Review Article
Austin J Chem Eng. 2014;1(1): 1003.
Hydrophobic and Superhydrophobic Organic-Inorganic Hybrids and their Applications
Nagappan S and Ha CS*
Department of Polymer Science and Engineering, Pusan National University, Republic of Korea
*Corresponding author: Ha CS, Department of Polymer Science and Engineering, Pusan National University, Busan 609-735, Republic of Korea
Received: May 22, 2014; Accepted: July 02, 2014; Published: July 07, 2014
Abstract
A material made from the combination of dissimilar organic or inorganic components could enhance properties of the component material. A range of materials can be prepared by mixing one or more dissimilar organic or inorganic precursors. Several hydrophobic and super hydrophobic surfaces were fabricated by these combinations and used in a variety of applications. This review focuses on the recent advancement and advantages of hydrophobic and super hydrophobic surfaces prepared by the combinations of organic-inorganic components and their applications such as anti-stain coating, highly stable substrate fabrication, hybrid monoliths, selective oil absorption, non-stick and self-cleaning coatings, blood typing etc.
Keywords: Hydrophobic; Anti-stain coating; Super hydrophobic; Superoleophilic; Oil and solvent absorption; Self-cleaning
Abbreviations
PMHS: Polymethyl hydrosiloxane; PVC: P o l y ( v i n y l chloride); LL: Lotus leaf (Nelumbo nucifera); TL: Tree of heaven leaf (Ailanthus altissima); HFBMA: 2,2,3,4,4,4 hexafluoro butyl methacrylate; TEOS: Tetraethoxysilane; AFM: Atomic force microscopy; FPMHS: Fluorinated polymethylhydrosiloxane; FPMS/silica hybrid: Fluorinated polymethylsiloxane/silica hybrid; PMHOS: Polymethylhydrosiloxane; PTES: Phenyltriethoxysilane; PSiOr: Phenyl substituted silica ormosil; LLPPSiOr: LL powder, PMHOS and PSiOr hybrid micro-nanocomposite; TLP suspension: TL powder and PMHOS in ethanol; TLPPSiOr: TL powder, PMHOS and PSiOr hybrid micro-nanocomposite; S1: FPMS/ silica hybrid with TEOS amount of (4.81 mmol); S2: FPMS/silica hybrid with TEOS amount of (9.62 mmol); S3: FPMS/silica hybrid with TEOS amount of (14.43 mmol); FSH3: FPMS/silica hybrid at various ratios of ethanol (130.24 mmol); FSH5: FPMS/silica hybrid at various ratios of ethanol (217.06 mmol); PVCFeS: poly(vinyl chloride-g-ferrotrimethylolpropane tris (3-thiopropionate)); PVCFeS-SiSH: mercaptosilica functionalized PVCFeS; PVCFeS-SiMe: methylsilica functionalized PVCFeS; apoMb: Apomyoglobin; micro-SPE: micro-solid phase extraction; SAs: Sulfonamides; DCA: Dynamic contact angle; SiNPs: Silica nanoparticles; LL25: Lotus leaf (Nelumbo nucifera) powder prepared at 25°C; LL50: Lotus leaf (Nelumbo nucifera) powder prepared at 50°C; LL100: Lotus leaf (Nelumbo nucifera) powder prepared at 100°C; MTMS: Methyltrimethoxysilane; TiO2: Titanium dioxide; HDPE: High density polyethylene; RBMCs: Rat bone marrow derived cells; SBF: Simulated body fluid; PGC-C: Poly(glycerol monostearate-co-ε-caprolactone); PEI: Poly(ethyleneimine); PVDMA: Poly (2-Vinyl-4,4-dimethylazlactone); TMR: Tetramethylrhodamine; FPOSS: Fluorinated polyhedral oligomeric silsesquioxane; PMC: Perfluoroalkyl methacrylic copolymer; RBCs: Red blood cells
Introduction
A hybrid material is defined generally from the combination of one or more dissimilar components such as organic or inorganic components to produce a single entity [1,2]. The obtained hybrid material should enhance a property of the component material over the pristine components or generate new properties for the material [2-4]. Organic-inorganic hybrid materials focused much attention in the recent days due to easy synthesis and ability to produce in large scale [1,2]. Hybrid materials were used in a variety of applications such as anti-stain coating, highly stable, selective oil and organic solvent absorption, non-stick and self-cleaning coatings, semi-conducting channels, flexible substrate fabrication, drug delivery, cell adhesion and transporting, micro-patterning, switchable surfaces, photovoltaic applications, and in storage batteries, etc [1,2,5-19]. Multi-functional hybrid materials have been synthesized successfully using various types of functional groups [20-22]. Sol-gel method is a versatile way to synthesize a multi-functional material. Functional silanes and other functional precursors were used in most situations of sol-gel method to synthesize the functional hybrid material. Several ways were processed such as spin-coating, dip-coating, spraying, electro spinning, micro emulsion, extrusion and casting (etc.,), for the development of stable hybrid substrates, fibers, foams, powders, optical devices, monoliths, flexible substrates, and films for various applications [23-27]. Multi-functional hybrid materials were also synthesized using macro initiators by various polymerization techniques such as surface initiated atom transfer radical polymerization, controlled/living radical polymerization and emulsion polymerization etc., [20,21]. Functional hybrid materials also exhibit various surface properties such as hydrophilic (contact angle (CA) < 90°), hydrophobic (CA ≥ 90°) and super hydrophobic surface (CA ≥ 150°) [7,8,28]. The surface properties of the hybrid material can be switchable, depending on the processing conditions such as UV light, pH, laser, plasma source and temperature [17].
