Research Article
Austin J Chem Eng. 2014;1(1): 1004.
Purification of Crude Glycerol using Acidification: Effects of Acid Types and Product Characterization
Nanda MR1, Yuan Z1, Qin W2, Poirier MA3 and Chunbao X1*
1Department of Chemical and Biochemical Engineering, Western University, Canada
2Department of Biology, Lake head University, Canada
3Imperial Oil Products Division, Sarnia Technology Applications and Research, Canada
*Corresponding author: Xu Chunbao (Charles), Department of Chemical and Biochemical Engineering, Institute for Chemicals and Fuels from Alternative Resources (ICFAR), Western University, London, ON, Canada, N6A 5B9
Received: May 25, 2014; Accepted: July 09, 2014; Published: July 14, 2014
Abstract
Purification of crude glycerol is essential for its applications for high-value products. In this study, crude glycerol was purified by acidification using sulfuric, hydrochloric or phosphoric acid, and the results were compared. Phosphoric acid was found to be the best purifying agent among the others. Acidification of a biodiesel plant waste crude glycerol (containing approximately 13 wt% glycerol and 6 wt% ash) for a total processing time of 1 h, produced a purified product containing approximately 96 wt% glycerol, and 0.7 wt% ash. Effects of pH values on the purification efficiency were investigated. The crude glycerol and the purified products were extensively characterized.
Keywords: Crude glycerol; Purification; Sulfuric acid; Hydrochloric acid; Phosphoric acid
Introduction
With the increased concern over the depletion of fossil fuels worldwide, the search for alternative energy/chemical sources has been becoming urgent more than ever before. Biodiesel produced from renewable animal or plant oil has been one of two most commonly explored bio-fuels (the other is bio-ethanol) that could effectively reduce the global dependence on the fossil fuels and the greenhouse gas emission.
Biodiesel is mainly produced by the transesterification of animal fats or vegetable oils (triglyceride) with methanol in presence of an alkali or acid catalyst [1,2]. During the transesterification process in a biodiesel plant, crude glycerol is the primary byproduct, accounting for about 10 wt% of the biodiesel product [3,4].
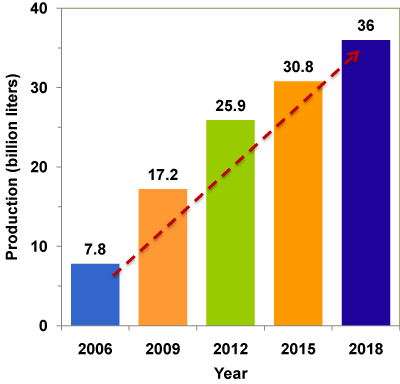
With the rapid growth of biodiesel industry all over the world, a large surplus of glycerol has been created [5], leading to the closure of several traditional glycerol production plants [6]. This huge amount of glycerol, once it enters into the market, would significantly affect the glycerol price. The current market value is US$ 0.27- 0.41 per pound of pure glycerol [7] and as low as US$ 0.04 - 0.09 per pound of crude glycerol (80% purity) [8]. The world scenario of glycerol production is given in Figure 1. It is predicted that by 2020 the global production of glycerol will reach 41.9 billion liters [9]. Thus, crude glycerol disposal and utilization has become a serious issue and a financial and environmental liability for the biodiesel industry. Economic utilizations of glycerol for value-added products are critically important for the sustainability of biodiesel industry.
Figure 1 : World's scenario of crude glycerol.
Research has been conducted for the conversion of glycerol to different value added chemicals such as; propane-1, 3-diol [10], propane-1,2-diol [11], acrolein [12], hydrogen [13,14], acetal or ketal [15,16], bio oil [17,18], poly hydroxyalkanoates [19], polyols and polyurethane foams [20], glycerol carbonate [21,22] etc.
Crude glycerol however has purity of 15-80% and it contains a large amount of contaminants such as water, methanol, soap/free fatty acids (FFAs), salts, and unused reactants. The common practice of using alkaline catalysts during the transesterification process results a high pH (above 10) of this byproduct. The presence of contaminants in this renewable carbon source creates certain challenges for the conversion processes, e.g., as it could plug the reactor, deactivate the catalysts, and inhibit bacterial activities (for bioconversion). Another major challenge for the utilization of crude glycerol is the inconsistency in its composition since it varies with the feedstock and production procedures. As such, it is of great significance and interest to purify crude glycerol for the aforementioned value-added applications of glycerol. High purity glycerol is also an important feedstock for various industrial applications in food, cosmetic and pharmaceutical industries.
