
Research Article
Austin Chem Eng. 2023; 10(2): 1101.
Removal of Uranium from Effluent Acidic Solution Using Manganese Oxide Coated Modified Talc (MOCMT)
MS Nagar*; KF Mahmoud; WM Yousef; AA Abdou
Nuclear Materials Authority, El Maadi, Cairo, Ehypt
*Corresponding author: MS Nagar Nuclear Materials Authority, PO Box 530, El Maadi, Cairo, Egypt. Email: mf_nagar@yahoo.com
Received: July 03, 2023 Accepted: August 11, 2023 Published: August 18, 2023
Abstract
In this study, the unique manganese dioxide coated modified talc (MOCMT) adsorbent was easily synthetic. (MOCMT) was evaluated as an adsorbent for the removal of U (VI) from effluent (=10 ppm) solution. To fit the adsorption results, the Langmuir and Freundlich isotherm models were applied, as well as the kinetic parameters of the adsorption process, which were measured and fitted. Five adsorption/desorption cycles with 0.3 M H2SO4 as an eluent were performed to test the reusability. The MOCMT was used to extract uranium from effluent solution produced after a four-cycle leaching/adsorption during the uranium milling process in the Gattar project.
Keywords: Uranium; Manganese dioxide; Talc; Adsorption
Introduction
Uranium is a naturally occurring radioactive element that has been mined and used for over a thousand years for its chemical properties. It is presently largely used as a fuel for electricity-generating nuclear reactors. Uranium is a non-degradable element with high fluidity and a lengthy history of chemical and biological contamination. Some uranium will enter the body through drinking water and the food chain, accumulating primarily in the liver, kidney, and bone [1]. Chemical poisoning and internal radiation will induce a variety of acute and chronic disorders [2]. The WHO has set a maximum contaminant value of 9μg L-1 for uranium, and the US EPA has set a recommended value of 30μg L-1 for a U (VI) maximum concentration limit in drinking water [3]. As a result, removing uranium from waste has become a pressing and vital issue for environmental and human health protection.
Several studies have been carried out to remove uranium from nonconventional sources such as seawater, industrial effluent, and other wastes [4-8]. On the other hand, the nuclear industry's activities have released excessive amounts of uranium into the environment [9]. For many years, uranium's toxicity has been a public health concern [10]. As a result, uranium must be removed, recovered, concentrated, and purified in order to meet future energy demand and avoid radioactive contamination of the environment. The removal of U (VI) ions from aqueous solutions has been reported using a variety of treatment approaches. Because it is environmentally acceptable, easy to purify, generally available, and highly efficient [11,12], the sorption process of U(VI) onto different solid materials has been thoroughly researched [13-16].
Due to their great removal capabilities for harmful ions for environmental remediation, nanostructured materials in environmental treatment have recently gained a lot of attention due to they introduce new functions that are absent for bulk materials [17-22].
Few investigations have been done on the adsorption properties of U(VI) in talc. Mg3Si4O10(OH)2 is the chemical formula for talc. It is made up of three layers: a magnesium hydroxide layer (MgOH2O) sandwiched between two silicate layers (SiO2) [23]. The platy structure of talc is created by weak van der Waals interactions connecting adjacent layers. The talc surfaces' low-energy silicate layers, [001] crystal domains, are hydrophobic, but the edges showing hydroxyl groups (–SiOH) and (–MgOH) are more hydrophilic [24,25]. It is clear that surface adsorption has a significant effect. Furthermore, specific surface functional groups of talc, such as Si-O-Si and O-Si-O, can bind with heavy metal ions and aid in the removal of heavy metal ions from talc [26]. In the manufacturing of paints, lubricants, plastics, cosmetics, medicines, and ceramics, talc is extensively employed as a filler, coating, and dusting agent [27].
Unmodified talc has been used in the chemical adsorption processes of many elements, and among these processes is the use of talc in the adsorption of heavy elements from water by Yunfeng Xu [28] investigated the adsorption of divalent lead ions in water using talc. According to studies, talc has a high rate of Pb2+ adsorption in lead-contaminated wastewater. In batch adsorption studies, Myroslav Sprynskyy et al., [29] investigated the adsorption of uranium in aqueous solution by talc. The study reveals that uranium adsorbs on talc in large clusters or molecules. Yunfeng Xu and colleagues [30] employed talc in their study of nickel-containing waste water adsorption in water, and they explored the impacts optimum conditions on nickel adsorption onto talc.