Hydrophobic and super hydrophobic surfaces fabricated from organic-inorganic hybrid materials gained much attention in many applications in the recent decades (Figure 1). This review deals with various organic-inorganic hybrid materials and their development for hydrophobic and super hydrophobic surface fabrication using poly methyl hydro siloxane (PMHS), poly(vinyl chloride) (PVC), fluorinated methacrylate and mercapto functional monomers, various functional silane precursors, lotus leaf (Nelumbo nucifera, LL) and tree of heaven leaf (Ailanthus altissima, TL) powder [5-11]. We also cover some of the recent advancements and their applications of hydrophobic and super hydrophobic surfaces. PMHS is a well known siloxane component with high softness, solubility in common organic solvents, non-toxicity, inertness to air and moisture, low surface energy, hydrophobicity and thermally stable properties [5,6]. PMHS was used in various applications such as reducing agent for organic synthesis, fabrication of stable hydrophobic and super hydrophobic surfaces, coatings on glass, micro-fluidic chips, electronic packages, actuators, and optical fibers [5,6,29-31]. On the other hand, PVC is the most widely used polymer in electronic applications due to the excellent electrical insulation property, inherent flame-retardance, weathering stability, process ability, energy recovery and recyclability [7]. Various silane precursors, fluorinated methacrylate, and mercapto functional monomer were used to enhance the properties of PMHS and PVC in the hybrid systems [7]. Recently, we also developed novel bio-inspired hybrid micro-nano composites by the use of LL powder, PMHS, and functional silica ormosils. The obtained hybrid suspension showed highly stable super hydrophobicity on any substrate by simply drying the solvent at room temperature for a few minutes [8-11]. The hybrid loaded melamine sponge showed selective oil absorption from the oil spill on the water surface. Moreover the super hydrophobic sponge can also absorb various organic solvents and can be recycled for several times for the absorption of larger amounts of oils and organic solvents on the water surface or in under-water. The practical applications of these hybrid materials were discussed in two approaches such as 1) hydrophobic surface in the field of anti-stain coating, highly stable substrate fabrication, protein encapsulation, monolith for micro-solid separation, and 2) super hydrophobic surface for selective oil and organic solvent absorption, non-stick and self-cleaning coatings, cell adhesion and proliferation, controlled drug delivery, controlled dye releasing, super hydrophobic and oleophobic coatings, and blood typing applications.
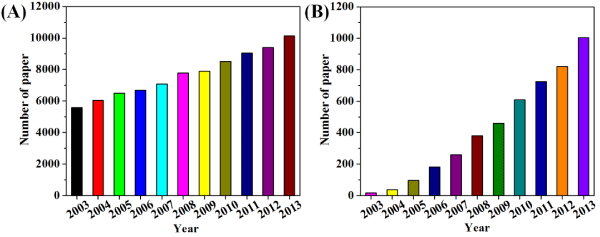
Figure 1 : Number of papers published in the last ten years under the topic of (A) hydrophobic/or hydrophobicity and (B) superhydrophobic/or superhydrophobicity (Source: ISI Web of Science).
Hydrophobic and Superhydrophobic Surfaces and their Applications
Hydrophobic surfaces for anti-stain coatings
Anti-staining is the ability to resist stains or easily erase the stains on the coated substrate [5-7,32,33]. This could be achieved by using low surface energy starting materials. On the other hand, the stability of the coated substrate is also important criteria for creating stable properties. We achieved stable substrate by using PMHS with 2,2,3,4,4,4 hexa fluorobutylmethacrylate (HFBMA) and tetraethoxysilane (TEOS) via a sol-gel reaction [5,6]. The spin coated PMHS (cured at 150°C for 24 h) showed a fractured surface morphology. On the other hand, the surface morphology of the PMHS was altered by the addition of HFBMA and TEOS. A smooth layered surface showed aggregated silica particle surface by increasing the TEOS content. The presence of micro/nanosilica particles on the fluorinated polymethylsiloxane/silica (FPMS/silica) hybrid showed hydrophobic properties [5,6]. The maximum roughness of the silica particles was ~27-35 nm, which depends on the concentration of TEOS content in the FPMS/silica hybrid surface. The mechanical hardness or scratch resistance of the coated substrate was checked by a pencil hardness tester. The spin coated organic-inorganic hybrid material showed good scratch resistance (≥ 4H) [5]. Meanwhile, the scratch resistance of the substrate was altered by changing the concentration of ethanol [5,6]. Increasing the concentration of ethanol would lead to enhance the scratch resistance (≥ 5H) of the hybrid substrate [5,6]. The enhanced scratch resistance is due to the presence of hexagonally packed low surface energy fluorine atom on the outer surface which is bounded strongly on the substrate by silica particles.
