
Research Article
Austin Chem Eng. 2023; 10(2): 1100.
Ultrasonic Method on the Extraction of Fucoxanthin from Marine Macro Algae Padina Australis
L Antony Catherine Flora¹; Kirubanandan Shanmugam²*; Renganathan Sahadevan³
¹Department of Chemical Engineering, AC Tech, Anna University, India
²Saveetha School of Engineering, SIMATS, Thandalam, India
³Department of Biotechnology, A.C.Tech, Anna University, India
*Corresponding author: Kirubanandan Shanmugam Saveetha School of Engineering, SIMATS, Thandalam, Chennai 602107, India. Email: Kirubanandan.shanmugam@gmail.com
Received: July 05, 2023 Accepted: August 07, 2023 Published: August 14, 2023
Abstract
Macro algae is the potential feedstock of fucoxanthin production. Fucoxanthin is a bioactive pigment found in the chloroplasts of marine algae and have a considerable pharmacological property. Fucoxanthin belongs to carotenoid which plays as an important ingredient in food and pharmaceutical industries. This study explains effect of ultrasonics on the extraction of fucoxanthin from marine macro algae Padina australis. Additionally, the effect of solvents and various extraction methods for effective isolation of fucoxanthin from algae have been investigated and compared. Method 1 of Extraction of fucoxanthin from Padina australis would give 8.12 mg of fucoxanthin/g of biomass with methanol, chloroform and methanol mixture (1:1 (v/v)) and then with chloroform. The ultrasonic assisted on the method 1 would yield 16.9 mg of fucoxanthin/g of biomass confirming that the ultrasonics increased the rate of extraction than that of extraction without ultrasonics. Thus, Ultrasonic assisted extraction is a potential method for isolation of fucoxanthin from marine macro algae Padina australis effectively.
Keywords: Padina australis; Fucoxanthin; Ultrasonics and type of methods for extraction
Introduction
Seaweeds or marine macro algae are potential renewable resources for many bioactive compounds in the marine environment. Seaweeds are photosynthetic-like plants that form the basic biomass in the intertidal zone. About 6000 species of seaweeds have been found and grouped into different classes as green (Chlorophytes), brown (Pheophytes) and red (Rhodophytes) algae depending on their nutrient, pigments and chemical composition. Like other plants, seaweeds have various inorganic and organic substances which can help human health. Seaweeds are a source of bioactive compounds as they can produce a great variety of secondary metabolites characterized by a broad spectrum of biological activities.
Compounds with antioxidant, antiviral, antifungal and antimicrobial activities are found in brown, red and green algae. The lipid content of seaweeds is low compared with that of microalgae. However, the lipids, especially brown seaweed lipids, have many types of bioactive compound such as omega-3 PUFAs, fucoxanthin, fucosterol and polyphenols. Among them, fucoxanthin is a key nutraceutical of brown seaweeds, since it shows specific physiological effects based on unique molecular mechanisms. Fucoxanthin is a major carotenoid present in the chloroplasts of brown seaweeds. It is the most abundant of all carotenoid, accounting for more than 10% of the estimated total natural production of carotenoid. It forms a complex with chlorophyll–protein and plays an important role in light harvesting and photo protection for effective light use and up-regulation of photosynthesis.
Fucoxanthin has a unique structure with an unusual allenic bond and 5, 6-monoepoxide in its molecule. Of 700 naturally occurring carotenoid, about 40 have this allenic bond. The principal allenic carotenoid are fucoxanthin from brown seaweeds, perinijin from microalgae, neoxanthin from higher plants and the fucoxanthin metabolites fucoxanthinol and amarouciaxanthin. Due to its distinct structure including an unusual allenic bond, fucoxanthin acts as antioxidant under anoxic conditions, when other carotenoids have no quenching abilities. These properties are very rare among naturally occurring food-derived products. Fucoxanthin had a variety of effects on human health such as anti carcinogen, anti inflammation, antioxidant and anti-obesity. A strong inhibitory effect on prostate cancer has been reported for fucoxanthin. The high anticancer activity of this carotenoid may be attributed to its antioxidant activities and its effect on biomolecules related to apoptosis. In addition, fucoxanthin has higher activity on human colon cancer cells compared to another carotenoid such as β-carotene and astaxanthin. Studies on fucoxanthin indicate that it has potential commercial value because it might be used to treat obesity and could reduce the risk of certain disorders such as type 2 diabetes through the promotion of uncoupling protein. Moreover, fucoxanthin can protect the liver, and blood vessels of the brain, bones, skin and eyes. Although chemical synthesis of fucoxanthin is possible there are some disadvantages such as high cost and low recovery, while brown algae have more than 3–10 g kg-1 fucoxanthin.