When talc is modified, its specific surface area can be raised to enhance the active functional group, and some features can be added, allowing the modified talc to be utilised for a variety of applications. Hannatu Abubakar Sani et al [31] used ZnO nanoparticles to modify talc to create ZnO/talc nanocomposites to examine the adsorption efficacy of Pb(II) in aqueous solution. According to the findings, ZnO/talc nanocomposites have a high adsorption capability. Cu(II), Ni(II), and Pb(II) ions were removed from aqueous solutions using Fe3O4/talc nanocomposites. The initial concentrations of heavy metal ions Cu (II), Ni (II), and Pb (II) were 100, 92, and 270 mg/L, respectively, according to the results [32]. To efficiently remove lead (II) and nickel (II) from aqueous solutions, a new polysulfone/Fe3O4-talc nanocomposite hybrid matrix membrane was used [33].
Through adsorption and co-precipitation processes, hydrous manganese oxides usually play a key role in limiting trace metal concentrations. They have a huge surface area, a microporous structure, and a high affinity for metal ions, which makes them an efficient heavy metal scavenging pathway [34]. Because manganese dioxide occurs in the form of fine particles, it is difficult to separate solids from liquids, making it unsuitable as a filtering medium [35]. Its doping on solid support will improve its ability to separate metal ions; for example, under column testing, manganese dioxide coated zeolite was employed to remove uranium (VI), copper (II), and lead (II) [36,37]. Table 1 summaries published results on the adsorption of various cations by manganese dioxide coated onto various sorbents [38-47].
Ions
Mn-coated material
Qmax (mg/g)
References
As+3
Manganese oxide-coated-alumina
42.5
38
Cd+2
Manganese oxide modified Diatomite
26.6
39
Cu+2
Manganese oxide modified bentonite
105.4
40
Pb+2
Manganese oxide coated bentonite
58.9
41
U+6
Manganese oxide coated zeolite
17.6
42
U+6
Manganese oxide coated sand
2.483
43
Mn+2
Manganese oxide coated zeolite
60
44
226Ra
Manganese oxide coated modified bentonite
94.28Bq/g
45
U+6
manganese oxide coated zeolite (MOCZ) modified with amine
99
46
U+6
Manganese oxide modified nanofiber
398
47
U+6
Manganese oxide coated modified talc
38
Present study
Table 1: Ions adsorption capacity by manganese oxide coated adsorbents.
The heat-based technique was used to create talc modified with MnO to form MnO/talc in this study. As a result, nano-flakes of Manganese Oxide Coated Modified Talc (MOCMT), as a novel sorbent for uranium sorption from aqueous solution was investigated in this study. Furthermore the effects of pH, sorbent doge, adsorption time, initial uranium concentration, ions strength, and temperature were examined. The kinetic characteristics and adsorption isotherms were used to assess the adsorption performance.
The goal of the study is to remove the uranium from the barren (effluent) solution after four cycles on the heap pad. Because the barren solution has high concentrations of elements that cause resin poisoning as a result of its repeated passage (4 cycles) on the heap pad, these solutions must be disposed about after removal of uranium contained in them using MOCMT.
Materials and Methods
A talc sample was obtained for the studies from a talc deposit near El-Atshan, Egypt, 60 kilometres southwest of El Qussier City. The raw material was manually ground and sieved at 11.27 μm, where the highest surface area (18.986 m2/g) was obtained at the lowest size (11.27 μm) [48].
All of the chemicals and reagents utilized are of the highest quality. UO2SO4 crystals were used to make a uranium stock solution (1000 mg/L). U(VI) was calculated using NH4VO3 and an oxidimetric titration [49]. Arsenazo III at = 655 nm validated the results spectroscopically [50].