Anti-staining property was checked by using water and oil based pens by simply writing and erasing on the coated substrate [5-7]. The FPMS/silica hybrid material showed good erasing property for both oil and water based pens for over 8 times by wiper paper [5,6]. The obtained results explain that the low surface energy matrix with good adhesive property is responsible for developing anti-stain coating. Transparency of the substrate is another important criterion for coating application. The FPMS/silica hybrid material showed 95% to 98% transparency in the visible region [5,6]. To enhance the anti-stain properties for practical applications, we also developed a novel metallopolymer hybrid material using PVC as a starting material [7]. A pre-synthesized iron-mercapto complex was grafted with PVC and then modified with mercapto silane precursor. The PVC metallopolymer showed very good hydrophobicity with CA ~143.27° ± 2.0° [7]. PVC has good transparency (98% at 400 nm wavelength) in the visible region (Figure 2a-c). The poly (vinyl chloride-g-ferrotrimethylolpropane tris(3-thiopropionate)) (PVCFeS) metallopolymer also maintains the transparency by drying up to 80°C for less than 24 h (Figure 2d-f). On the other hand, the transparency of the substrate was decreased by further increasing the drying temperature to 150°C (Figure 2g-k). Mercapto silane precursor and methyl silane precursor were introduced separately into the PVCFeS metallopolymer (mercapto silica functionalized PVCFeS (PVCFeS-SiSH), and methylsilica functionalized PVCFeS (PVCFeS-SiMe) in order to enhance the transparency (Figure 2l and m) [7]. The hybrid metallopolymer maintained the transparency and enhances scratch resistance and anti-stain properties even by increasing the drying temperature to 150°C. This is due to higher boiling point of the mercapto silane precursor which maintains the colouration of metallopolymer and enhances other properties. PVC metallopolymer hybrid showed improved scratch resistance (> 8H) and anti-staining properties (more than 10 times of writing and erasing test) as compared to the hybrid material surface made from PMHS [5-7]. Due to excellent lubrication of PVC metallopolymer hybrid substrate, the surface can easily drag away the water droplet at the sliding angle. This can lead to the use of the surface in self-cleaning coatings for practical applications. The self-cleaning properties of the PVC metallopolymer hybrid substrate was checked by spreading activated charcoal powder on the surface followed by dropping water droplets on the substrate. The bare glass showed the adhesion of charcoal on the substrate. On the other hand, the adhesion property was reduced on the PVC metallopolymer and their hybrid substrate. This proved the excellent anti-stain property of the PVC metallopolymer hybrid [7].
Figure 2 : (a-c) Optical images of the spin coated poly(vinyl chloride) (PVC) substrate cured at room temperature, 80°C, and 150°C for 24 h. (d-f) poly(vinyl chloride-g-ferrotrimethylolpropane tris(3-thiopropionate)) (PVCFeS) metallopolymer substrates cured at room temperature, 40°C, 80°C for 1 h, (g) 80°C for 24 h, (h and i) 100°C for 1 h and 7 h, (j) 120°C for 1 h and (k) 150°C for 30 min. (l and m) Mercaptosilica functionalized PVCFeS (PVCFeS-SiSH) (SiSH-0.05 phr), and methylsilica functionalized PVCFeS (PVCFeS-SiMe) (SiMe-0.01 phr) metallopolymer substrates cured at 150oC for 24 h. Reproduced with permission from [7], Nagappan S. et al. J Mater Chem A 2013; 1: 12144-12153. © 2013, Royal Society of Chemistry.
Hydrophobic surfaces for other applications
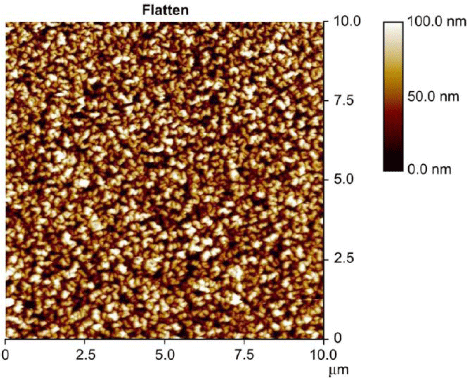
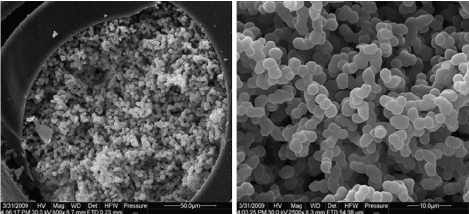
Hydrophobic surfaces also favoured for protein encapsulation in the organically modified porous substrate [34]. Meena et al. studied the effect of protein encapsulation on various types of organically modified hydrophobic surfaces using two types of proteins such as apomyoglo bin (apoMb) and ribonuclease A [34]. They also studied the protein encapsulation on the untreated glass substrate. In most cases, apoMb is not favourable to hydrophobic environment due to the phase separation. Interestingly, they found that organically modified porous hydrophobic surface showed better encapsulation behavior to the proteins than hydrophilic untreated glass substrate. They predicted the encapsulation behavior mainly due to the concentration of organic component (~5-15%) treated on the hydrophilic glass substrate. The concentration may vary depending on the type of organic component used for surface treatment. Increasing the concentration of organic components may lead to phase separation or reduce the transparency of the treated surfaces. The increase in protein encapsulation efficiency is due to the increase in the fraction of properly folded functional enzymes. The surface morphology was changed by encapsulation of protein to the hydrophobic hybrid glass. This was confirmed from the atomic force microscopy (AFM) surface mean roughness value (Figure 3). The mean roughness was increased by the encapsulation of apoMb from 0.21 nm to 2.64 nm (Figure 3). Zheng et al. prepared hydrophobic organic-inorganic hybrid silica monolith with cation exchange functional group as a sorbent for micro-solid phase extraction (micro-SPE) [35]. The hybrid monolith showed aggregated spherical particles interconnected with macro pores with pore size around 5 μm and narrow distribution (Figure 4). Macro porous structure is highly favourable for the permeability in the extraction application. A testing analysis such as sulfonamides (SAs) was mixed with milk and then separated by using micro-SPE. The separation efficiency of the mixture was monitored by high performance liquid chromatography (HPLC). The hydrophobic hybrid silica monolith showed excellent recyclability and separation efficiency of SAs and milk from the mixture [35].
Figure 3 : AFM image of a 10% ethyl-modified glass with encapsulated apoMb. Spiral chains of interconnected silica particles are evident. Reproduced with permission from [34], Menaa B. et al. Biomaterials 2008; 29: 2710-2718. © 2008, Elsevier Ltd.
Figure 4 : Scanning electron microscope images of the cross section of the hybrid silica monolith: wide view (left) and close-up view (right). Reproduced with permission from [35], Zheng MM. et al. J Chromatogr A 2009; 1216: 7739-7746. © 2009, Elsevier Ltd.