Different purification processes have been developed and reported in the literature, among which the most common processes include those using ion exchange resin [23], nano-cavitation technology [24], membrane separation technology (MST), simple distillation under reduced pressure [25], and acidification, followed by neutralization and solvent extraction [26,27], etc. Nevertheless, the purification processes using ion exchange resin and simple distillation are limited because of these processes generally produce a very low yield of pure glycerol (<15 wt%). The use of nano-cavitation technology for the purification of crude glycerol has been demonstrated, but its large-scale operation is very challenging [24]. MST could yield ultra-high purity glycerol provided that the crude glycerol undergoes prior purification that reduces salts and matter organic non glycerol (MONG, such as methyl ester) [28]. Compared with other processes, the processes using acidification demonstrated to be more promising due to higher yields and their relatively low costs [26].
Kongjao et al (2010) [26] reported the purification of crude glycerol (30 wt% glycerol content) from a waste used-oil methyl ester plant using 1.19 M H2SO4 followed by neutralization and solvent extraction to get purifies glycerol of around 93 wt% purity. In a similar work, Ooi et al (2001) [29] demonstrated that crude glycerol was upgraded from purity of 34 wt% to 52 wt% by using sulphuric acid. However, the main issue in these processes is the use of sulphuric acid that has corrosive nature of sulphuric acid and the non-biodegradability of the produced sulfate salts [30].
In this work, purification of crude glycerol obtained from a multi-feed biodiesel plant was carried out using different acids (sulfuric acid, hydrochloric acid, phosphoric acid) in order to investigate the effects of acid types and pH value on crude glycerol purification.
Materials and Methods
Crude glycerol was obtained from a biodiesel plant of Methes Energies Canada Inc (Mississauga, Ontario). All chemicals were purchased from Sigma Aldrich, including phenolphthalein, reagent grade HNO3, concentrated H2SO4, concentrated HCl, concentrated H3PO4, KOH, methyl orange, methanol and dimethyl sulfoxide.
Purification process
As the crude glycerol received is solid at room temperature, around 200 g of the crude glycerol was melted at 55OC in a 500 ml beaker placed on a magnetic hot plate. The molten crude glycerol under gentle stirring was acidified with different acids (sulfuric acid, hydrochloric acid, and phosphoric acid, respectively) to a desired pH level and was kept for a sufficiently long time to allow the formation of three separate layers. The top layer is fatty acid phase, the middle one is glycerol rich phase and the bottom one is inorganic slat phase. The bottom phase was separated by simple decantation. The fatty acid-rich top phase was separated from the glycerol-rich phase by using a separator funnel. The extracted glycerol was neutralized using 12 M KOH solution followed by evaporation of water at 110°C for 2 h and filtration to remove the precipitated salt.
The obtained glycerol was further purified by solvent extraction process using methanol as solvent to promote the precipitation of dissolved salts. The precipitated salts were separated by filtration and passed through a column of activated charcoal to de-color and remove odor and some metal ions.
Characterization of crude and purified glycerol
The crude and purified glycerol samples were characterized for the density, alkalinity, moisture content, glycerol content, ash content, metal content and the color intensity.
Density
The density was determined according to ASTM D 891-95 (2004). First, the weight of the dried pycnometer was recorded. Water was added into the pycnometer at room temperature (22 ± 1°C) and its mass and hence the volume of the pycnometer was recorded. Again, crude or purified glycerol was filled in the dried pycnometer at same temperature and the mass of the crude glycerol was reported. The density of the crude glycerol was obtained by taking the ratio between the mass of the sample and the volume of the pycnometer.
Alkalinity
The alkalinity of crude or purified glycerol was calculated according to IUPAC- ACD 1980(6th edition) method using the following formula
where V is the volume (ml) of the HCl solution consumed in the titration, N is the normality of HCl solution and W is the weight (g) of crude glycerol used for titration.
pH
Around 1.00 g of crude or purified glycerol was dissolved in 50.0 ml of deionized (DI) water. The pH of the solution was measured by a pH meter (SymphonyTM 89231-608, VWR) at room temperature (22± 1°C) after calibration of the apparatus with buffer solutions of pH 7 and 10.
Water content
The water content of crude or purified glycerol was measured following the standard method ISO 2098-1972 by using the Karl- Fisher titrator V20.
Ash content
Ash content was analyzed according to standard method ISO 2098-1972 by burning 1 g of glycerol in muffle furnace at 750°C for 3 h.
