Review Article
Austin J Chem Eng. 2014;1(2): 1009.
Overview of CO2 Capture from Flue Gas Streams by Vacuum Pressure Swing Adsorption Technology
Jianghua Ling,sup>1,2
, Augustine Ntiamoah2, Penny Xiao2*, Dong Xu3, Paul A Webley2, Yuchun Zhai11School of Materials and Metallurgy, Northeastern University, China
2Department of Chemical and Bimolecular Engineering, University of Melbourne, Australia
3Guodian New Energy Technology Research Institute, China Guodian Corporation, China
*Corresponding author: Penny Xiao, Department of Chemical and Bimolecular Engineering, University of Melbourne, Victoria 3010, Australia
Received: May 31, 2014; Accepted: August 08, 2014; Published: August 12, 2014
Abstract
With increasing concerns about CO2 emissions and their impact on global warming, CO2 capture technologies have been widely studied. Adsorption technology, as an important process for gas separation, has also been studied for CO2 capture from flue gas for more than two decades. Because the pressure of most flue gas streams is approximately atmospheric, vacuum swing adsorption process (VSA) is preferred. This paper provides an overview of the development of VSA processes for CO2 capture based on commercial adsorbent materials. We discuss the general trends in process performance with respect to adsorbent characteristics, cycle design and operating conditions. We have also discussed the impact of impurities in feed gases on VSA processes and strategies for dealing with the negative impacts. Finally, energy consumption in CO2VSA processes is summarized. The review of process performance is mainly based on simulation and laboratory scale work.
Keywords: CO2 capture; Vacuum swing adsorption; TSA
Introduction
CO2 is a major greenhouse gas and hence, contributes significantly to global warming. The development and deployment of CCS (CO2 capture and storage) technologies is considered the most important option to make much deeper cuts in greenhouse gas emissions, and CO2 concentration of greater than 95% is commonly required for sequestration [1]. Currently three major separation technologies namely absorption, membranes and adsorption are being developed to capture and concentrate CO2 from flue gases for CSS applications [2, 3]. Absorption is the most mature of these technologies, and has long been used for CO2 capture, though not from power plants. However, it results in high energy consumption during the high temperature absorbent regeneration [4].
Adsorption technology is increasingly becoming popular for CO2 capture because of its potential low energy consumption, simple operation, easy maintenance and flexibility in design to meet different demand requirements [5-7]. Each of the different adsorption processes such as TSA (temperature swing adsorption), PSA (pressure swing adsorption), VSA (vacuum swing adsorption), and ESA (electrical swing adsorption) may be most suitable for treating feed gases with different CO2 concentrations [8]. The TSA can be designed to directly utilize cheaper, low-grade thermal energy resources from power plants for regeneration to reduce the operating cost. However, the longer time required for heating/cooling limits its application for CO2 capture. With the long cycle time, productivity will be lower compared to other adsorption technologies. The product may also be diluted by the purge gas if regeneration is performed by direct hot gas purge as used in conventional systems [9-12]. Because the pressure in flue gas streams is approximately equal to 1.0 bar and CO2 concentration in the feed gas is commonly higher than 10%, VSA is considered more economical for CO2 capture than PSA (where significant compression of the feed gas is required) [13,14]. The temperature range of flue gases varies by their sources and pre-treatment processes that may be available. Taking a typical coal-based power plant for instance, the flue gas stream may be available at a temperature ranging from 30 to 80°C or even higher, depending on the extent of heat recovery from the stream [15]. The VSA performance is sensitive to the feed gas temperature, so a further heat treatment may be required to condition the flue gas before feeding it to the VSA plant, which will inevitably impact on the separation efficiency and economics of the process.
Adsorbents play a key role in adsorption technology. The adsorbent determines the overall CO2 capture performance in VSA technology [16,17]. The key elements for a good adsorbent in CO2VSA technology are high selectivity of CO2 over N2, high adsorption capacity of CO2, rapid adsorption/desorption kinetics, stable adsorption capacity after repeated cycles, and adequate mechanical strength of the particles [18]. Many adsorbents with high CO2 adsorption capacity and selectivity have been developed recently such as MOFs, amine modified adsorbents, etc. [19]. While the number of new adsorbent materials reported has proliferated, only a very select few will undergo bench-top testing and even fewer will pass on to pilot testing stage, partly due to limited availability of production materials since large scale production is often not the goal of initial materials research. Therefore, VSA process design for CO2 capture still focuses on commercially available materials such as zeolites, activated carbon and CMS which can be purchased in bulk and tested in pilot or field installations [20-24]. This paper provides a review of VSA process development for CO2 capture, and specifically discusses how adsorbent characteristics, process design and operating conditions impact the overall process performance.