Stable superhydrophobic surfaces for selective oil and organic solvent absorption
We successfully synthesized super hydrophobic polymethyl hydroxysiloxane (PMHOS) powder in one-pot using PMHS, ethanol and sodium hydroxide and hydrophobic phenyl substituted silica ormosil (PSiOr) using phenyltriethoxysilane (PTES) in the presence of oxalic acid and aqueous ammonia [8-10,13,24]. PMHOS powder can be used in various applications due to super hydrophobic and superoleophilic properties. The above properties of PMHOS gave an idea to use the material for the preparation of super hydrophobic hybrid micro-nanocomposites sponge for selective oil and organic solvent spill capture applications. The use of lotus leaf (LL) powder can also enhance the absorption behavior of the hybrid system. Introducing hydrophobic silica ormosil to the system can make the material more stable. Based on the ideas, a novel bio-inspired super hydrophobic hybrid micro-nanocomposite (LLPPSiOr) was prepared from the combination of LL powder, PMHOS, and PSiOr [8-10]. LLPPSiOr hybrid suspension showed excellent super hydrophobicity by the evaporation of solvents at room temperature on a range of substrate such as glass, laminating film, tree leaf (Ailanthus altissima), cotton cloth and laminating film with CA over 175° [8]. The super hydrophobicity was very stable (more than 6000 s) on the coated substrate even the substrate treated at 500°C for 5h. On the other hand, the substrate became super hydrophilic by treating at 600°C for 5h. The substrate at room temperature also showed superoleophilicity by contact with dodecane (<5°) and soybean oil (<5°). On the other hand, the surface become switchable (super hydrophobic (θadv = 173.92° ± 1.5°, θrec = 169.94° ± 1°, and H = 3.98°)) by the evaporation of dodecane. These switchable properties may play a key role in the absorption and separation of large amounts of low surface energy oils from water. The hybrid micro-nanocomposites loaded melamine sponge also exhibited super hydrophobicity. The super hydrophobic sponge was used for the absorption of various oils and organic solvent spills on the water surface or in under water.
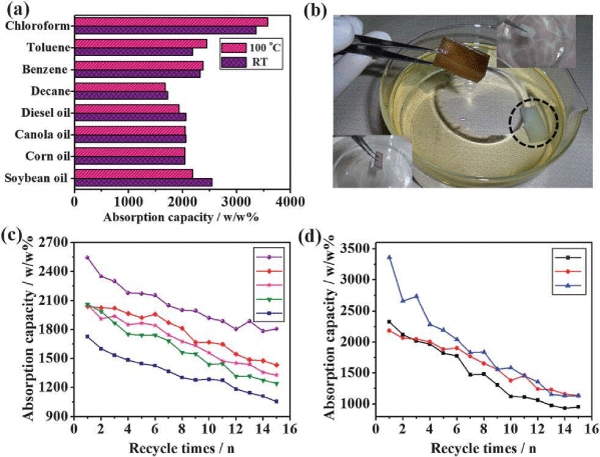
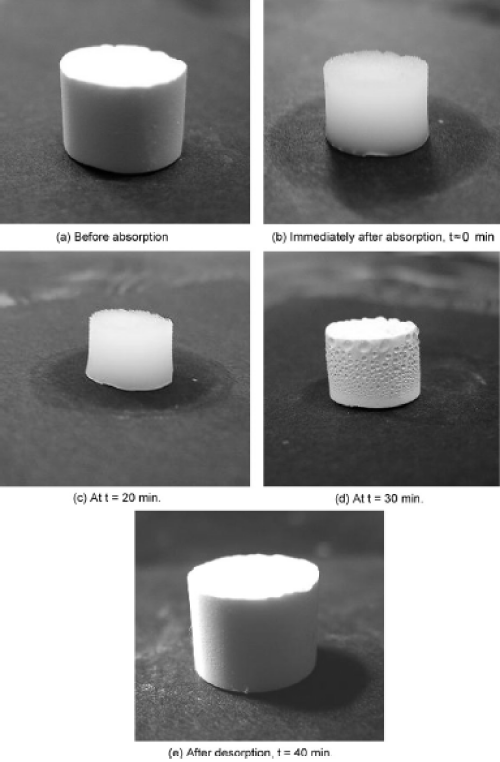
The absorption capacity (k) = [(Wb-Wa)/Wa x 100 (w/w %)] of various oils and organic solvents were calculated from the amount of oils and organic solvents absorbed before and after absorption [8]. Wb indicates the amount of oil or solvent absorbed by the absorbent, while Wa indicates the initial weight (before absorption) of the hybrid/PDMS sponge. The super hydrophobic hybrid sponge showed selective absorption of oils and solvents from the spills. The absorption behavior was tested for two types of hybrid sponge prepared at room temperature and at 100°C. The super hydrophobic hybrid sponge showed very high absorption to soybean oil (2544 w/w% for hybrid/ PDMS sponge) and various oils (corn, canola, diesel, and decane) and organic solvents (benzene, toluene, and chloroform) at room temperature and 100°C (Figure 5a-d). The absorption capacity was enhanced for the super hydrophobic hybrid sponge prepared at room temperature as compared with the sponge cured at 100°C (soybean oil, 2185 w/w% for hybrid/PDMS sponge). Several methods were used for the absorption and separation of oil spills on the water surface. On the other hand, super hydrophobic sponges and magnetically separable absorbents were used widely for absorption and separation of oil spills [8,36-39]. These absorbents also showed the maximum absorption and separation efficiency due to the excellent recyclable properties. Rao et al. fabricated super hydrophobic silica aerogels using methyltrimethoxysilane (MTMS) in the presence of acid and base catalyst [40]. The super hydrophobic silica aero gels showed excellent and uniform absorption and desorption behavior to various organic (alkenes, aromatics, alcohols) liquids (Figure 6) [40].