The occurrence of algal blooms disturbs the ecosystems by modifying food chains and faunal community structure. The accumulation of algal biomass moving natural communities of sea grasses and higher plants [9]. Similarly, macro algal blooms cause changes in the main biogeochemical cycles of C, N, P and S [10]. As a result of this problem, there is much attention needed to clean up the macro algae biomass. Hence this biomass is used for the extraction of fucoxanthin. As a result, utilization of marine macro algae for fucoxanthin extraction provides dual benefits. It serves as a biomass for the extraction of fucoxanthin and protects our environment from detrimental effects.
The aim of this preliminary study is to investigate and evaluate the fucoxanthin content of, Padina australis. This paper investigates to determine the extraction method for fucoxanthin from marine macro algae Padina australis and to determine the effect of ultrasonication assisted extraction of fucoxanthin from seaweed Padina australis.
Materials and Methods
Materials
Organic solvents of analytical grade were bought, and they were used for extraction.
Methodology
Collection of Sample
Samples of brown seaweed Padina australis, was collected from Rameshwaram sea coast and these samples were taken.
Scientific classification of Padini australis
Kingdom Chromista
Phylum Ochrophyta
Class Phaeophyceae
Order Dictyotales
Family Dictyotaceae
Genus Padina
Species australis
Padina australis is found mostly on reef flats and tide pools, in places with plenty of sunlight and shallow water. These algae is light brown in color, with white tips because of the calcium in it. It has flat round blades that are rolled into circular disks. It grows by itself, or on rocks. It has fine little hairs on the edges of the blades.
Preparation of Sample
Samples obtained were first washed with fresh water to remove dirt’s such as sand, rock and mud, and then they were drained. All samples were packed in black polyethylene bags and put in cool box before transported to laboratory for further analysis.
Selection of Extraction Method
Extraction of fucoxanthin from marine macro algae Padina australis in dried powder form was carried out using three different methods as described below.
Method I
The extraction procedure used in this experiment has been proposed [1]. Padina australis algae collected were washed thoroughly with freshwater, transported to the laboratory immediately in an iced condition and shade dried (38±2oC) in a drier for about 20 hour. The shade dried seaweeds were powdered and used for further experiments. First extraction of each seaweed was prepared by pouring methanol into the bottle having 50 g of seaweed powder at the ratio of 10:1 (v/w), the mixture was kept under orbital shaking incubator at room temperature (29±2oC) for 24 h under dark condition. Likewise, second extraction with chloroform and methanol mixture (1:1 (v/v)) and third extraction with chloroform were prepared from the same powder. Three extracts (standing for both lower polar, polar and non-polar components) of each sample were pooled together and evaporated under reduced pressure using rotary flash evaporator. The crude extract of each sample was weighed. Resulting fractions including aqueous were evaporated to dryness.
Method II
A slight modification of the method described [5] was adopted for the extraction and purification of fucoxanthin. Padina australis algae collected were washed thoroughly with freshwater, transported to the laboratory immediately in an iced condition and shade dried (38±2oC) in a drier for about 20 hour. The shade dried seaweeds were powdered and used for further experiments. Acetone-methanol (7:3 v/v) was added to a 1 L flask containing dried and ground brown seaweed Padina australis. These mixtures were homogenized on ice for 10-15 min, and then mixtures filtered through a filter paper. The steps were repeated at least three times. The acetone: methanol extracts were pooled and left at room temperature and in the dark. The extract was evaporated to dryness at 30 to 35°C on a rotary evaporator, and the residue was dissolved in methanol.