Commercial CO2 Adsorbents
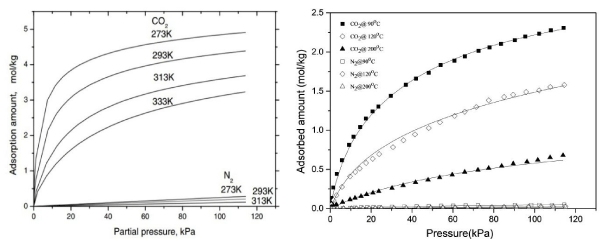
In capture processes of CO2 from flue gas streams, it is assumed that the impurities in the feed gases including water are removed through flue gas pre-treatment processes so that CO2 separation from flue gases can be represented as that from a mixture of CO2 and N2. Zeolite materials have been widely studied for CO2 capture from flue gases in fossil fuel power plants, natural gas and biogas facilities [5,14,25,26]. According to the literature on CO2 capture using different types of natural and various types of synthetic zeolites such as Y, X, A, 13X zeolite shows superior performance for CO2 separation from N2 at relatively low temperatures [27]. Some researchers have considered 5A in TSA processes because of its higher volumetric capacity and high working capacity between low and high temperatures [28,29]. Zeolite 13X, studied for more than two decades, is the benchmark material for CO2VSA systems because of its high working capacity and selectivity for CO2 at the prevailing conditions of the process. From the isotherms of CO2 and N2 on 13X [22,30], both CO2 and N2 adsorption amount increases as their partial pressures increase (Figure 1) in the usual way as expected for a physic-sorbent. As the temperature increases, the adsorption amount for both CO2 and N2 decreases, indicative of exothermic adsorption. Although 13X is a physic-sorbent for CO2 and as such should be restricted to modest temperature application, we have found that it presents a relatively high CO2 working capacity even over 120°C. Therefore, it is an ideal adsorbent for CO2 and N2 separation as it has a wide operating temperature range.
Figure 1 : Isotherms of CO2 and N2 on zeolite 13X at low temperature [22](left) and high temperature [30] (right).
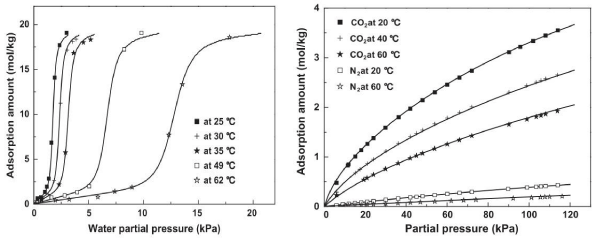
CO2 adsorption capacity on 13X is reduced significantly by water vapor because water is be strongly adsorbed onto the hydrophilic sodium cations within the supercage, displacing sites for CO2 adsorption. As a result, a guard layer must be employed to avoid moisture contamination of 13X zeolite. Traditional activated carbon is also a commonly used adsorbent for CO2 adsorption but contains both hydrophobic and hydrophilic sites on its surface. Water vapor can be adsorbed onto the surface by hydrogen bonding to the surface functional groups. However, this bonding is very weak so that the water vapor can be removed by reducing its partial pressure and as a result activated carbons are often regarded as more suitable for wet flue gas treatment [31]. The isotherms of water vapor and CO2 on activated carbon are shown in (Figure 2) [32]. CO2 adsorption capacity on activated carbon is much lower compared to that of zeolite 13X. However, its ability to moderately withstand water vapor makes it a promising material in the application of CO2 capture from wet (real) flue gases. Current research is focused on modifying the activated carbon surface with desired functional groups in order to improve its CO2 adsorption capacity and selectivity [33, 34].
Figure 2 : Isotherms of water vapor and CO2 on activated carbon [32]: water vapor (left) and CO2 (right).