Figure 5 : (a) Absorption of various oils and organic solvents by the superhydrophobic hybrid/PDMS sponge cured at room temperature (RT) and 100oC. (b) Optical image of the hybrid/PDMS sponge after the absorption of diesel oil. The complete superhydrophilic properties of the pristine sponge illustrated at the right corner of the inset figure and at the bottom (black dotted circle) of the water bath. The left corner inset figure shows the complete superhydrophobic properties of the hybrid/ PDMS sponge when immersed in water (silvery shine). (c,d) Graph of the absorption capacity to various oils (soybean (Ο), corn (♦), canola (✶), diesel (∇), and decane oils (⌋)) and various solvents (benzene (⌋), toluene (Ο) and chloroform (Δ)) of the hybrid loaded and surface treated sponge cured at room temperature Reproduced with permission from [8], Nagappan S. et al. J Mater Chem A 2013; 1: 6761-6769. © 2013, Royal Society of Chemistry.
Figure 6 : Photographs showing various stages of shrinkage and spring back during desorption of hexane from the aerogel. Reproduced with permission from [40], Rao AV. et al. J Colloid Interface Sci. 2007; 305: 124-132. © 2007, Elsevier Ltd.
Superhydrophobic surfaces for non-stick and self cleaning applications
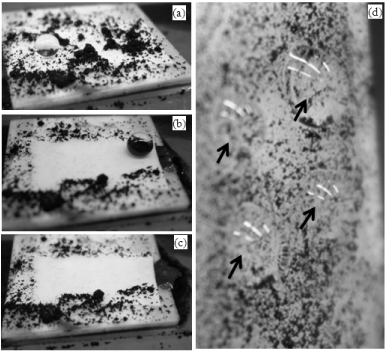
Non-stick and self-cleaning properties are depending on the sliding angle of the substrate surface. The free rotation and displacement of water droplets on the substrate surface can exhibit excellent self-cleaning behavior [8-11]. The non-stick property was tested on the super hydrophobic hybrid substrate by a water droplet (surface energy of water, 72 (mN/m)) or from the movements of water droplets on the substrate. Due to very high CA of the hybrid substrate can easily resist water droplets on the substrate. Super hydrophobic substrate can also easily drag away the water droplets and keep the substrate clean. This self-cleaning behavior was checked further by placing various dusts (zinc dust (<10μm), Portland cement, sea sand and carbon dust) on the drop coated or spin coated substrate followed by dropping water droplets [9,10,41]. The pollutants were collected by the droplets and drag away from the substrate (Figure 7). This self-cleaning lotus leaf behavior was also achieved from the lotus leaf powder hybrid micro-nanocomposites. Similarly, Xu et al. fabricated super hydrophobic self-cleaning surface using titanium dioxide (TiO2)/high density polyethylene (HDPE) [41]. TiO2 in the HDPE have enhanced the self-cleaning properties of the substrate due to photo-oxidation of TiO2 in UV light [41]. The stability of the bio-inspired hybrid micro-nanocomposites was also checked by varying the compositions of the starting materials [8-11]. The introduction of lotus leaf powder prepared at various drying temperatures (LL25°C, LL50°C and LL100°C) or hydrophilic silica nanoparticles (SiNPs) to the hybrid suspension (LL powder, PMHOS, and SiNPs (LLPSiNPs) hybrid micro-nanocomposites) showed stable hydrophobicity with spherical shape particles on the substrate [10]. Increasing the hydrophilic silica nanoparticles to the hybrid suspension also maintained the hydrophobicity on the coated substrate. We also prepared the bio-inspired hybrid micro-nanocomposites using another leaf powder such as TL powder [11]. The tree leaf powder showed better compatibility with the super hydrophobic PMHOS. The addition of hydrophobic PSiOr to the TLP suspension (TL powder and PMHOS in ethanol) also produced super hydrophobicity on any substrates. The surface property of the hybrid was tested at various temperatures of water droplets. The hybrid suspension maintains the super hydrophobicity to cold and hot water. The addition of water droplet at 0oC or below 0°C maintains the super hydrophobicity with stable contact angle as obtained for water droplet at room temperature. The obtained results show the anti-icing property of the substrate. The super hydrophobicity was maintained on the substrate by placing water droplet temperature up to 50°C. Further increasing the water droplets temperature to 60°C to 80°C, the surface CA was reduced to hydrophobic by the nucleation of the hot water droplet on the substrate. Meanwhile, the super hydrophobicity can retain on the substrate by replacing the hot water droplet and placing the room temperature water droplet on the same place. The TL powder, PMHOS and PSiOr hybrid micro-nanocomposites (TLPPSiOr) also showed excellent non-stick and self-cleaning properties.
Figure 7 : The self-cleaning effect of water droplets on the TiO2-HDPE composite superhydrophobic surface (a-c), and sticky water droplets (marked) on a normal flat HDPE surface put vertically (d). The black contaminates are fine carbon particulates with an average size of 1 μm. Reproduced with permission from [41], Xu QF. et al. ACS Appl Mater Interfaces 2013; 5: 8915-8924. © 2013, American Chemical Society.