Method III
The extraction procedure used here has been propsosed [3]. The marine algae Padina australis was collected. The samples were washed three times with tap water to remove the salt, epiphytes, and sand attached to the surface, then carefully rinsed with fresh water, and maintained in a medical refrigerator at 20oC. Then, the frozen samples were lyophilized and homogenized with a grinder prior to extraction. The marine algae samples were pulverized into powder using a grinder. The algal powder (1g) was extracted with 80% methanol (100 ml) at room temperature for 24h and filtrated. After filtration, the methanolic extracts were evaporated to dryness under vacuum.
Ultrasonication Assisted Exraction
Cell disruption is often necessary for recovering intracellular products from biomass. Disruption greatly enhances the bioavailability of and the assimilation of the pigments from the cells.The dried and powdered marine macro algae Padina australis was ultrasonicated while extracting to improve the extraction yield of fucoxanthin. The enhancement in extraction obtained by using ultrasound is mainly attributed to the effect of acoustic cavitations produced in the solvent by the passage of an ultrasound wave. Ultrasound also exerts a mechanical effect, allowing greater penetration of solvent into the tissue, increasing the contact surface area between the solid and liquid phase. As a result, the solute quickly diffuses from the solid phase to the solvent.
The three extraction methods for fucoxanthin were performed with the aid of ultrasonication. 1g of dried powdered marine macro algae was kept in a glass flask and the volume was made upto 10 ml with the extraction solvent. The solid to solvent ratio used was 1:10. Contents were mixed using magnetic stirrer. Ultrasound-assisted extraction was performed in a sonication water bath. The working frequency and power were fixed at 40 kHz and 250 W respectively. The extract was filtered and then it was analysed for fucoxanthin content.
Species Selection
The species of marine macro algae is Padina australis, used for extraction. The extraction was carried out using the optimal method selected from the above three methods with the aid of ultrasonication. The extract was analysed to determine the fucoxanthin content.
Selection of Solvent System
Different solvent system for extraction was selected from literature and the extraction was carried out using the optimal extraction method selected from above three methods with the aid of ultrasonication. The nine-different solvent systems selected are listed below. The final extract was then analysed for fucoxanthin extraction.
S.NO
SOLVENT
RATIO / PERCENTAGE
1
Methanol
-
2
Chloroform
-
3
Methanol and Chloroform
1:2
4
Acetone and Methanol
7:3
5
Aqueous Methanol
80%
6
Acetone
-
7
n-hexane
-
8
Ethyl Acetate
-
9
Ethanol
-
Table 1: Different Solvent System selected.
Results and Discussion
Effect of Extraction Methods
Fucoxanthin pigment was extracted from the marine macro algae Padina australis using three different methods and the amount of fucoxanthin extracted was found out. Figure 4 shows the amount of fucoxanthin extracted Padina australis using three different methods. From this it can be observed that high content of fucoxanthin can be extracted by Method I.

Figure 1: Padina australis.
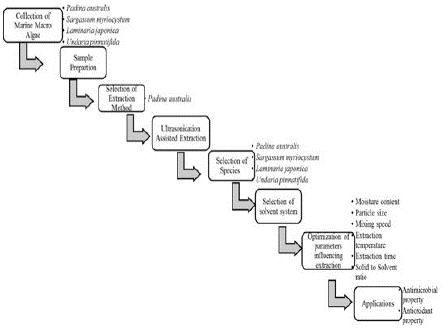
Figure 2: Experimental Procedure for fucoxanthin from marine macro algae Padina australis.
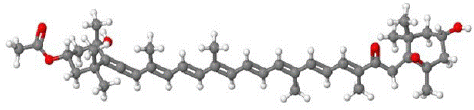
Figure 3: Structure of fucoxanthin- Molecular Weight of 658.92 g/mol and formula of C42H58O6.

Figure 4: Dried Algal biomass.

Figure 5: Fucoxanthin Extract.
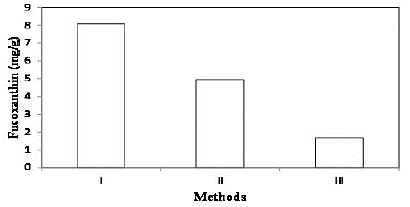
Figure 6: Fucoxanthin extracted using different methods.