VSA Process Development
Cycle design
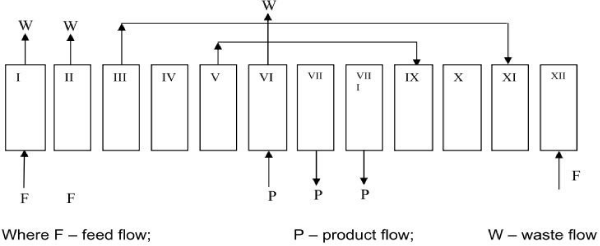
There are basic five steps employed in most VSA process designs [21,26], which include adsorption, desorption, re-pressurization, pressure equalization and product purge (Figure 3). In the adsorption step, CO2 is adsorbed onto the adsorbent packed in the column as the feed gas (flue gas) passes through the column, while CO2-lean gas stream exits to the atmosphere. The amount of CO2 adsorption depends on the operating pressure and temperature. In the desorption step, the adsorbed CO2 is desorbed from the bed and extracted into a CO2-rich gas phase by reducing the pressure to vacuum (or sub-atmospheric) levels. In the re-pressurization step, feed gas or part of the CO2-lean gas is used to pressurize the adsorption bed, from the bottom or top of the bed, until the pressure in the bed is equal to that in the feed step for the next cycle. Pressure equalization is used in multiple-bed VSA systems. A bed at higher pressure (usually after adsorption) transfers gas to a bed at lower pressure (after vacuum desorption) until their pressures equalize. This can bring energy savings by reducing the amount of re-pressurization required by the low-pressure bed, and also improve CO2 product purity. For product purge, part of the extracted product or a CO2-rich gas is used to purge the bed before the desorption step. However, in order to obtain high CO2 product purity, the pressure at the top of the bed needs to be controlled to the same pressure as in the last equalization step. Because CO2 adsorption capacity is much higher than N2, most N2 trapped in the void spaces of the adsorbent or adsorbed in the bed can be displaced by the purge gas, leading to a more pure CO2 product in the subsequent vacuum desorption step.
Figure 3 : VSA cycle design [26].
A light reflux step can also be included in the cycle design [35], but this step must be carefully controlled to avoid breakthrough of the nitrogen into the CO2 product since the proportionate pattern profile in this step will lead to rapid N2 propagation. CO2 adsorption on zeolite 13X is much stronger (and non-linear) than N2 so that the light refluxes gas flow (a N2-rich gas stream) cannot easily clean the CO2 front but rather dilutes the final CO2 product.
Rapid-swing adsorption, characterized by faster cycling permits the adsorbent to be used more frequently and, therefore, leads to CO2 productivity and relatively smaller plant sizes. However, recoveries may be lower due to kinetic limitations. It is often used in O2 concentrators, where high purity and/or recovery rate is not essential and feed gas can be discarded without concern [36]. Therefore, this is not discussed further in this review.
Operating parameters in CO2VSA processes
Optimizing operating parameters is important for the VSA process to achieve satisfactory performance. Pressure and temperature are the major operating parameters, and affect CO2 product purity and recovery by changing working capacity. CO2 working capacity (WC) and selectivity (S) can be estimated from isotherm of single component on adsorbent by equations 1, 2 and 3 below:
WC (mol/kg) = CO2PH - CO2PL (1)
SWC = WCCO2/WCN2 (2)
S = CO2PH/N2PH (3)
Where WC is CO2 working capacity and S is CO2 selectivity; CO2PH is the amount of CO2 that is adsorbed at the highest CO2 partial pressure (i.e. CO2 partial pressure in the feed) and CO2PL is the amount of CO2 that is adsorbed at the lowest CO2 partial pressure (i.e. CO2 partial pressure reached at the end of desorption); SWC is the selectivity for working capacity of CO2 and N2. Although selectivity S is often used to assess adsorbent performance, selectivity based on working capacity is a more accurate assessment metric.
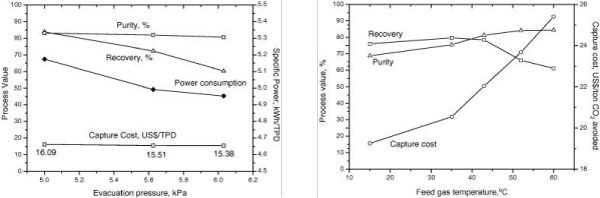
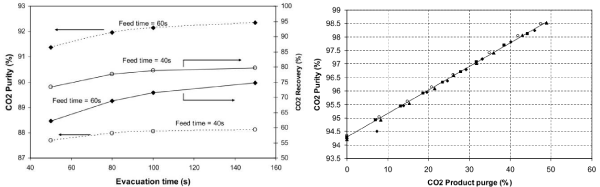
As working capacity varies with CO2 partial pressure, higher pressure at the same CO2 concentration in feed gas will result in an increase of CO2 adsorption in the bed. However, more energy will be required for compressing the feed gas. Therefore, most studies are conducted for constant pressure feed steps. The pressures at the end of vacuum desorption and equalization is most important in VSA testing as shown in Figure 4. During the pressure equalization step, some of the CO2 adsorbed in the sorbent can be released into the gas phase so that N2 in the void spaces of the bed ahead of the CO2 front or even some co-adsorbed N2 can eluted from the bed (which is transferred onto another bed). Thus, after several pressure equalizations, SWC will increase, leading to increase in CO2 product purity [26]. At the same time, the pressure in the bed (before the evacuation step) will decrease, which means the vacuum pump will perform less work during desorption, and this can bring some amount of energy savings. The vacuum pressure applied is very important and it affects both CO2 purity and recovery. Many previous studies [22,26,35,37], have shown that deep vacuum levels are needed to achieve high recoveries and purities for CO2 capture from post-combustion flue gas streams. However, the deeper vacuum requires multistage pump units which can be very expensive and also consume much power. The deep vacuum also results in very large suction and valve line sizes [38].
