and more disability to the patients.
No studies have been conducted on the risk factors associated with ineligibility to rTPA administration, as well as those associated with reluctance to give rTPA in eligible patients, in Lebanon and the region. Hence, this study aims to look into factors associated with the underutilization of rTPA. Shedding light on the reasons for exclusion from giving rTPA can help tacklethem to ultimately increase the proportion of patients treated with thrombolysis. This would eventually enhance patients’quality of life, presuming that the treatment would be administered safely and effectively [3].
Methods
Design, Inclusion Criteria and Categorization
This is a retrospective, cross-sectional, single-center study, approved by the institutional review board at the American university of Beirut (BIO-2019-0302). We reviewed the charts of patients who presented to the Emergency Department (ED) of AUBMC between January 2015 and October 2019. We included patients older than 18 years who presented to the ED within 48 hours of symptom onset and were diagnosed with AIS. No informed consent was required.
All included patients were referred to as Stroke Patients (SP) and were divided into candidates for rTPA administration (labelled as Target Patients, TP) and non-candidates (labelled as Non-Target Patients, NTP). Based on our chart review and the guidelines for rTPA administration, the eligibility criteria for receiving rTPA (i.e. being a target or a non-target patient) were an NIHSS ≥ 6 and no absolute or relative Contraindications (CIs), including arrival to the ED beyond 4.5 hours since symptom onset. TP were further categorized into those who actually received rTPA (labelled as Target Patients Yes, TPY) and those who did not (labelled as Target Patients No, TPN). The disposition of studied patients is shown in (Figure 1).
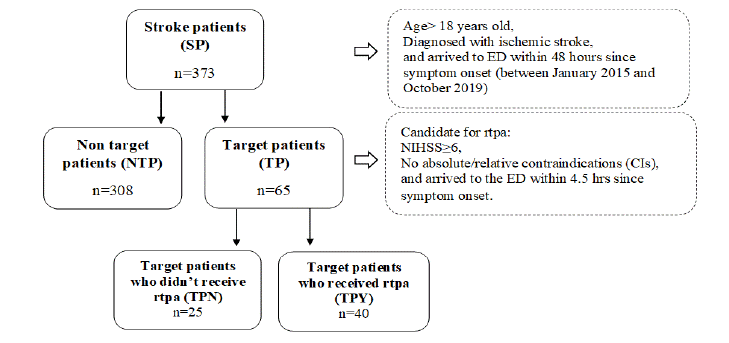
Figure 1: Patient’s disposition.
Statistics
We performed descriptive statistics on the SP and bivariate and multivariate analyses on the TP/NTP and TPY/TPN groups.
The categories explored in this study were patient-related factors (age, gender, NIHSS, time since symptom onset, presenting symptoms, type of stroke, and use of anti-platelets); logistic factors (method of transportation); and ED-related factors (code activation, and time till CT acquisition). Variables were explored for normality using the Shapiro-Wilk test. Categorical variables were summarized using frequency distributions, and their differences were examined using the chi-squared test or Fisher’s exact test, as appropriate. Continuous variables were compared using the Mann-Whitney test.
Multivariate analyses were performed to determine the relevant factors associated with being in the target patient group, as well as in the target patient group who received rTPA. Logistic regression models were built with “TP” as the outcome variable, and different clinical, demographic, and logistic variables as covariates. Another logistic regression model was built with “TPY” as the outcome. The final models included only variables that showed statistical significance or any potential confounding effect. Adjusted Odds Ratios (OR), their corresponding 95% Confidence Intervals (CI) and two-sided P-values were reported. Log likelihood ratio was used to assess the goodness of fit of the model. Significance was set at the 5% level and all statistical analyses were performed using the Statistical Package for Social Sciences (IBM Corp. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
Results
Stroke Patients
Clinical and demographic characteristics: Out of the 373 patients included, 59% of the patients were males and the average age was 76 years. The most common comorbidity was hypertension (69%), followed by dyslipidemia (37%), diabetes mellitus (36%), Coronary Artery Disease (CAD) (24%), and a trial fibrillation (Afib) (21%). A prior history of ischemic stroke or Transient Ischemic Attack (TIA) was seen in 19.6% of the patients. 47% of the patients were current or ex-smokers. 37% were on anti-platelets, out of which 17% were on dual anti-platelets. However, anti-platelets were not given in 71 of 169 patients with indications like CAD, carotid stenosis or a history of stroke and they were given in 38 of 204 patients with no indications. 12% of the patients were on anti-coagulants; however, the latter were not given to 64 of 107 patients with indications like Afib and were given to 6 of 266 patients with no indications.