Super hydrophobic surfaces for other applications
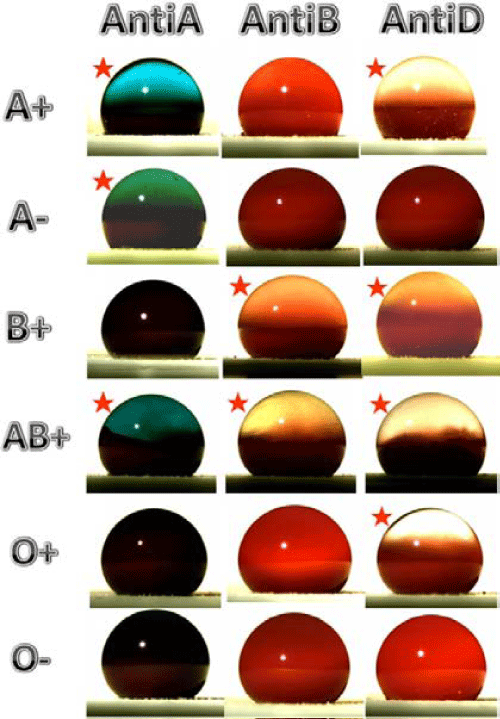
Super hydrophobic surfaces were also used in other applications such as cell adhesion and proliferation, drug delivery, organic dye loading and release, self-healing coatings, anti-corrosion, anti-fouling, and blood typing etc., [42-50]. Alves et al. developed novel bio-inspired hydrophobic (controlled) and super hydrophobic membrane surfaces using poly (L-lactic acid) (PLA) by casting the PLA solution into the Petri dish and checked the cell adhesion and proliferation of the film [43]. They found that super hydrophobic membrane surface showed excellent repellency to cell adhesion and proliferation to bone marrow derived cell. The cell adhesion behavior was also checked by dipping in simulated body fluid (SBF) for more than 20 days. The hydrophobic surface showed smooth layer on the controlled membrane surface (Figure 8). On the other hand, super hydrophobic membrane surface maintained the surface properties even dipped in SBF solution for 21 days. In some cases cells can adhere to the super hydrophobic surfaces depending on the type of cell used for adhesion. Yohe et al. developed electrospun mesh using poly (glycerol monostearate-co-ε-caprolactone) (PGC-C18) [44]. 7-ethyl-10-hydroxycamptothecin (SN-38, anticancer agent) drug was loaded in the PGC-C18 mesh and observed the controlled drug delivery behavior. The entrapped air present in the electrospun nanofiber mesh can maintain the super hydrophobicity of the mesh in a buffer solution and releases the drug in a controlled fashion of intervals. In another case, Manna et al., fabricated a novel super hydrophobic films using a mixture of branched poly (ethylene eimine) (PEI) and poly (2-Vinyl-4,4-dimethylazlactone) (PVDMA) by layer by layer technique [45]. The fabricated PEI/PVDMA film was loaded with fluorophore tetramethylrhodamine (TMR) dye and observed the dye releasing behavior from the super hydrophobic surface. TMR dye was loaded quickly on the polymer matrix (within 80 s). On the other hand, the polymer film showed slower release behavior (over one year). The obtained results showed the controlled release behavior of the dye loaded substrate. The author's also explained the self-healing behavior of the PEI/PVDMA film [46]. The PEI/PVDMA film losses the super hydrophobic behavior under crushing. On the other hand, the super hydrophobicity was recovered by dipping the PEI/PVDMA film in water for few seconds (15 s). This self-healing, self-cleaning and controlled dye releasing behavior can lead to use of the material for textile and other applications. Dong et al. fabricated super hydrophobic and oleophobic surface using fluorinated polyhedral oligomeric silsesquioxane (FPOSS) by spin coating method [49]. The spin coated surface resisted water droplet on the surface and was dragged away from the substrate in a fraction of seconds. More recently, Li et al. used super hydrophobic surface for the identification of six types of blood groups from their aggregation behavior with antibodies [50]. Blood types were identified by haemagglutination reaction in a conventional method. The reaction was carried out by placing a blood droplet on the super hydrophobic surface followed by the addition of anti-body. The interaction of anti-body and antigens in haemagglutination reaction results to the phase separation of red blood cells (RBCs) (Figure 9). The separation of agglutinated RBCs was observed clearly from the plasma phase by the introduction of anti-body to the antigen (Figure 9). The use of super hydrophobic paper surface by this method is quite simple, low-cost and environmental friendly to detect blood types. Super hydrophobic materials were used in numerous fields of applications in the recent decades. More and more novel materials are also continuously developed by several research groups and their applications will be extendable for the future applications.
Figure 8 : RBMCs onto (A) control films and (B) superhydrophobic substrates after 21 days of culture. The white arrow identifies the only cell that was observed in the later surface. Reproduced with permission from [43], Alves NM. et al. J Biomed Mater Res A 2009; 91A: 480-488. © 2009, Wiley-VCH, Weinheim, Germany.
Figure 9 : Photos of the blood agglutination of the six blood samples (A+, A-, B+, AB+, O+ and O-) when they are respectively mixed with three antibodies (Anti-A, Anti-B and Anti-D) on superhydrophobic surface. The red star represents blood aggregation was found in that photo. The blood type can be determined by observing the aggregation of blood with different antibodies. Reproduced with permission from [50], Li L. et al. Colloids Surf B: Biointerfaces. 2013; 106: 176-180. © 2013, Elsevier Ltd.
Conclusions and Future Perspectives
In this review, we discussed the fabrication of hydrophobic and super hydrophobic surfaces from PMHS, PVC, lotus leaf and tree of heaven leaf powders and from silane precursors (etc.) and their vast applications in anti-stain coatings, highly stable surfaces fabrication, protein encapsulation, monolith for micro-solid separation, selective oil and organic solvent absorption, non-stick and self-cleaning coatings etc. We also discussed various applications of hydrophobic and super hydrophobic surfaces. Hydrophobic and super hydrophobic properties are important surface properties for practical applications in a wide range of field. Today, the growth of coating materials was established in various applications. The dramatic growth of the materials with hydrophobic or super hydrophobic property on the coated substrate can lead to extend the life of the substrate for several years. On the other hand, cost, easy processability, environmental-friendness and stability of the substrate for air and moisture are among the most important criteria to consider for the establishment of hydrophobic or super hydrophobic materials for practical applications.