Figure 4 : CO2 purity and recovery with three-bed CO2VSA system affected by vacuum pressure (left) and operation temperature (right) [22].
Temperature is another important parameter in the VSA system as shown in Figure 4. For zeolite 13X (Figure 1), CO2 working capacity varies at different temperatures: the working capacity is 0.35mol/kg at 0°C as CO2 partial pressure changes from 15kPa to 10kPa, but 0.4mol/ kg at 40°C and 0.35mol/kg at 60°C. Selectivity normally increases as temperature increases in the temperature range from 40 to 90°C on zeolite 13X. Therefore, CO2 purity in CO2 product increases as the operation temperature increases, which is confirmed by our experiments (Figure 4).
Flow-rates in the feeding and desorption steps are also important. Firstly, a high flow-rate will cause large pressure drop (Δp) which can be described by the Ergun equation [39] (equation 4):
Where L is the height of the bed, μ is the fluid viscosity ε is the void space of the bed, u0 is the fluid superficial velocity, dp is the partial diameter and ρ is the density of the fluid.
The pressure drop increases as the velocity increases, which will affect CO2 adsorption and also desorption. Higher pressure is required to overcome the pressure drop for the feed gas to reach a higher flow-rate and the pressure drop also causes insufficient vacuum level at the top of bed during desorption step so that adsorbed CO2 cannot be desorbed effectively. The flow-rate also impacts adsorption/ desorption kinetics. CO2 adsorption on 13X beads is macropore diffusion controlled, both under Knudsen and molecular diffusion regimes [40]. Thus, the flow-rate will affect CO2 recovery during VSA process as well (Figure 5 left). The data in Figure 5 left were obtained by fixing the level of vacuum pressure reached at the end of desorption, while varying desorption time, so that velocity decreases as desorption time increases. Improvements in product purity by CO2 product purge also depend on the amount of product used for purge [26,35]. Our study [26] found that CO2 product purity increased almost linearly from 94.3 to 98.5% when the CO2 purge percentage increased from 0 to 50% (Figure 5 right).
Figure 5 : CO2VSA performance affected by flow-rates and CO2 product percentage [26].
Re-pressurization also affects the process performance. The source of the re-pressurization gas can be either the feed gas or the exhausted gas (CO2-lean gas). Re-pressurization using CO2-lean gas can improve CO2 product recovery [35]. Because the CO2-lean gas can push the CO2 front downward to clean the top of the bed, less CO2 is emitted from the bed during the next adsorption step. However, the effect of re-pressurization is diminished for multiple-bed VSA systems. After several pressure equalizations between the beds, less gas is required for re-pressurization because of the small gap in pressures after equalization and adsorption steps.
CO2 Capture from Different Sources (Different Inlet CO2 Concentrations)
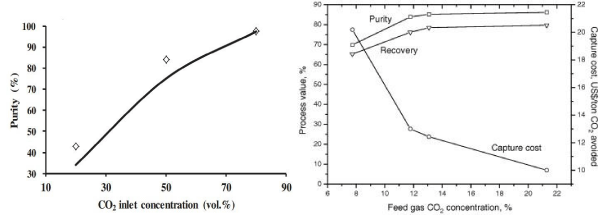
A typical flue gas from a fossil fuel power plant contains CO2 of 10-15% and this emission represents about 50% of all greenhouse gas emissions [41]. With a well-designed VSA process and a good adsorbent such as zeolite 13X, a high CO2 purity at a high recovery rate can be obtained from flue gases for CCS applications [22,37]. However, a much deeper vacuum pressure <= 5kPa (with higher power consumption), or a two-stage VSA process (which also lead to additional equipment cost) would be needed in order to reach such high CO2 product purity and recovery [6,14]. Apart from CO2 emissions from fossil fuel power plants, there are also emissions from other industrial sources where CO2 concentrations vary over a large range: 15-33% from cement industries, 20-30% from iron and steel industries [42]. The VSA process will show better results when employed to capture CO2 from these sources. CO2 product recovery increases at the same vacuum pressure as CO2 concentration in the feed gas increases (Figure 6 left) [43]. Deep vacuum pressure during desorption steps in CO2VSA processes may be avoided when dealing with the gas feeds with higher CO2 concentrations because of their higher working capacities. Thus, capture cost will decrease as CO2 concentration in feed gas increases (Figure 6 right) [22].