The majority of patients (37%) presented with a Partial Anterior Circulation Infarct (PACI), followed by Lacunar Cerebral Infarct (LACI, 23%), Posterior Circulation Infarct (POCI, 17%), Total Anterior Circulation Infarct (TACI, 16%), and bilateral numerous infarcts (7%). Typical AIS symptoms including aphasia, unilateral limb weakness and numbness (upper or lower extremity), facial weakness and numbness, and loss of visual field were seen in 73% of patients; with the most common being unilateral upper extremity weakness. The average NIHSS of total patients was 6.48.
The majority of the SP were not candidates for rTPA administration, with 73% presenting with absolute CIs (with or without relative CIs) (Figure 2) and 27% presenting with relative CIs only (Figure 3). The most common absolute CI was arriving to the ED beyond 4.5 hours (81%) followed by bleeding diathesis (43%). The most common relative CI was having a minor or a rapidly resolving stroke (84%).
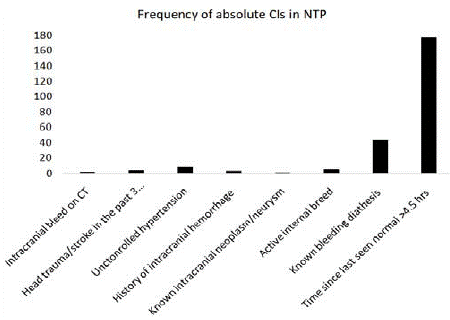
Figure 2: Absolute contra-indications in the non-target population.
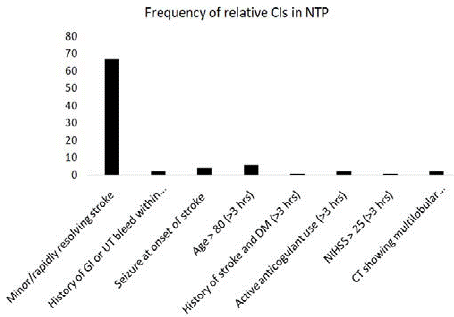
Figure 3: Relative contra-indications in the non-target population.
Admission conditions: Most patients (36.1%) presented to the ED between 12pm and 7pm and only 27% were transported by ambulance. However, patients seemed to be more frequently transported to ED by ambulance between 12am and 7am and on Fridays and Saturdays. In addition, the mean NIHSS was higher (10.3) in patients transported by an ambulance compared to those transported by other means (5.09). The frequency of the yearly stroke code activation in the TP increased from 39% to 78% while the frequency of rTPA administration fluctuated between 55% and 66% between 2015 and 2019. To note, the stroke code at AUBMC is activated whenever the ED physician recognizes stroke symptoms, such as aphasia, limb weakness or sensory deficit, visual disturbances, or other neurologic symptoms, regardless of time from symptom onset.
Target and Non-Target Patients (TP vs NTP)
Patient-related factors: Compared with the NTP, the TP had a similar age and gender distribution, a shorter median time since symptom onset, and a higher median NIHSS score. Common ischemic stroke symptoms, including aphasia, unilateral limb weakness or numbness, facial asymmetry or numbness, and visual field loss, were more common in TP. The TP were also more likely to have total or partial anterior circulation ischemia compared with the NTP. There was no difference in the proportion of patients on antiplatelets between both groups (Table 1).