Modification Methods of Talc
1- Talc powder pretreatment: The talc powder samples were pretreated as follows before being used in the studies. The combinations of different mesh sizes of talc powder were combined with 300 mL distilled water and shaken for 1 hour. After stirring, all combinations were allowed to stand for 1 hour before removing the tiny contaminants suspended in the supernatant with a pipette. This was done again and again until the supernatant was clear. The finished products were then dried at 105°C.
2- Acid modified of talc: the surface modification reactions of talc were carried out by reacting talc powder (10g) with 0.2M HCl, heated to 80°C for 2h. The treated talc was then filtered, washed with deionized water, and dried in a vacuum oven at 105°C for 48 h.
3- 10g of the dried modified talc was added to 300 mL of hot KMnO4 (0.5 M) solution at a room temperature and pH of 6.5; they were mixed using a mechanical stirrer for 4h. The pulp obtained was washed using deionized water, filtered and dried at 70°C in an electric oven. The aim of this stage was to precipitate MnO onto the modified talc surface to obtain the MnO-coated modified talc (MOCMT).
Sorption and Desorption Studies
Batch Studies (static)
Batch experimental were carried out to investigate the effect of various effective parameters on the removal of U+6 from liquid waste effluent onto the synthesized adsorbent. At room temperature, the following factors were investigated: working solution pH, adsorbent dosage, interfering elements, and reaction time. In a typical adsorption experiment, 50 mL U(VI) solution (5, 10, 15,20, 25 and 30 mg L-1) and 0.5 g adsorbent was placed into a 100 mL conical flask at a predetermined pH (2, 3, 4, 5, 8, 10, and the initial pH=7). The admixture-containing conical flasks were vibrated for 180 min at room temperature in a water-bathing constant temperature vibrator. After that, the mixtures were filtered through proper filter papear. The solutions were then examined by a UV-VIS spectrophotometer. The loaded MOCMT was filtered and the sorbed uranium metal were desorbed by shaking with the appropriate solution of eluting agents and subsequently analyzed spectroscopically.
Column Studies (dynamic)
A glass column with a length of 300 mm and a diameter of 10 mm was loaded with 1.00 g 0.0001 g of MOCMT adsorbent. A peristaltic pump was used for passing the effluent sample through the column after adjusting its pH at (4.5) and flow rate at 1.0ml/2min and the operation was performed by the downstream flow. Recovery experiments were carried out after the sorption procedure. The column was first rinsed with water, and then a certain volume of the eluting agent was allowed to percolate through it. The sorbed metal ions get eluted from MOCMT and subsequently determined by spectrophotometric analysis.
Adsorption % (adsorption percentage of U(VI)) and qe (mg/g) (the adsorption capacity) and distribution coefficient (Kd) were calculated according to the following equations [51].
00Sorption rate=
where Co and Ce stand for the initial uranium concentration and that at equilibrium (mg/L), respectively. V is the volume of aqueous solution (L) and m is the dry composite weight (g).
Gattar Effluent (barren) Sample
The uranium-contaminated solution obtained after 4 cycles of leaching/adsorption on a heap pad came from a uranium-processing milling plant in Egypt's Gattar region. In site of Gattar-II, the presence of uranium minerals near fractures, faults, and joints favours hydrothermal solutions and/or groundwater circulation. This ore material has a high concentration of related elements such as Ca, Mg, Mn, Si, and Fe, with a concentration of about 300 (mg U/Kg). The systematically work is to leach uranium from its mineralization and obtain pregnant uranium leaching solutions to be suitable for uranium recovery by the quaternary ammonium resins. The produced effluent (barren) of our studies is the outlet of adsorption process prior to discharge to environment.