Glycerol content
Crude and purified glycerol samples were identified by gas chromatograph, equipped with a mass selective detector [Varian 1200 Quadrupole GC/MS (EI), Varian CP-3800 GC equipped with VF-5 MS column (5% phenyl/95% dimethyl-polysiloxane, 30 m x 0.25 mm x 0.25 μm)], using helium as the carrier gas at a flow rate of 5 x 10-7 m3/s. The oven temperature was maintained at 120°C for 2 min and then increased to 200°C at a ramp rate of 40°C/min. Injector and detector block temperature were maintained at 300°C. The component was identified using the NIST 98 MS library with the 2002 update. The concentration of the glycerol in the samples was analyzed quantitatively on a GC-FID (Shimadzu -2010) under the similar conditions as used for the GC-MS measurement.
Infrared Spectroscopy
Fourier transform infrared spectra (FT-IR) were obtained using the KBr method on a Nicolet Magna-IR 560 spectrometer operating at 1 cm-1 resolution in the 400-4000 cm-1 region.
Metal composition
Inductively coupled plasma- atomic emission spectrometry (ICP-AES) was conducted to quantify the metal content present in the samples, when standard calibration for each metal was made in the concentration range of 0 -400ppm.
UV-Visible spectroscopy
For the crude and purified glycerol samples, their absorbance of light were examined by using Varian Cary 300 Bio UV Visible spectrophotometer (Lab Commerce, Inc. USA).The wavelength of incident light was chosen between 800-200 nm, out of which 800-400 nm accounts for visible light and 400-200 nm accounts for the UV region of light.
In addition, the heating value and viscosity of the glycerol samples were also measured to confirm the purity of the glycerol in the crude and purified glycerol samples.
NMR spectroscopy
1H NMR and 13C NMR (nuclear magnetic resonance) spectra of resin disolved in d6-DMSO were acquired at 25°C on a Varian Inova 600 NMR spectrometer equipped with a Varian 5 mm triple-resonance indirect-detection HCX probe.
Results and Discussion
Crude glycerol analysis
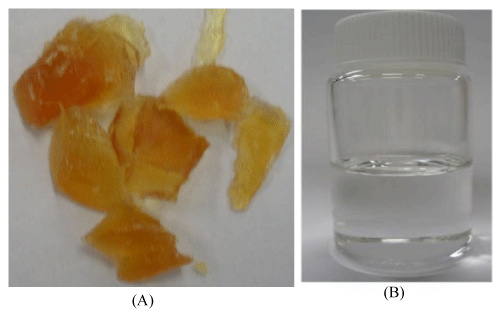
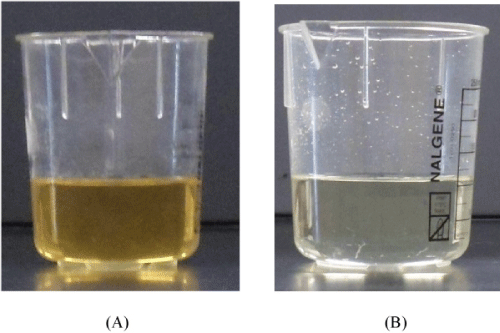
The crude glycerol obtained from the biodiesel plant was a dark brown solid (Figure 2A) with a high pH (10.43) and low density (1.05 g/mL) as compared to the commercially available pure glycerol (Figure 2 B, pH: 6.97, density: 1.26 g/ml). The glycerol content was found to be very low in the range of 12-15 wt%, but it has high matter organic non glycerol (MONG ~ 70 wt%), and high ash (~ 6 wt%) and water (~ 10 wt%) contents (Table 1). The presence of high MONG is due to the presence of soap, methanol and methyl esters generated during the biodiesel production process, and the high ash content was mainly originated from the KOH catalyst during the transesterification process.
Figure 2 : Pictures of crude glycerol (A) and pure glycerol (B).
Properties
Commercial glycerola
Crude glycerol
Density (at 20 °C,g/mL)
1.27 ±0.01
1.05 ± 0.26
pH
6.97 ± 0.03
10.30± 0.26
Water (wt%)
0.01± 0.00
9.20 ± 1.04
Ash (wt%)
0.0 ±0.00
5.6 ± 0.51
Glycerol (wt%)
99.9 ± 0.00
12.0 ± 2.38
MONG (%)
0.0 ± 0.00
70.2± 4.37
Alkalinity
---
56.0 ± 1.02
K (ppm)
870 ± 40
45762 ± 3240
Na (ppm)
28± 10
140.5±23.7
Viscosity (in cp at 50 °C, 250 rpm)
142 ± 1
---
Table 1: Composition and physical properties of various glycerol samples.