Figure 6 : CO2VSA performance at varying CO2 inlet concentration [22,43].
Effects of Impurities on the CO2VSA Process Performance
Water vapor, SOx and NOx are common impurities in flue gases from power plants, and these negatively affect CO2 adsorption capability on zeolites [44,45]. Thus, capturing CO2 from flue gas at coal-fired power stations by pressure/vacuum swing adsorption may be complicated by the existence of these impurities when commercial CO2 selective adsorbents are used. Conventional process designs rely on using a pre-treatment process to remove water, SOx and NOx, which adds considerably to the overall cost.
A wash tower is used to reduce the concentration levels of SOx and NOx in our CO2 capture demonstration plant [46]. The trace amounts still remaining may not significantly affect the CO2 capture process [47,48]. This leaves water vapour as the main impurity and therefore, the discussion on this section focuses on CO2 capture in the presence of water vapour. Flue gas streams usually contain 8-10% water vapour.
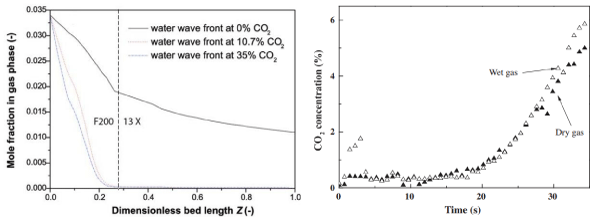
In study conducted in our research group [49], a multiple adsorbent layered column was designed to remove water and CO2 at the same time from the feed gas. The first layer can be a water reversible adsorbent such as sorbead or activated alumina. This protects the main layer-13X from the negative effects of water vapour on its performance [47,49-51]. Higher CO2 concentration can also help remove water by internal purge during vacuum desorption (Figure 7 left). As discussed above, activated carbon shows good performance in the presence of water vapour [32]. We found that the effect of water vapour on CO2 adsorption is not significant on activated carbon, and CO2 can accumulate at the bottom of bed due to its high adsorption. However, more energy is required to remove water from the bed. Figure 7 right shows CO2 concentration in the bed outlet gas during the adsorption step for dry gas and wet gas containing 4.6% water.
Figure 7 : Water and CO2 concentration in double layered adsorption bed (left) [49] and CO2 concentration in emission gas using activated carbon as adsorbent (right) [32].
Power Consumption
Power consumption in PSA/VSA is often calculated according to adiabatic power law [26].
where Qvac is an instantaneous flow-rate (m3/s) and Δtis time interval (s); η (pump coefficient) equals 0.7 and k (specific heat ratio) is 1.28 for CO2 and 1.4 for air, Qfeed represents an instantaneous flue gas flow rate (m3/s), Patm (atmospheric pressure 101.325 kPa), Pfeed and Pvac (kPa) represent instantaneous pressures during adsorption (feeding) and desorption (vacuum) stages of the process. In this equation, the vacuum power is made up of two parts: power due to desorption and power due to the product purge step (to keep the bed pressure equal to that in the last step).
Most power in VSA process is consumed by vacuum pumps during desorption. The value increases with deeper vacuum desorption. However, CO2 productivity also increases with deeper vacuum levels. Thus, there is an optimum value of power consumption in the VSA process [26,35]. Measured power consumption is much higher than calculated or simulated values. It is found that the measured power consumption of VSA processing corresponded to theoretical values based on 30% pump efficiency, whereas 70% is often assumed in calculations [37]. From single stage CO2VSA, deep vacuum is conducted in order to obtain CO2 product purity of 95%. It must be noted that pump efficiency, ?, may decrease with deeper vacuum level because volumetric velocity decreases nonlinearly as deeper vacuum levels are required. When the system is operated at moderate vacuum pressure, the error between experimental result and calculation value may be less.