Ultrasound Assisted Extraction
The three different extraction methods where again carried out using the aid of ultrasonication and it was found that ultrasonication increased the fucoxanthin yield to two fold times as shown in Figure 7. Ultrasound exerts a mechanical effect, allowing greater penetration of solvent into the tissue, increasing the contact surface area between the solid and liquid phase. As a result, the solute quickly diffuses from the solid phase to the solvent.
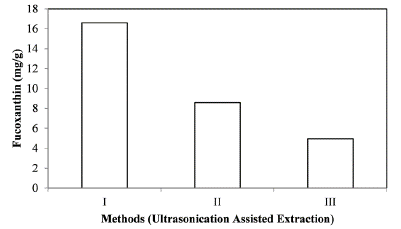
Figure 7: Fucoxanthin extracted using different methods assisted by ultrasonication.
Conclusion
Extraction of fucoxanthin from marine macro algae padina australis using ultrasound assisted extraction gave higher yield of fucoxanthin when compared with other methods studied. The optimum method confirms that it can be used for scaling up and process development for the separation of fucoxanthin from the sea weeds. This bioactive molecule can be used in the treatment of cancer cells via inducing apoptosis in various types of cancer cells, having anti-tumour activities in cancer cells, having potential of inhibitory effect against invasion of cancer cell and a potential of inhibiting enzymes in cancer cells.
References
- Chandini SK, Ganesan P, Bhaskar N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008; 107: 707-13.
- Conde E, Reinoso BD, Román-Figueroa C, Balboa EM, Moure A, Domínguez H et al. Supercritical CO2 extraction of fucoxanthin from Sargassum muticum.
- Heo SJ, Yoon WJ, Kim KN, Ahn GN, Kang SM, Kang DH, et al. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem Toxicol. 2010; 48: 2045-51.
- Kanazawa K, Ozaki Y, Hashimoto T, Das SK, Matsushita S, Hirano M, et al. Commercial-scale Preparation of biofunctional Fucoxanthin from Waste Parts of Brown Sea Algae Laminalia japonica. Food Sci Technol Res. 2008; 14: 573-82.
- Dedi N, Jaswir I, Salleh HMohd, Taher M, Miyashita K, Ramli N. Fucoxanthin extraction and fatty acid analysis of Sargassum binderi and S. duplicatum. J Med Plants Res. 2011; 5: 2405-12.
- Roh M, Uddin MS, Chun B. Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using supercritical carbon dioxide with co-solvent. Biotechnol Bioprocess Eng. 2008; 13: 724-9.
- Shang YF, Kim SM, Lee WJ, Um B-H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J Biosci Bioeng. 2011; 111: 237-41.
- Mise T, Ueda M, Yasumoto T. Production of Fucoxanthin-Rich Powder from Cladosiphon okamuranus. Adv J Food Sci Technol. 3: 73-6.
- Taylor D, Nixon S, Granger S, Buckley B. Nutrient limitation and the eutrophication of coastal lagoons. Mar Ecol Prog Ser. 1995; 127: 235-44.
- Viaroli P, Azzoni R, Bartoli M, Giordani G, Taje L. Evolution of the trophic conditions and dystrophic outbreaks in the St Acca di Goro lagoon (northern Adriatic Sea). In: Faranda FM, Guglielmo L, Spezie G, editors. Mediterranean ecosystems. Structure and processes. Italia, Milano: Springer Verlag. 2001; 467-75.
- Zailanie K, Purnomo H. Fucoxanthin content of five species brown seaweed from Talango District, madura island. J Agric Sci Technol. 2011: 1103-5.
- Lim SN, Cheung PCK, Ooi VEC, Ang PO. Evaluation of antioxidative activity of extracts from a brown seaweed Sargassum siliquatrum. J Agric Food Chem. 2002; 866: 3862-3.
- Yan X, Chuda Y, Suzuki M, Nagata T. Fucoxanthin as the major antioxidant in Hijika fusiformis a common edible seaweed. Biosci Biotechnol Biochem. 1999; 63: 605-7.
- Hii S-L, Choong P-Y, Woo K-K, Ching-Lee W. Stability studies of fucoxanthin from Sargassum binderi. Aust J Basic Appl Sci. 2010; 4: 4580-4.