The main compounds detected by GC-MS analysis are listed in Table 2. In crude glycerol, propan-1-ol, hexanoic acid, glycerol, octanoic acid, dodecanoic acid, methyl tetradecanoate, 7-hexadecanoic acid, methyl ester, octadecanoic acids are the main components. In purified glycerol the main component was found to be dominantly glycerol (> 96%).
Retention Time (min)
Compounds
Molecular weight (MW)
15.375
Glycerol (propane-1,2,3-triol)
92
29.333
propaneoctanoic acid, 2-hexyl-, methyl ester
282
29.592
methyl tetradecanoate
242
31.108
heptacosanoic acid, methyl ester
424
31.275
tetradecanoic acid, 12-methyl-, methyl ester
256
31.883
methyl stearate
298
33.158
eicosanoic acid, methyl ester
326
33.358
9-octadecenoic acid, methyl ester
296
33.425
9- hexadecenoic acid, methyl ester
268
33.9
hexadecanoic acid, methyl ester
270
35.208
heneicosanoic acid, methyl ester
340
35.208
triacontanoic acid, methyl ester
466
35.925
heptadecanoic acid, methyl ester
284
37.192
9,12- octadecadienoic acid, methyl ester
294
Table 2: Main compounds in crude glycerol detected by GC-MS analysis.
Effects of acid type and pH value
The performance of different mineral acids such as hydrochloric acid, sulfuric acid and phosphoric acid in the purification process was evaluated and compared. In this study, a given amount of crude glycerol was acidified individually as mentioned earlier using different acids to a fixed pH (pH=1) and the reactions are given in the following equations.
Table 3a compares the performance of different acids in purification of crude glycerol, with respect to the glycerol products purity, phase separation time, precipitation time and ash content of the purified glycerol products.
Acids
Gly content
(wt%)
Phase separation time
(min)
Precipitation time (min)
Amount of Ash contents (%)
H3PO4
96 ±1.02
30-45
10-15
1.4 ± 0.31
HCl
93 ±2.00
180-240
120-180
1.6 ± 0.53
H2SO4
94 ±1.06
600-720
120-180
1.7 ± 0.25
Table 3a: Performance of different acids in purification of crude glycerol.
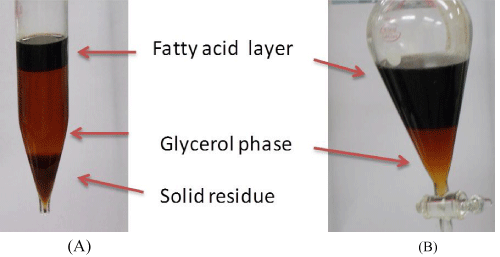
From the above results, all acids resulted in a purified glycerol product of very similar properties such as the glycerol content (96-93 wt%) and ash content (1.4-1.7 wt%). However, the time required for separation of the three distinct phases (glycerol, fatty acids and solid phases) was the shortest with phosphoric acid (30-45 min), medium (180-240 min) with HCl acid and the longest (600-720 min) with H2SO4 acid. Also, the precipitation time was least (10-15 min) with H3PO4 acid. Unlike the precipitates using sulfuric and hydrochloric acids (shown in equations 3 and 4, respectively), the well formed precipitates with H3PO4 acid (equation 2) were found to be easily separated by filtration. This may be attributed to the well developed and poorly soluble phosphate salts in the glycerol phase. Due to its superior performances in the process, phosphoric acid was chosen as acidifying agent for all further works. Moreover, the biogenic nature of phosphorous is an added advantage to the process. Being even better, the obtained phosphates could be directly used as a fertilizer and as buffer solution. The roles of the phosphoric acid in the crude glycerol acidification process may be described in more details as follows. In the first step of purification, crude glycerol was acidified by H3PO4, when the acid reacts with the soap molecules to form free fatty acids and less soluble sodium/potassium salts according to the reaction: The acidification formed three distinct phases as pictured in Figure 3A. The middle glycerol-rich phase was obtained by decantation of solid residues, followed by separation of fatty acid layer from the glycerol rich phase (Figure 3B).
Figure 3 : Photos showing the formation of three phases (A) and separation of purified glycerol phase from fatty acid layer (B).