Patient-related factors
NTP (308)
TP (65)
p-value
Male gender
183 (59.4%)
36 (55.4%)
0.549
Median age (range)
79 (19-100)
79 (36-100)
0.967
Median time since symptom onset in hours (range)
6 (0.1-48)
1.8 (0.5-4)
<0.0001
Median NIHSS (range)
4 (0-31)
10 (6-31-)
<0.0001
Presenting symptoms
Common symptoms
216 (70.1%)
56 (86.2%)
0.008
Uncommon symptoms
92 (29.9%)
9 (13.8%)
Anti-platelet
90 (29.2%)
23 (35.3%)
0.434
Type of stroke
TACI
37 (12%)
22 (33.8%)
<0.0001
PACI
104 (33.8%)
31 (47.7%)
POCI
61 (19.8%)
3 (4.6%)
LACI
81 (26.3%)
6 (9.2%)
Multiple territories
25 (8.1%)
3 (4.6%)
Logistic factors
NTP (308)
TP (65)
Method of transportation to ED
Ambulance
60 (23.2%)
26 (44.8%)
0.001
Other means
199 (76.8%)
32 (55.2%)
ED factors
NTP (n=279)
TP (n=56)
Code activation
66 (23.7%)
36 (64.3%)
<0.0001
Code activation
Common symptoms
51 (77.3%)
34 (94.4%)
0.026
Uncommon symptoms
15 (22.7%)
2 (5.6%)
Median time from arrival to CT (range)
243 (1-330)
23 (1-122)
<0.0001
Table 1: Bivariate analysis of patient-related and logistic variables.
Logistic factors: TP were more likely to be brought to the ED by ambulance, as compared with the NTP. There was no difference in the month or day of presentation, nor the time at presentation to ED between the TP and NTP (Table 1).
ED factors: The stroke code was activated more frequently in TP than NTP. Moreover, among patients presenting with common symptoms, the stroke code activation was more common among the TP. Mean time from ED arrival to brain CT scan initiation was longer in non-eligible than eligible patients (Table 1).
Patient-related factors
TPY (n=40)
TPN (n=25)
p-value
Male gender
24 (60%)
12 (48%)
0.344
Median age (range)
72 (36-94)
86 (36-100)
0.018
Median time since symptom onset in hours (range)
1.5 (0.4-4)
2 (0.5-4)
0.218
Median NIHSS (range)
11.5 (6-31)
7 (6-22)
0.032
Presenting symptoms
Common symptoms
5 (12.5%)
4 (16%)
0.691
Uncommon symptoms
35 (87.5%)
21 (84%)
Anti-platelet
9 (22.5%)
14 (56%)
0.021
Type of stroke
TACI
15 (37.5%)
7 (28%)
0.774
PACI
19 (47.5%)
12 (48%)
POCI
2 (5%)
1 (4%)
LACI
3 (7.5%)
3 (12%)
Multiple territories
1 (2.5%)
2 (8%)
Logistic factors
Method of transportation to ED
Ambulance
18 (51.4%)
8 (34.8%)
0.212
Other means
17 (48.6%)
15 (65.2%)
ED factors
Code activation
27 (79.4%)
9 (40.9%)
0.011
Median time from arrival to CT (range)
21 (1-45)
24 (1-122)
0.2
Table 2: Bivariate analyses of patient-related and logistic variables.
Multivariate analysis: After performing multivariate logistic regression, three variables were statistically associated with corresponding to the TP. For every point increase in the NIHSS, there was 22% higher odds of being in the TP (OR 1.22 (1.14-1.31), P<0.0001). TACI and PACI stroke subtypes had higher odds of corresponding to the TP than the NTP (OR 3.58 (1.49-8.6), p=0.004), and a longer duration since symptom onset (in hours) was associated with corresponding to the the NTP (OR 0.14 (0.07-0.27, p<0.0001).
Target Patients Having Taken or Not rTPA (TPY vs TPN)
Patient-related factors: In patients who were candidate for IV rTPA administration with no absolute or relative contraindications, we looked at possible risk factors that could be associated with having received or not rTPA. Gender distribution was similar between TPY and TPN groups, but patients who received rTPA were younger. The mean time since symptom onset did not differ between both groups, but mean NIHSS was higher in TPY. Both groups had similar frequencies of common and uncommon symptoms, and the type of stroke did not differ between them. More patients who did not receive rTPA were on single or dual anti-platelets. New hypodensities on brain Computed Tomography (CT) were more commonly seen in patients who were not treated with rTPA, however, having a hyperdense Middle Cerebral Artery (MCA) sign was not associated with having received rTPA.