Acknowledgement
This study was supported by the National Research Foundation of Korea (NRF) Grant funded by The Ministry of Science, ICT & Future Planning, Korea (Pioneer Research Center Program (2010- 0019308/2010-0019482) and BK 21 plus Program (21A2013800002)).
References
- Sanchez C, Julián B, Belleville P, Popall M. Applications of hybrid organic-inorganic nanocomposites. J Mater Chem. 2005; 15: 3559-3592.
- Sanchez C, Belleville P, Popall M, Nicole L. Applications of advanced hybrid organic-inorganic nanomaterials: from laboratory to market. Chem Soc Rev. 2011; 40: 696-753.
- Nicole L, Rozes L, Sanchez C. Integrative approaches to hybrid multifunctional materials: from multidisciplinary research to applied technologies. Adv Mater. 2010; 22: 3208-3214.
- Fahmi A, Pietsch T, Mendoza C, Cheval N. Functional hybrid materials. Mater Today 2009; 22: 44-50.
- Nagappan S, Choi MC, Sung G, Park SS, Moorthy MS, Chu SW, et al. Highly transparent, hydrophobic fluorinated polymethylsiloxane/silica organic-inorganic hybrids for anti-stain coating. Macromol Res. 2013; 21: 669-680.
- Nagappan S, Park JJ, Park SS, Hong SH, Jeong YS, Lee WK, et al. Polymethylhydrosiloxane-based organic-inorganic hybrids for amphiphobic coatings. Compos Interfaces. 2013; 20: 33-43.
- Nagappan S, Park SS, Yu EJ, Cho HJ, Park JJ, Lee WK, et al. A highly transparent, amphiphobic, stable and multi-purpose poly (vinyl chloride) metallopolymer for anti-fouling and anti-staining coatings. J Mater Chem A. 2013; 1: 12144-12153.
- Nagappan S, Park JJ, Park SS, Lee WK, Ha CS. Bio-inspired, multi-purpose and instant superhydrophobic-superoleophilic lotus leaf powder hybrid micro-nanocomposites for selective oil spill capture. J Mater Chem A. 2013; 1: 6761-6769.
- Nagappan S, Park JH, Sung AR, Ha CS. Superhydrophobic hybrid micronanocomposites for non-stick and self-cleaning coatings. Compos Interfaces. 2014; 21: 597-609. DOI: 10.1080/15685543.2014.928108 [In press].
- Nagappan S, Sung AR, Ha CS. Preparation of stable hydrophobic bio-based silica hybrid micro-nanocomposites. J Bio-based Mater Bioenergy. 2014; 8: 175-183.
- Nagappan S, Park SS, Ha CS. Recent advances in superhydrophobic nanomaterials and nanoscale systems. J Nanosci Nanotechnol. 2014; 14: 1441-1462.
- Kagan CR, Mitzi DB, Dimitrakopoulos CD. Organic-inorganic hybrid materials as semiconducting channels in thin-film field-effect transistors Science. 1999; 286: 945-947.
- Yildirim A, Budunoglu H, Yaman M, Guler MO, Bayindir M. Template free preparation of nanoporous organically modified silica thin films on flexible substrates. J Mater Chem. 2011; 21: 14830-14837.
- Xing L, Zheng H, Cao Y, Che S. Coordination polymer coated mesoporous silica nanoparticles for pH-responsive drug release. Adv Mater. 2012; 24: 6433-6437.
- Luo SC, Liour SS, Yu HH. Perfluoro-functionalized PEDOT films with controlled morphology as superhydrophobic coatings and biointerfaces with enhanced cell adhesion. Chem Commun (Camb). 2010; 46: 4731-4733.
- Ueda E, Levkin PA. Micropatterning hydrophobic liquid on a porous polymer surface for long-term selective cell-repellency. Adv Healthc Mater. 2013; 2: 1425-1429.
- Liu MJ, Jiang L. Switchable adhesion on liquid/solid interfaces. Adv Funct Mater. 2010; 20: 3753-3764.
- Son J, Kundu S, Verma LK, Sakhuja M, Danner AJ, Bhatia CS, et al. A practical superhydrophilic self cleaning and antireflective surface for outdoor photovoltaic applications. Solar Energy Mater Solar Cells. 2012; 98: 46-51.
- Zhu Q, Chen N, Tao F, Pan QM. Improving the lithium storage properties of Fe2O3@C nanoparticles by superoleophilic and superhydrophobic polysiloxane coatings. J Mater Chem. 2012; 22: 15894-15900.
- Hui CM, Pietrasik J, Schmitt M, Mahoney C, Choi J, Bockstaller MR, et al. Surface-initiated polymerization as an enabling tool for multifunctional (nano-) engineered hybrid materials. Chem Mater. 2014; 26: 745-762.
- Pyun J, Matyjaszewski K. Synthesis of nanocomposite organic/inorganic hybrid materials using controlled/“living” radical polymerization. Chem Mater. 2001; 13: 3436-3448.
- Yan Z, Ma L, Zhu Y, Lahiri I, Hahm MG, Liu Z, et al. Three-dimensional metal-graphene-nanotube multifunctional hybrid materials. ACS Nano. 2013; 7: 58-64.
- Pandey S, Mishra SB. Sol-gel derived organic-inorganic hybrid materials: synthesis, characterizations and applications. J Sol-Gel Sci Technol. 2011; 59: 73-94.
- Yang DJ, Li JP, Xu Y, Wu D, Sun YH, Zhu HY, et al. Direct formation of hydrophobic silica-based micro/mesoporous hybrids from polymethylhydrosiloxane and tetraethoxysilane. Microporous Mesoporous Mater. 2006; 95: 180-186.