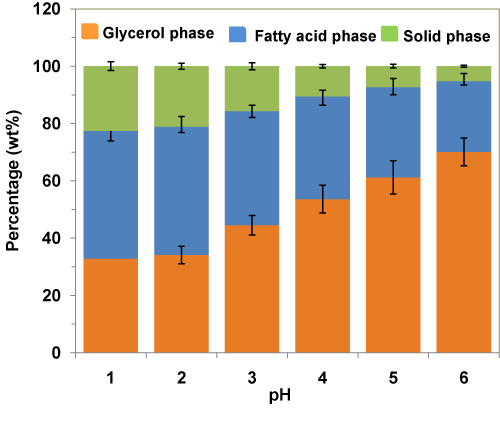
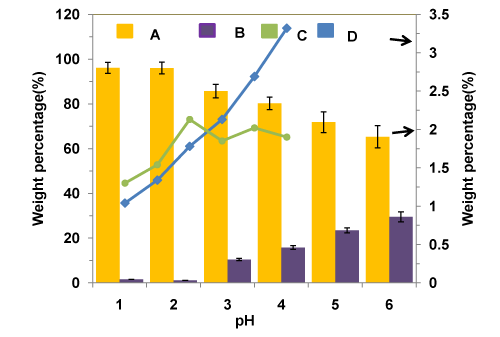
Effects of pH levels on the weight percentages of various phases during acidification of crude glycerol using H3PO4 acid are given in Figure 4. From the figure, it can be seen that decreasing the pH from 6 to 1, in the acidification step led to a decrease in the weight fraction of glycerol-rich phase from 70 wt% to 33 wt%, accompanied by an increase in the weight fraction of fatty acid (from 25 wt% to 45 wt%) and solid residues (from 5 wt% to 23 wt%). This was likely attributed to the fact that under strong acidic conditions, the acid neutralizes almost all the alkali species present in the crude glycerol to precipitateout as solid residue (salt) at the bottom and reacts with the soap to form free fatty acids as the top phase.
Figure 4 : Effects of pH levels on the weight percentages of various phases during acidification of crude glycerol using H3PO4 acid.
The effects of pH on the composition of purified glycerol products are shown in Figure 5. The ash contents of the purified glycerol at any pH values are lower than that of the original crude glycerol (5.6 wt%), as expected. As clearly shown in the Figure, there is a decreasing trend of both ash and MONG contents with decreasing pH (from 6 to 1). More solid phase can be produced while lowering the pH level of the crude glycerol during the acidification step. On the other hand, all purified glycerol products (at all pH values) have a much lower content of MONG (0-30 wt%), compared with approximately 70wt% MONG for the crude glycerol. Thus, a decrease in the pH in the process resulted in a lower content of organic impurities in the purified glycerol products. It should be noted that some short chain and medium chain fatty acids are soluble in the glycerol phase; hence complete elimination of MONG from the purified glycerol products is very difficult. The metal contents (mainly Na and K) of crude glycerol, commercially available glycerol and purified glycerol are given in the Tables 1 and 3. The very high concentration of K in the crude glycerol is owing to the use of alkali catalysts in the biodiesel process.
Figure 5 : Composition of purified glycerol vs. pH (A: Glycerol B; MONG C: Water D: Ash).
Analysis of purified glycerol products
Physical properties
Composition and physical properties of purified glycerol (obtained with H3PO4 acid at pH = 1.0) and commercial glycerol are comparatively shown in Table 3b. All properties including density, pH, water/ash/glycerol/MONG contents, K and Na concentration and viscosity are very similar, suggesting the success of the purification process using acidification.
Properties
Commercial glycerola
Purified glycerol
Density (at 20 °C,g/mL)
1.27 ±0.01
1.26 ± 0.02
pH
6.97 ± 0.03
6.98± 0.06
Water (wt%)
0.01± 0.00
1.30 ± 0.03
Ash (wt%)
0.0 ±0.00
1.04 ± 0.31
Glycerol (wt%)
99.9 ± 0.00
96.0 ±1.02
MONG (%)
0.0 ± 0.00
1.09 ± 0.02
Alkalinity
---
0
K (ppm)
870 ± 40
1165± 110
Na (ppm)
28± 10
82±22.0
Viscosity (in cp at 50 °C, 250 rpm)
142 ± 1
140 ±2
Table 3b: Composition and physical properties of purified glycerol and commercial glycerol.