Logistic factors: No difference was seen in the method of transportation (ambulance or other means), the month, day of presentation and time of arrival to ED between TPY and TPN groups.
ED factors: The neurology resident’s post-graduate level and gender did not differ between having received rTPA or not, as well as the mean years since graduation of the attending neurologist overseeing the case. Nevertheless, the stroke code was more commonly activated in the TPY group.
Multivariate analysis: Three variables showed statistically significant associations with having received rTPA or having been excluded from treatment in the multivariate analysis. The older the patient’s age, the lower his chances of receiving rTPA (OR 0.95 (0.91- 0.99), p=0.029). Stroke code activation was strongly associated with TPY (OR 2.81 (1.16-6.81), p=0.022) and the higher the patients’ NIHSS at presentation, the higher their odds of being treated with rTPA (OR 1.12 (1.01-1.25), p=0.041).
Discussion
Despite the progressively-increasing rate of rTPA administration reported by prior studies, a large number of patients with acute ischemic stroke does not fit the criteria to receive it, and up to 20% of potentially eligible patients do not get treated [5]. This retrospective analysis of stroke patients presenting to the biggest tertiary center in Lebanon sheds light on some patient-related and logistic factors thatare associated with not being eligible for rTPA administration and not receiving it when eligible.
The main absolute contraindication excluding patients from rTPA treatment was arriving to the ED after more than 4.5 hours since last seen normal. In fact, the longer the duration was since symptom onset, the lower the chances were of being eligible to treatment. Intravenous thrombolysis was initially proven to be successful in the first 3 hours since symptom onset in the NINDS trial in 1995 [1], and the window was then extended up to 4.5 hours based on the ECASS III trial published in 2008 [6]. Recently, rTPA was proven to improve the functional outcome at 90 days in patients with AIS presenting beyond 4.5 hours from symptom onset or with an unknown last-seen well or wake-up stroke, given they had Diffusion-Weighted Image (DWI)/ Fluid-Attenuated Inversion Recovery (FLAIR) mismatch on brain Magnetic Resonance Imaging (MRI) [7]. However, this new concept was not applied in our study since data collection stopped by the time the trial results were published.
Another factor associated with not being candidate for rTPA is the type of stroke. Having Posterior Circulation (PC) ischemia or a lacunar stroke were associated with a higher risk of not being eligible for rTPA. PC ischemiadoes not present with classical stroke symptoms, and the relatively rich collateral system render the clinical symptoms highly variable [8]. Nonspecific manifestations such as dizziness, imbalance, slurred speech, headache, nausea, and vomiting, could constitute the initial presentation of PC strokes, making their recognition by ED physicians and even neurologists hard [9]. Furthermore, decision-making heavily relies on the NIHSS, which reflects stroke severity. However, this scale has limitations in posterior circulation ischemia, where fewer points are attributed to cranial nerve deficits and ataxia, while higher points are assigned to anterior circulation stroke [8]. This could possibly explain why patients with PC stroke are less eligible to receive rTPA and end up with less favorable functional outcomes at 3 months. On the other hand, lacunar strokes are small sub cortical infarcts located in the deep structures such as the brainstem, thalamus, basal ganglia, internal capsule and corona radiate [10]. Their manifestations consist of typical lacunar syndromes such as pure motor or sensory deficits, sensorimotor stroke, dysarthria-clumsy-hand syndrome or ataxic hemiparesis. Those symptoms frequently do not cross the NIHSS threshold for giving rTPA and this category of strokes might be considered as the most benign subtype, which could explain why these patients are usually less eligible for treatment [10].