Results and Discussions
Characterization of MnO Coated Natural Talc and Surface Modification
The talc elementary sheet is composed of octahedral magnesium hydroxide structures sandwiched between sheets of silicon–oxygen tetrahedral in which the components are linked by ionic and covalent bonds. The silicate layers of talc sheets are bonded together by weak Van der Waals forces between surface oxygen atoms. Face surfaces present hydrophobic character
by the presence of –Si–O–Si– groups. On the other side, edge surfaces contain hydrophilic groups as –SiOH and –MgOH. It is clearly that confirmation of HCl broke siloxane bonds at the face surfaces, increasing the –OH groups content. In (Figure 1), the FTIR spectra of talc and MOCMT are shown. The FTIR spectra of talc and freshly synthesized MOCMT revealed substantial differences in peaks, including weakening of the bands at 1420 and 3640 cm-1, which correspond to talc's –OH group. In addition, after MnO2 impregnation, the bands at 710 and 870 cm-1 in talc, which correspond to Mg–O and Si–O bands, were diminished. The FT-IR bands of MOCMT shift to lower wave numbers, indicating that MnO particles are interacting with the talc surface. This demonstrates that MnO are present in the talc surface. Furthermore, the stretching vibrations of Mn-OH and Mn-O are allocated to the MnO2 bands at 1370 and 559 cm-1.

Figure 1: FT-IR chart of the Talc and (MOCMT).
SEM pictures and EDX spectra for talc and the synthesized MOCMT are shown in (Figure 2) it can be seen that the talc's aky form is coated in MnO nanoparticles. The presence of Mn in the EDX spectrum indicates that a MnO/talc nanocomposite has formed.
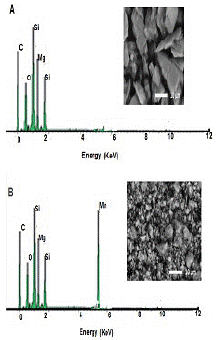
Figure 2: SEM image and energy dispersive X-ray spectra for A) Talc and B) MOCMT.
Batch Study
Effect of pH
To find the optimum pH value for maximum sorption, series of adsorption measurements were carried out by contacting a fixed weight of the prepared MOCMT (0.5g) with 10 ppm of uranium standard solution at 25 °C for 180 minutes. The investigated pH ranged from 2 to 7. The obtained data were shown in (Figure 3). The results showed that the adsorption of uranium increases from about 10 to 94% with an increase in pH of the solution from 2.0 to 3.5 and then decreases to 70% at pH of 5. The uranium adsorption mechanism was affected by the solution pH through the hydrolysis of uranyl ions in aqueous solution as well as the properties of the active sites on the surface of the sorbent [52]. At low pH value, the uranium is present in the solution mainly in the form of free UO22+ ions and the availability of free uranium ions are maximum at pH 3.5. Low adsorption of the positively-charged uranyl ions at pH values less than 3.0 may be caused by strong protonated silanol groups of the talc layer with slightly positive charge. As the pH of a uranium solution increases, the uranylions are easily hydrolyzed, and these hydrolysis products are also polymerized.
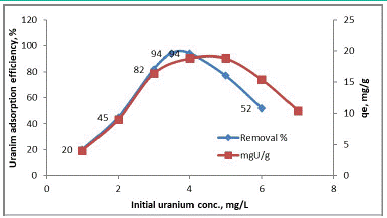
Figure 3: Influence of pH on the adsorption efficiency of uranium by MOCMT. C0(U) = 10 mg/l,adsorbent dosage = 0.5 g, T = 298 K, t = 90 min.
Effect of Adsorbent Dosage
The capacity of an adsorbent for a given initial concentration of the adsorbate is determined by the dosage of the adsorbent. The results reveal that as the adsorbent concentration increases, percentage removal increases, but the amount adsorbed per unit mass of the adsorbent falls significantly. The decrease in unit adsorption as the dose of adsorbent is increased is due to unsaturated adsorption sites remaining during the adsorption reaction. The maximum removal rate of MOCMT reached about 94% when their dosage was 0.5 g/l. (Figure 4) shows the effect of dosage on U(VI) ion elimination.
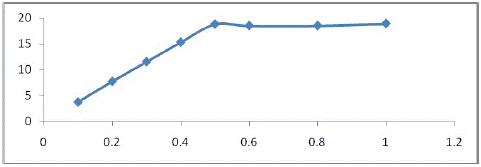
Figure 4: Influence of adsorbent dosage on the sorption efficiency of uranium by MOCMT. pH = 3.5, C0(U) = 20 mg/l, T = 298 K, t = 90 min.