There are no operating large scale commercial CO2 capture processes by adsorption technology so far. Published estimates for CO2 capture power vary widely, mainly as a result of different assumptions regarding technical factors related to plant design and operation. We show some of the published energy consumption of CO2VSA processes in Table 1.
Process
type
Purity, %CO2
Recovery, %CO2
Sp. power,
MJ/kg CO2
Reference
VPSA
90
85
1.674
[52]
VPSA
85
79
2.37[exp]
[53]
VPSA
85
79
0.535[sim]
[53]
2- stage VPSA
96
91
0.6444
[14]
2- stage VPSA
95.3
74.4
0.7236
[54]
VSA
93
90
0.432
[55]
Duplex PSA
97.5
0.746
[56]
VSA
90 -95
60 - 70
0.518-0.864
[22]
2-stage VPSA
2.455
[57]
2-stage VPSA
96.54
93.35
0.528
[58]
Table 1: Purity, recovery and specific power consumption of some VSA studies for CO2 capture.
Conclusions and Future Development
CO2 product of high purity can be obtained from fossil fuel flue gas streams, at high recovery rates using VSA cycles; however, very low vacuum pressure levels are required during the desorption step. Two-stage VSA processes can be used, which may avoid operating at deeper vacuum with each unit operating at moderate vacuum pressure. However, capital cost will increase because of the extra VSA unit. TVSA may be promising (VSA with slight increase in bed temperature during desorption) since it combines the merits of rapid vacuum swing and effects of temperature rise on the adsorption equilibrium. In this case, the system will not need to operate at deep vacuum. High performance may be generally achieved at intermediate vacuum levels as CO2 concentration in feed gas streams increases. Therefore, VSA technology is very promising for CO2 capture from CO2 sources where the inlet CO2 concentrations are higher such as in cement, iron and steel, etc. manufactories.
Both multiple-layered adsorption beds and adsorbents with hydrophobic characteristics can be applied for eliminating the negative impact of impurities, such as water vapour, traced SOx and NOx, on CO2 capture performance, but more energy may be consumed for removing the collected impurity from the system. Therefore, new adsorbents, which have higher CO2 working capacity, high selectivity, and the characteristics of easy desorption and high tolerance for impurities are still required.
References
- EASAC. CO2 Capture and Storage (CCS) in energy-intensive industries: The Advisory Council of the European Technology Platform for Zero Emission Fossil Fuel Power Plants. 2013.
- D'Alessandro DM, Smit B, Long JR. Carbon dioxide capture: prospects for new materials. Angew Chem Int Ed Engl. 2010; 49: 6058-6082.
- Duke MC, Ladewig B, Smart S, Rudolph V, Diniz da Costa JC. Assessment of postcombustion carbon captures technologies for power generation. Front Chem Eng Chin. 2010; 4: 184-195.
- Yu CH, Huang CH, Tan CS. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual Res. 2012; 12: 745-769.
- Chue KT, Kim JN, Yoo YJ, Cho SH, Yang RT. Comparison of activated carbon and zeolite 13X for CO2 recovery from flue gas by pressure swing adsorption. Ind Eng Chem Res. 1995; 34: 591-598.
- Park JH, Beum HT, Kim JN, Cho SH. Numerical analysis on the power consumption of the PSA process for recovering CO2 from flue gas. Ind Eng Chem Res. 2002; 41: 4122-4131.
- Zhang J, Webley PA. Cycle development and design for CO2 capture from flue gas by vacuum swing adsorption. Environ Sci Technol. 2008; 42: 563-569.
- Grande CA, Ribeiro RP, Rodrigues AE. Challenges of electric swing adsorption for CO(2) capture. ChemSusChem. 2010; 3: 892-898.
- Pirngruber GD, Cassiano-Gaspar S, Louret S, Chaumonnot A, Delfort B. Amines immobilized on a solid support for postcombustion CO2 capture-A preliminary analysis of the performance in a VSA or TSA process based on the adsorption isotherms and kinetic data. Energy Procedia. 2009; 1: 1335-1342.
- Zaman M, Lee JH. Carbon capture from stationary power generation sources: A review of the current status of the technologies. Korean J Chem Eng. 2013; 30: 1794-1526.
- Clausse M, Bonjour J, Meunier F. Adsorption of gas mixtures in TSA adsorbers under various heat removal conditions. Chem Eng Sci. 2004; 59: 3657-3670.
- Clausse M, Bonjour J, Meunier F. Influence of the Presence of CO2 in the Feed of an Indirect Heating TSA Process for VOC Removal. Adsorption. 2003; 9: 77-85.