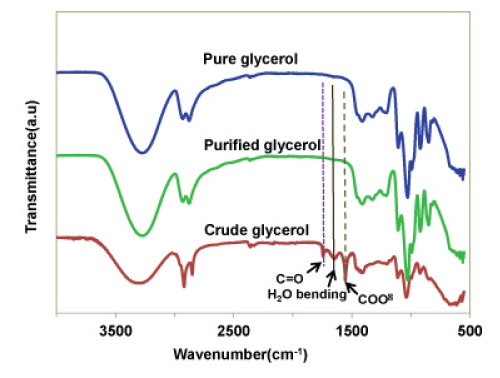
FTIR analysis
The presence of different functional groups in the crude glycerol and purified glycerol were analyzed by FTIR and compared to those of a pure glycerol available commercially (Figure 6). In the crude glycerol, some additional peaks at 1580 cm-1, 1740 cm-1 and 3050 cm-1 were observed. The absorbance peak at 1580 cm-1 clearly indicates the presence of impurities containing carboxylate ions (COO-) (likely originated from soap) in the crude glycerol and the peak at 1740 cm-1 indicates the presence of carbonyl group (C=O) of an ester or carboxylic acids.
Figure 6 : FTIR spectra of pure, purified and crude glycerol.
The FTIR spectrum of the purified glycerol at pH = 1 clearly shows the absence of peaks at 1580, 1740, and 3050 cm-1, indicating the complete removal of impurities like free fatty acid and methyl esters compounds, owing to the fact that the mineral acid could convert the soap molecules to fatty acids to be separated out via phase separation.
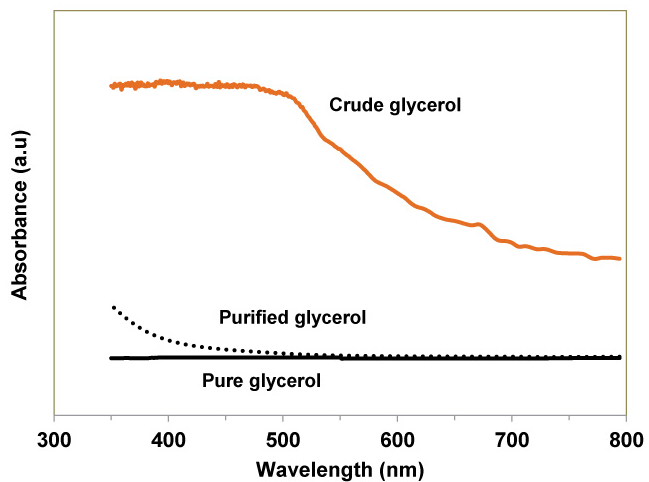
UV-VIS spectral analysis
UV -VIS spectroscopy gives an idea about the color and transparency of the liquid products. The greater the absorbance of radiation, the lesser is the transmittance and therefore the lesser the transparency. The spectroscopic results for crude glycerol, pure glycerol and the purified glycerol (after declaration with activated charcoal) are illustrated in Figure 7. Since pure glycerol is very transparent it has negligible absorbance. On the contrary, due to the presence of contaminants like fatty acids, salts, soap and other impurities, crude glycerol is almost opaque and therefore has a very high absorbance. During purification process of crude glycerol most of the contaminants were removed from the crude glycerol and after activated charcoal declaration treatment most of the impurities were adsorbed. Hence the purified glycerol has an absorbance closer to pure glycerol in visible light region (400-800 nm). The UV-VIS spectra are in agreement with the naked eye observation. Photographs of the purified glycerol before and after activated charcoal declaration treatment are displayed in Figure 8.
Figure 7 : UV-Vis spectra of pure, purified and crude glycerol.
Figure 8 : Purified glycerol before (A) and after (B) charcoal treatment.
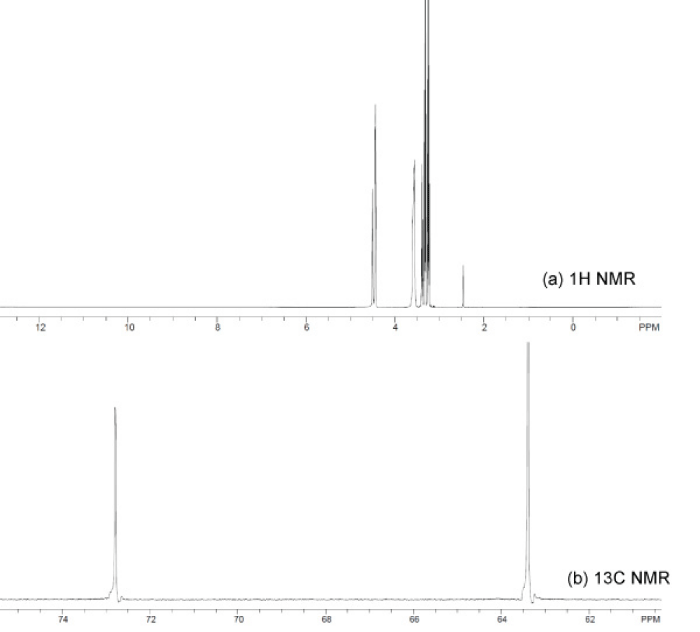
Figure 9 : Spectra of 1H NMR (a) and 13C NMR for the purified glycerol.