A lower NIHSS not only renders acute ischemic stroke patients less eligible for rTPA but is also associated with less frequent treatment in those who are candidate. The most recent guidelines from the American Heart Association/American Stroke Association for early management of patients with acute ischemic stroke state that an eligible patient should have an NIHSS between 6 and 25, except those with minor (NIHSS<6) but functionally-impairing symptoms [11]. Thus, this study contributes to the reporting of prespecified contraindications excluding patients from treatment. Unfortunately, we did not categorize minor symptoms into functionally and non-functionally-impairing deficits to analyze the rate of rTPA administration in these sub-categories. Furthermore, in patients who are eligible and have no contraindications, lower NIHSS was strongly associated with a lower likelihood of receiving rTPA. This was found to be the most common cause for not treating otherwise candidate patients, though numerous studies reported poor long-term prognosis even with mild untreated stroke symptoms [12]. Despite excluding those patients from older rTPA trials, a recent post hoc analysis of mild acute ischemic stroke patients from the International Stroke Trial 3 (IST3) randomized controlled trial and multiple metaanalyses proved the efficacy of rTPA in this sub-population, especially if treated within 3 hours of onset [13,14].
One of the relative contraindications in patients presenting within 3 and 4.5 hours from symptom-onset, is age above 80 years. Our study showed that even in eligible patients presenting with neither absolute nor relative contraindications, the older they are, the less likely they are to receive rTPA treatment. Multiple meta-analyses and the IST3 have recently demonstrated sustained improved outcomes after thrombolysis in the elderly and even in the very elderly [15,16]. However, a recent survey of neurologists showed that 4 out of 5 were less encouraged to give rTPA in elderly patients, especially those coming from nursing homes or those more than 80 years of age [17]. This could be explained by the fear of inducing intracerebral or systemic bleeding in this fragile population [18].
Among eligible patients, stroke code activation was more common is those who received treatment with rTPA compared to those who did not. In the emergency department, stroke code activation heavily relies on the fast recognition of stroke symptoms by ED nurses, medical students, residents and fellows. It entails a rapid arrival of the neurology team with the goal of recanalization and reperfusion, as well as a fast sequence of imaging and laboratory testing. Hence, stroke code activation results in reduction of in-hospital delays [19], which is why it is crucial to train the ED staff for an accurate and safe identification of stroke manifestations.
Multiple independent factors were also associated with not being a target patient; one of which was having uncommon stroke symptoms. Manifestations such as headache, dysarthria, dizziness, gait instability, and altered behavior are non-specific and can occur in a variety of neurological illnesses, rendering them less recognizable as stroke symptoms [20]. In fact, stroke code activation with common symptoms was independently more associated with being a target patient in our study. In addition, arriving to the hospital via ambulance and a shorter time from ED arrival to brain CT acquisition, were associated with being a candidate for rTPA. However, that could partially be mediated by more recognizable stroke symptoms and stroke code activation [21,22].
Being on anti-platelet treatment (single or dual) was independently associated with not receiving rTPA in otherwise eligible patients. Although blood thinners may be thought to increase the risk of symptomatic intracerebral hemorrhage (sICH) after rTPA in patients with AIS, a study on 11,865 patients showed that the absolute excess of sICH in patients on anti-platelets is very small compared with the advantages of thrombolysis [23]. Caution is needed in patients taking dual anti-platelets, especially if they are older and have severe white matter disease, but treatment with anti-platelet should not be considered a contra-indication to rTPA administration.
There are several limitations to this study. First, this is a retrospective chart-review study, which limits our access to some information like comorbidities, time intervals (e.g. neurologist arrival and time from arrival to brain CT), and reasons behind not receiving rTPA, especially in candidate patients. This might mask the existence of important non-documented factors associated with exclusion from treatment. Furthermore, we defined eligible patients as those presenting within 4.5 hours from symptom onset, knowing that patients with AIS can still be eligible based on specific MRI findings even if their last-seen well was unknown. In addition, we focused on factors related to exclusion from thrombolysis, but not on those associated with treatment delays. Finally, we did not control for baseline dementia and disability in the study sample, or other variables that might have played a role in discouraging the neurologist to treat with rTPA certain patients, and many of these reasons were not documented in the charts.