- Meisen A, Shuai X. Research and development issues in CO2 capture. Energy Convers Mgrnt. 1997; 38: 37-42.
- Liu Z, Grande CA, Li P, Yu J, Rodrigues AE. Multi-bed Vacuum Pressure Swing Adsorption for carbon dioxide capture from flue gas. Sep Purif Technol. 2011; 81: 307-317.
- Rackley SA. Carbon Capture and Storage. 1st ed. Oxford: Butterworth-Heinemann. 2010.
- Harlick PJE, Tezel FH. An experimental adsorbent screening study for CO2 removal from N2. Microporous and Mesoporous Materials. 2004; 76: 71-79.
- Chaffee AL, Knowles GP, Liang Z, Zhang J, Xiao P, Webley PA. CO2 capture by adsorption: Materials and process development. Int J Greenh Gas Con. 2007; 1: 11-18.
- Yang RT. Adsorbents: fundamentals and application. New Jersey: John Wiley & Sons. 2003.
- Wang Q, Luo J, Zhong Z, Borgna A. CO2 capture by solid adsorbents and their applications: current status and new trends. Energy Environ Sci. 2011; 4: 42-55.
- Siriwardane RV, Shen MS, Fisher EP, Poston JA. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels. 2001; 15: 279-284.
- Na BK, Lee H, Koo KK, Song HK. Effect of rinse and recycle methods on the pressure swing adsorption process to recover CO2 from power plant flue gas using activated carbon. Ind Eng Chem Res. 2002; 41: 5498-5503.
- Zhang J, Webley PA, Xiao P. Effect of process parameters on power requirements of vacuum swing adsorption technology for CO2 capture from flue gas. Energy Convers Manage. 2008; 49: 346-356.
- Plaza MG, Garcìa S, Rubiera F, Pis JJ, Pevida C. Post-combustion CO2 capture with a commercial activated carbon: Comparison of different regeneration strategies. Chem Eng J. 2010; 163: 41-47.
- Plaza MG, Pevida C, Pis JJ, Rubiera F. Evaluation of the cyclic capacity of low-cost carbon adsorbents for post-combustion CO2 capture. Energy Procedia. 2011; 4: 1228-1234.
- Cavenati S, Grande CA, Rodrigues AE. Separation of CH4, CO2 and N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem Eng Sci. 2006; 61: 3893-3906.
- Xiao P, Zhang J, Webley P, Li G, Singh R, Todd R. Capture of CO2 from flue gas streams with zeolite 13X by vacuum-pressure swing adsorption. Adsorption. 2008; 14: 575-582.
- Samanta A, Zhao A, Shimizu GKH, Sarkar P, Gupta R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind Eng Chem Res. 2012; 51: 1438-1463.
- Liu Z, Grande CA, Li P, Yu J, Rodrigues AE. Adsorption and Desorption of Carbon Dioxide and Nitrogen on Zeolite 5A. Sep Scie Technol. 2011; 46: 434-451.
- Merel J, Clausse M, Meunier F. Experimental Investigation on CO2 Post-Combustion Capture by Indirect Thermal Swing Adsorption Using 13X and 5A Zeolites. Ind Eng Chem Res. 2008; 47: 209-215.
- Xiao G, Xiao P, Lee S, Webley PA. CO2 capture at elevated temperatures by cyclic adsorption processes. RSV Adv. 2012; 2: 5291.
- Rodrìguez-Mirasol J, Bedia J, Cordero T, Rodrìguez JJ. Influence of Water Vapor on the Adsorption of VOCs on Lignin-Based Activated Carbons. Sep Sci Technol. 2005; 40: 3113-3135.
- Xu D, Xiao P, Zhang J, Li G, Xiao G, Webley PA, et al. Effects of water vapour on CO2 capture with vacuum swing adsorption using activated carbon. Chem Eng J. 2013; 230: 64-72.
- Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A. A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrolysis. 2010; 89: 143-151.
- Caglayan BS, Aksoylu AE. CO2 adsorption on chemically modified activated carbon. J Hazard Mater. 2013; 252-253: 19-28.
- Haghpanah R, Nilam R, Rajendran A, Farooq S, Karimi IA. Cycle synthesis and optimization of a VSA process for postcombustion CO2 capture. AIChE J. 2013; 59: 4735-4748.
- Chai SW, Kothare MV, Sircar S. Rapid Pressure Swing Adsorption for Reduction of Bed Size Factor of a Medical Oxygen Concentrator. Industrial & Engineering Chemistry Research. 2011; 50: 8703-8710.