NMR spectral analysis
The purity of the purified glycerol was analyzed using 13C and 1H-NMR spectra and the results were compared with that of the pure glycerol available on-line [31]. The 13C-NMR of purified glycerol demonstrated two signals at 63.4 and 72.8ppm for the presence of primary and secondary aliphatic carbon atoms, respectively. The 1H-NMR spectra showed the presence four types of different signals at 4.5, 3.45, 3.4 and 3.3ppm for the hydrogen from hydroxyl groups, secondary carbon atom , and two types of primary carbon atoms respectively. These results reflect that the physicochemical purification demonstrated in this work is efficient enough to enhance the glycerol level in the purified glycerol close to that of the commercial one. Figure-9.
Cost and margin analysis
Cost analysis is of the key factors determining the potential of the crude glycerol purification process for commercialization. Table 4 shows the gross margin analysis for the crude glycerol purification process based on 1.0 tone of purified glycerol, in which the operational cost is not considered. Methanol and activated carbon can be recycled and reused in the process. Thus, the loss of these materials was assumed at 10 wt%. From the Table, for every tonne of purified glycerol produced, the sales revenue of all products (purified glycerol, KH2PO4 and KH2PO4 (as fertilizers)) is $2500, while the total purification cost, considering raw materials costs only, is $1955. This gives a gross profit of $545 per tonne of purified glycerol and a gross margin 21.8%. Moreover, there is potential of using KH2PO4 and K2HPO4 in high-value applications like food additives (in food industry), and the purified glycerol in different value-added applications in diverse fields [32]. This would make the process more economically promising.
Raw materials or products
Unit price ($/t)a
Amount of products produced or raw materials consumed (t)
Sales or costs ($)
Purified glycerol
900
1.0
900
KH2PO4c
1200
0.40
480
K2HPO4c
1400
0.80
1120
Total revenue
2500
Crude glycerol
50d
6.67
334
H3PO4
800
1.07
856
KOH
900
0.20
180
Methanol
400
1.33
532
Activated carbon
750
0.07
53
Total purification cost
1955
Gross profit
545
Gross margin
21.8%
Table 4: Margin analysis for the crude glycerol purification process based on 1.0 tonne of purified glycerol.
Conclusion
Phosphoric acid was found to be the best acidifying agent among the other mineral acids tested for crude glycerol acidification for purification. Glycerol content was increased from approximately 13 wt% in the crude glycerol to > 96 wt% in the purified glycerol products. The density, viscosity, pH and metal contents of the purified glycerol products were analyzed and found to be very close to that of the commercially available pure glycerol. The purity of the purified products was confirmed by FTIR and GC-MS/FID measurements. UV-VIS spectroscopy demonstrated a nearly equal absorbance of the purified glycerol to that of pure glycerol. The biogenic nature of phosphorous, the high value applications of the phosphates with easy scalability of the process make it very promising for commercialization.
Acknowledgement
The authors cordially acknowledge the financial support provided by Imperial Oil via University Research Award and the Discovery Grant from NSERC for one of the authors (C. Xu). We are also thankful to Professors Yasuo Ohtsuka and Guus Van Rossum from Akita University (Japan) and University of Twente (the Netherlands), respectively, for their invaluable suggestions on some aspects of this research.
References
- Behr A, Eilting J, Irawadi K, Leschinski J, Lindner F. Improved utilization of renewable resources: New important derivatives of glycerol. Green Chemistry. 2008; 10: 13-30.
- Lin L, Cunshan Z, Vittayapadung S, Xiangqian S, Mingdong D. Opportunities and challenges for biodiesel fuel. Applied Energy. 2011; 88: 1020-1031.
- Zhou CH, Beltramini JN, Fan YX, Lu GQ. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem Soc Rev. 2008; 37: 527-549.
- Johnson DT, Taconi KA. The glycerol glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007; 26: 338-348.
- Biofuel production 2010-2019. OECD-FAO Agricultural Outlook.
- McCoy M. Glycerol surplus: Plants are closing and New Uses for the chemical are being found. Chem Eng News. 2006; 84: 7.
- Yang F, Hanna MA, Sun R. Value-added uses for crude glycerol--a byproduct of biodiesel production. Biotechnol Biofuels. 2012; 5: 13.
- Sims B. Clearing the way for byproduct quality. Biodiesel Magazine. 2011.