Effect of Initial Uranium Concentration
For studying the effect of initial uranium concentration on the adsorption efficiency and quantity of the adsorbed uranium per unit weight of adsorbent of (MOCMT). A series of measurements were carried out by contacting a fixed weight (1.0g) for 90 minutes at room temperature and pH3.5. The studied initial uranium concentrations ranged from 5 up to 70 mg/l. The obtained results were plotted in figure [32]. From the obtained data, it is clearly obvious that uranium adsorption efficiency decreases with increasing its initial concentration. The (experimental) uranium adsorption capacity of the (MOCMT) was determined from (Figure 5) to be about 38.0 mg U/g (MOCMT).
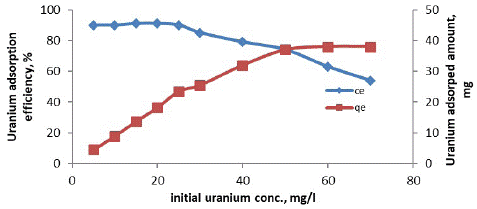
Figure 5: Effect of initial uranium concentrations on uranium adsorption onto MOCMT.
Sorption Isotherm
Isotherm of adsorption gives information on mechanisms of adsorption, properties of the surface and affinity of an adsorbent towards heavy metal ions. The adsorption data were analyzed using Langmuir and Freundlich adsorption isotherm models. The Langmuir model assumes that the monolayer adsorption which happens at fixed numbers of homogeneous sites on the adsorbent. Thus, the Langmuir model is given by the following equation:
Where: Ce (mg/ L) is the equilibrium concentration of U(VI) in the solution, qe (mg /L) is the amount adsorbed at equilibrium, Qo and b, the Langmuir constants, are the saturated monolayer sorption capacity and the sorption equilibrium constant, respectively. A plot of Ce/qe versus Ce would result in a straight line with a slope of (1/Qo) and intercept of 1/bQo as shown in (Figure 6a).
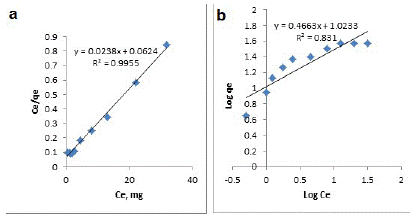
Figure 6: Fitting lines of Langmuir (a) and Freundlich (b) isotherm models of MOCMT.
Freundlich Isotherm
The Freundlich model stipulates that the ratio of solute adsorbed to the solute concentration is a function of the solution. The empirical model was shown to be consistent with exponential distribution of active centers, characteristic of heterogeneous surfaces. The amount of solute adsorbed at equilibrium, qe, is related to the concentration of solute in the solution, Ce, as in the following equation:
This expression can be linearized as follow:
log
Where: KF and n are the Freundlich constants, which represent sorption capacity and sorption intensity, respectively. A plot of “logqe” versus “logCe” would result in a straight line with a slope of (1/n) and intercept of “log KF” as shown in (Figure 6b). The Langmuir and Freundlich constants are given in Table (2). The experimental data shows that the adsorption of uranium onto MOCMT fits well with Langmuir than Freundlich isotherm.
Metal
Adsorbent
Langmuir model parameters
Freundlich model parameters
Q° (mg/g)
b(L/mg)
R2
1/n
Kf (mg/g)
R2
Uranium
MOCMT
38
0.063
0.998
0.564
1.6
0.831
Table 2: Langmuir and Freundlich parameters for uranium adsorption onto MOCMT.
Effect of Contact Time and Sorption Kinetics
The effect of contact time on the adsorption efficiency of uranium onto MOCMT was examined. The duration of the study ranged from 15 to 180 minutes. According to the results in (Figure 7), the uranium adsorption effectiveness was around 63% when measured at 45 minutes of contact time. High uranium adsorption efficiencies were obtained when the contact duration was increased from 45 to 90 minutes. The reaction has reached equilibrium because there is no substantial variation in % removal. The initial quick adsorption rate is caused by the abundance of active sites on the nanocomposite's surface.

Figure 7: Pseudo first order and second reaction kinetics for the adsorption of Uranium on MOCMT.