- Krishnamurthy S, Rao VR, Guntuka S, Sharratt P, Haghpanah R, Rajendran A, et al. CO2 capture from dry flue gas by vacuum swing adsorption: A pilot plant study. AIChE J. 2014; 60: 1830-1842.
- Webley PA. Adsorption technology for CO2 separation and capture: a perspective. Adsorption. 2014; 20: 225-231.
- Welbley PA, He J. Fast solution-adaptive finite volume method for PSA VSA cycle simulation; 1 single step simulation. Comput Chem Eng. 2000; 23: 1701-1712.
- Hu X, Mangano E, Friedrich D, Ahn H, Brandani S. Diffusion mechanism of CO2 in 13X zeolite beads. Adsorption. 2014; 20: 121-135.
- International Energy Agency. CO2 Emissions from Fuel Combustion-Highlights. 2012.
- Gale J. IPCC Special Report on Carbon dioxide Capture and Storage, Chapter 2: Sources of CO2. 2005 ed: Cambridge University Press. 2005; 105-178.
- Huang Q, Eic M. Commercial adsorbents as benchmark materials for separation of carbon dioxide and nitrogen by vacuum swing adsorption process. Sep Purif Technol. 2013; 103: 203-215.
- Li G, Xiao P, Webley P, Zhang J, Singh R, Marshall M. Capture of CO2 from high humidity flue gas by vacuum swing adsorption with zeolite 13X. Adsorption. 2008; 14: 415-422.
- Sayari A, Belmabkhout Y, Serna-Guerrero R. Flue gas treatment via CO2 adsorption. Chem Eng J. 2011; 171: 760-774.
- CO2CRC. Australian Government's Cooperative.
- Zhang J, Xiao P, Li G, Webley PA. Effect of flue gas impurities on CO2 capture performance from flue gas at coal-fired power stations by vacuum swing adsorption. Energy Procedia. 2009; 1: 1115-1122.
- Ruiz E, Sánchez JM, Marono M, Otero J. CO2 capture from PCC power plants using solid sorbents: Bench scale study on synthetic gas. Fuel. 2013; 114: 143-152.
- Li G, Xiao P, Zhang J, Webley PA, Xu D. The role of water on postcombustion CO2 capture by vacuum swing adsorption: Bed layering and purge to feed ratio. AIChE J. 2014; 60: 673-689.
- Xu D, Xiao P, Li G, Zhang J, Webley P, Zhai Y. CO2 Capture by Vacuum Swing Adsorption Using F200 and Sorbead WS as Protective Pre-layers. Chin J Chem Eng. 2012; 20: 849-855.
- Xu D, Guo H, Liu HQ, Zhang J, Xiao P. CO2 and H2O Capture from High Humidity Flue Gas by Single Multi-Layer Vacuum Swing Adsorption Unit. Adv Mater Res. 2012; 529: 402-407.
- Agarwal A, Biegler LT, Zitney SE. A superstructure-based optimal synthesis of PSA cycles for post-combustion CO2 capture. AIChE J. 2010; 56: 1813-1828.
- Liu Z, Wang L, Kong X, Li P, Yu J, Rodrigues AE. Onsite CO2 Capture from Flue Gas by an Adsorption Process in a Coal-Fired Power Plant. Ind Eng Chem Res. 2012; 51: 7355-7363.
- Shen C, Liu Z, Li P, Yu J. Two-Stage VPSA Process for CO2 Capture from Flue Gas Using Activated Carbon Beads. Ind Eng Chem Res. 2012; 51: 5011-5021.
- Delgado JA, Uguina MA, Sotelo JL, Águeda VI, Sanz A, Gómez P. Numerical analysis of CO2 concentration and recovery from flue gas by a novel vacuum swing adsorption cycle. Comput Chem Eng. 2011; 35: 1010-1019.
- Sivakumar SV, Rao DP. Modified Duplex PSA. 1. Sharp Separation and Process Intensification for CO2-N2-13X Zeolite System. Ind Eng Chem Res. 2011; 50: 3426-3436.
- Cho SH, Park JH, Beum HT, Han SS, Kim JN. A 2-stage psa process for the recovery of CO2 from flue gas and its power consumption. Stud Surf Sci Catal. 2004; 153: 405-410.
- Wang L, Liu Z, Li P, Wang J, Yu J. CO2 capture from flue gas by two successive VPSA units using 13XAPG. Adsorption. 2012; 18: 445-459.