- Wen J, Wilkes GL. Organic/inorganic hybrid network materials by the sol-gel approach. Chem Mater. 1996; 8: 1667-1681.
- Hoffmann F, Cornelius M, Morell J, Fröba M. Silica-based mesoporous organic-inorganic hybrid materials. Angew Chem Int Ed Engl. 2006; 45: 3216-3251.
- Rahane SB, Hensarling RM, Sparks BJ, Stafford CM, Patton DL. Synthesis of multifunctional polymer brush surfaces via sequential and orthogonal thiol-click reactions. J Mater Chem. 2012; 22: 932-943.
- Li XM, Reinhoudt D, Crego-Calama M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem Soc Rev. 2007; 36: 1350-1368.
- Keinan E, Greenspoon N. Highly chemoselective reductions with PMHS and Palladium (0) catalyst. J Org Chem. 1983; 48: 3545-3548.
- Lee SJ, Goedert M, Matyska MT, Ghandehari EM, Vijay M, Pesek JJ. Polymethylhydrosiloxane (PMHS) as a functional material for microfluidic chips, J Micromech Microeng. 2008; 18: 1-9.
- Thami T, Nasr G, Bestal H, Der Lee AV, Bresson B. Functionalization of surface-grafted polymethylhydrosiloxane thin films with alkyl side chains. J Polym Sci: Part A: Polym Chem. 2008; 46: 3546-3562.
- Choi MC, Sung G, Nagappan S, Han MJ, Ha CS. Transparent anti-stain coatings with good thermal and mechanical properties based on polyimide-silica nanohybrids. J Nanosci Nanotechnol. 2012; 12: 5788-5793.
- Sung G, Choi MC, Nagappan S, Lee WK, Han MJ, Ha CS. Polynorbornene/fluorosilica hybrids for hydrophobic and oleophobic coatings. Polym Bull. 2013; 70: 619-630.
- Menaa B, Herrero M, Rives V, Lavrenko M, Eggers DK. Favourable influence of hydrophobic surfaces on protein structure in porous organically-modified silica glasses. Biomaterials. 2008; 29: 2710-2718.
- Zheng MM, Ruan GD, Feng YQ. Hybrid organic-inorganic silica monolith with hydrophobic/strong cation-exchange functional groups as a sorbent for micro-solid phase extraction. J Chromatogr A. 2009; 1216: 7739-7746.
- Li A, Sun HX, Tan DZ, Fan WJ, Wen SH, Qing XJ, et al. Superhydrophobic conjugated microporous polymers for separation and adsorption. Energy Environ Sci. 2011; 4: 2062-2065.
- Tang Y, Yeo KL, Chen Y, Yap LW, Xiong W, Cheng W. Ultralow-density copper nanowire aerogel monoliths with tunable mechanical and electrical properties. J Mater Chem A. 2013; 1: 6723-6726.
- Ge B, Zhang ZZ, Zhu XT, Ren G, Men XH, Zhou XY. A magnetically superhydrophobic bulk material for oil removal. Colloids Surf A: Physicochem Eng Aspects. 2013; 429: 129-133.
- Zhang L, Wu J, Wang Y, Long Y, Zhao N, Xu J. Combination of bioinspiration: a general route to superhydrophobic particles. J Am Chem Soc. 2012; 134: 9879-9881.
- Venkateswara Rao A, Hegde ND, Hirashima H. Absorption and desorption of organic liquids in elastic superhydrophobic silica aerogels. J Colloid Interface Sci. 2007; 305: 124-132.
- Xu QF, Liu Y, Lin FJ, Mondal B, Lyons AM. Superhydrophobic TiO2-polymer nanocomposite surface with UV-induced reversible wettability and self-cleaning properties. ACS Appl Mater Interfaces. 2013; 5: 8915-8924.
- Nagappan S, Park SS, Ha CS. Superhydrophobic and self-cleaning natural leaf powder/poly (methylhydroxysiloxane) hybrid micro-nanocomposites. Macromol Res. 2014; 22: 843-852. [In press]..
- Alves NM, Shi J, Oramas E, Santos JL, Tomás H, Mano JF. Bioinspired superhydrophobic poly(L-lactic acid) surfaces control bone marrow derived cells adhesion and proliferation. J Biomed Mater Res A. 2009; 91: 480-488.
- Yohe ST, Colson YL, Grinstaff MW. Superhydrophobic materials for tunable drug release: using displacement of air to control delivery rates. J Am Chem Soc. 2012; 134: 2016-2019.
- Manna U, Kratochvil MJ, Lynn DM. Superhydrophobic polymer multilayers that promote the extended, long-term release of embedded water-soluble agents. Adv Mater. 2013; 25: 6405-6409.
- Manna U, Lynn DM. Restoration of superhydrophobicity in crushed polymer films by treatment with water: self-healing and recovery of damaged topographic features aided by an unlikely source. Adv Mater. 2013; 25: 5104-5108.
- Isimjan TT, Wang T, Rohani S. A novel method to prepare superhydrophobic, UV resistance and anti-corrosion steel surface. Chem Eng J. 2012; 210: 182-187.
- Miyagawa H, Yamauchi K, Kim YK, Ogawa K, Yamaguchi K, Suzaki Y. Fabrication of transparent antifouling thin films with fractal structure by atmospheric pressure cold plasma deposition. Langmuir. 2012; 28: 17761-17765.
- Dong F, Ha CS. Superhydrophobic and oleophobic surfaces fabricated from incompletely condensed polyhedral oligomeric silsesquioxane. Macromol Res. 2011; 19: 101-104.
- Li L, Tian J, Li M, Shen W. Superhydrophobic surface supported bioassay--an application in blood typing. Colloids Surf B Biointerfaces. 2013; 106: 176-180.