In conclusion, a proportion of patients with AIS were excluded from rTPA treatment. Posterior circulation or lacunar strokes and a longer time since symptom onset render the patients less eligible to treatment, while an older age and no stroke code activation as was associated with exclusion from treatment. Lower NIHSS scores were associated with treatment exclusion, regardless if the patient was a candidate or not. In order to improve the rate of rTPA administration, public health efforts should be exerted to educate the public about the typical and atypical stroke symptoms and the need to be transported by an ambulance, if stroke is suspected. Logistic factors related to access to the hospital and routing like helicopters and shortcuts are also important .Moreover, the ED staff must also be thoroughly trained to activate stroke codes whenever appropriate, in order to increase the chances of patients being treated.
References
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333: 1581-7.
- Lahr MMH, Luijckx GJ, Van der Zee DJ, Buskens E. Proportion of Patients Treated With Thrombolysis in a Centralized Versus a Decentralized Acute Stroke Care Setting. Stroke. 2012; 43: 1336-40.
- Barber PA, Zhang J, Demchuk AM, Buchan A. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001; 56: 1015-20.
- Kleindorfer D, Kissela B, Scheinder A, Woo D, Khoury J, Miller R, et al. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population-based study. Stroke. 2004; 35: e27-9.
- Messé SR, Khatri P, Reeves M, Smith E, Saver JL, Bhatt DL, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016; 87: 1565-1574.
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetto D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359: 1317-29.
- Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med. 2018; 379: 611-622.
- Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Frontiers in neurology. 2014: 5: 30-30.
- Dorňák T, Král M, Šaňák D, Kaňovský P. Intravenous Thrombolysis in Posterior Circulation Stroke. Frontiers in neurology. 2019; 10: 417-417.
- Pantoni L, Fierini F, Poggesi A. Thrombolysis in acute stroke patients with cerebral small vessel disease. Cerebrovasc Dis. 2014; 37: 5-13.
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019; 50: e344-e418.
- Khatri P, Conaway MR, Johnston KC. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke. 2012; 43: 560-2.
- Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012; 379: 2352-63.
- Khatri P, Tayama D, Cohen G, Lindley R, Wardlaw JM, Yeatts SD, et al. Effect of Intravenous Recombinant Tissue-Type Plasminogen Activator in Patients With Mild Stroke in the Third International Stroke Trial-3: Post Hoc Analysis. Stroke. 2015; 46: 2325-7.
- Mishra NK, Diener HC, Lyden PD, Bluhmki E, Lees KR. Influence of age on outcome from thrombolysis in acute stroke: a controlled comparison in patients from the Virtual International Stroke Trials Archive (VISTA). Stroke. 2010; 41: 2840-8.
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014; 384: 1929-35.
- Shamy MC, Jaigobin CS. The complexities of acute stroke decision-making: a survey of neurologists. Neurology. 2013; 81: 1130-3.
- Alshekhlee A, Mohammadi A, Mehta S, Edgell RC, Vora N, Feen E, et al. Is thrombolysis safe in the elderly?: analysis of a national database. Stroke. 2010; 41: 2259-64.
- Hsiao CL, Su YC, Yang FY, Liu CY, Chiang HL, Chen GC, et al. Impact of code stroke on thrombolytic therapy in patients with acute ischemic stroke at a secondary referral hospital in Taiwan. J Chin Med Assoc. 2018; 81: 942- 948.
- Newman-Toker DE, Moy E, Valente E, Coffey R, Hines AL. Missed diagnosis of stroke in the emergency department: a cross-sectional analysis of a large population-based sample. Diagnosis. 2014; 1: 155-166.
- Xu H, Xian Y, Woon FP, Bettger JP, Laskowitz DT, Ng YY, et al. Emergency medical services use and its association with acute ischaemic stroke evaluation and treatment in Singapore. Stroke and vascular neurology. 2020; 5: 121-127.
- Barbour V, Thakore S. Improving door to CT scanner times for potential stroke thrombolysis candidates - The Emergency Department’s role. BMJ quality improvement reports. 2017; 6: u211470.w4623.
- Diedler J, Ahmed N, Sykora M, Uyttenboogaart M, Overgaard K, Luijckx GJ, et al. Safety of intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy at stroke onset. Stroke. 2010; 41: 288-94.