- Nanda MR, Yuan Z, Qin W, Ghaziaskar HS, Poirier M, Xu C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel. 2014; 117: 470-477.
- Kraus G A. Synthetic methods for the preparation of ,3-propanediol. Clean. 2008; 36: 648-651.
- Kurosaka T, Maruyama H, Naribayashi I, Sasaki Y. Production of ,3-propanediol by hydrogenolysis of glycerol catalysed by Pt/WO3/ZrO2. Catal. Commun. 2008; 9: 1360-1363.
- Cheng L, Liu l, Ye P. Acrolein production from crude glycerol in sub- and super- critical water. J. Am. Oil Chem. Soc. 2013; 90: 601-610.
- Ito T, Nakashimada Y, Senba K, Matsui T, Nishio N. Hydrogen and ethanol production from glycerol-containing wastes discharged after biodiesel manufacturing process. J Biosci Bioeng. 2005; 100: 260-265.
- Sabourin-Provost G, Hallenbeck PC. High yield conversion of a crude glycerol fraction from biodiesel production to hydrogen by photofermentation. Bioresour Technol. 2009; 100: 3513-3517.
- Nanda MR, Yuan Z, Qin W, Ghaziaskar HS, Poirier M, Xu C. A new continuous-flow process for catalytic conversion of glycerol to oxygenated fuel additive: Catalyst screening Appl. Energ. 2014; 123: 75-81.
- Nanda MR, Yuan Z, Qin W, Ghaziaskar HS, Poirier M, Xu C. Catalytic conversion of glycerol to oxygenated fuel additive in a continuous flow reactor: Process optimization. Fuel. 2014; 128: 113-119.
- Gan J, Yuan W, Nelson NO, Agudelo SC. Hydrothermal conversion of corn cobs and crude glycerol. Biol. Eng. 2010; 2:197-210.
- Xiu S, Shahbazi A, Shirley VB, Wang L. Swine manure/crude glycerol co-liquefaction: physical properties and chemical analysis of bio-oil product. Bioresour Technol. 2011; 102: 1928-1932.
- Mothes G, Schnorpfeil C, Ackermann J. Production of PHB from crude glycerol. Eng Life Sci. 2007; 7: 475-479.
- Hu S, Wan C, Li Y. Production and characterization of biopolyols and polyurethane foams from crude glycerol based liquefaction of soybean straw. Bioresour Technol. 2012; 103: 227-233.
- Nguyen N, Demirel Y. Biodiesel-glycerol carbonate production plant by glycerolysis. Journal of Sustainable Bioenergy Systems. 2013; 3: 209-216.
- Nguyen N, Demirel Y. A novel biodiesel and glycerol carbonate production plant, Int. Journal of Chemical Reactor Engineering. 2011; 9: 1-25.
- Ferreira MO, deSousa EMBD, Pereira CG. International Journal of Chemical Reactor Engineering. 2013; 11: 1-8.
- Nano cavitation: A proven new concept. 2013.
- Yong KC, Ooi TL, Dzulkefly K, Wan Yunus WMZ, Hazimah AH. Characterization of glycerol residue generated from a palm kernel oil methyl ester plant. Journal of Oil Palm Research. 2001; 13:1-6.
- Kongjao S, Damronglerd S, Hunsom M. Purification of crude glycerol derived from waste used-oil methyl ester plant, Korean J Chem Eng. 2010; 27: 944-949.
- Hazimah A H, Ooi TL, Salmiah A. Recovery ofglycerol and diglycerol from glycerol pitch. Journal of Oil Palm Research. 2003; 15: 1-5.
- Manosak R, Limpattayanate S, Hunsom M. Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process. Technol. 2011; 92: 92-99.
- Ooi TL, Yong KC, Dzulkefly K Wanyunus WMZ, Hazimah AH. Crude glycerine recovery from glycerol residue waste from a palm kernel oil methyl ester plant. J Oil Palm Res. 2001; 13: 16-22.
- Human and Environmental Risk Assessment-2006. Retrieved from : https://www.heraproject.com/files/39-F-06_Sodium_Sulfate_Human_and_Environmental_Risk_Assessment_V2.pdf.
- Available online at SDBS Web: http//sdbs.riodb.aist.go.jp (National Institute of Advanced Industrial Science and Technology) (Accessed on June 27, 2014).
- Javani A, Hasheminejad M, Tahvildari K, Tabatabaei M. High quality potassium phosphate production through step-by-step glycerol purification: A strategy to economize biodiesel production, Bioresour Technol. 2012; 104: 788-790.