Adsorption kinetic is used to predict the rate at which uranium ions is removed from the barren solutions. The adsorption data of U(VI) at different time intervals are fit for to pseudo-first-order kinetic model and pseudo-second-order kinetic model. Pseudo first order kinetic model describe mechanisms of metal species adsorption by an adsorbent and can be expressed as:
Pseudo-second-order kinetic model
where qt is the amount of U(VI) adsorbed at time t (mg/ g); qe is equilibrium solid phase concentration and k1 and k2 are first-order and second-order rate constant for adsorption (L min-¹) respectively. The adsorption kinetic studies of U(VI) ions onto nanocomposite are shown in (Figure 7) and summarize in Table 3.
Material
Pseudo-second-order model
Pseudo-first-order model
MOCMT
R2
K2
qe (mg/g)
R2
K1
qe (mg/g)
0.992
0.00824
38.8
0.988
0.0364
32.9
Table 3: Pseudo-first-order and pseudo-second-order model parameters for MOCMT.
The theoretical adsorption capacity value (38.8 mg/g) for pseudo second-order kinetics is in good agreement with the experimental adsorption capacity value (38 mg/g). The regression coefficient R² of second order is higher than that of the first order and hence the data best fitted to pseudo-second-order than pseudo-first-order and hence confirming chemisorption involving chemical bonding between uranyl ions and the adsorbent active sites [53,54].
As explained previously, the adsorbents can efficiently adsorb uranium from aqueous solutions containing mainly uranium. In the Gattar uranium barren solution, iron is one of the most concentrated elements in the solution as which have a significant impact on the uranium adsorption from acidic uranium effluent. To investigate the impact of iron as a high foreign ion concentration on the adsorption efficiency of uranium onto MOCMT, a series of adsorption experiments were carried out onto a constant sample dose of MOCMT (0.5g each) were contacted with uranium solution (20 mg/L) with different additions of iron ranged from 50 to 2,200 mg/L. The studies were conducted out at room temperature (25oC) with a 90 min contact time and a pH of 3.5. As shown in (Figure 8), increasing the amount of iron additional reduces uranium adsorption (from roughly 94–5%) due to adsorption competition between iron and uranium. The decrease in MOCMT capacity after contact foreign ions could be due to competition between uranium and other ions in the solution, particularly ferric iron, which can form anionic complexes, [Fe(SO4)n]3-2n or [Fe(OH)(SO4)2]2- that compete with uranium for MOCMT sites during acid leach liquor recovery. To overcome the high percentage of iron in the solution, it must be precipitated at pH 3.5 with some other impurities before loading onto the adsorbent.
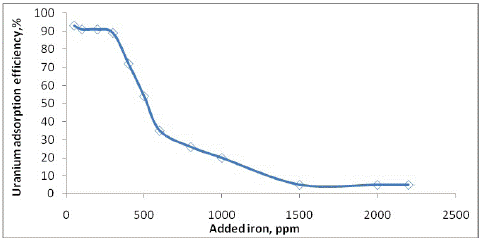
Figure 8: Effect of added iron amounts upon uranium adsorption efficiency onto MOCMT.
Desorption Studies
Desorption is a critical step in sorption research work because it enhances the economic value of the sorption process. Desorption studies will enable in the adsorbent's regeneration, allowing it to be reused. For this objective, three mineral acids (H2SO4, HCl, and HNO3) were examined. 1.0g of loaded adsorbent was eluted for 35 minutes in a 10 mL solution with a var1ied acid content ranging from 0.1 to 0.5 M. The observed data, (Figure 9) demonstrate that increasing acid increased uranium elution efficiency. With 10 mL of 0.4 MH2SO4 and 1.0g loaded adsorbent, it reached 91%. The unused fraction of sulphuric acid is maintained on the barren adsorbent during the elution phase, and then it is returned to the leaching system, where it is recycled back into the leaching process, resulting in a reduction in acid consumption.

Figure 9: Elution efficiency using different eluent reagents.
Application
Case Study (Barren Solution from Milling Plant in Gattar Project, Egypt)
The novel sorbent MOCMT was used to separate UO22+ ions in barren samples obtained from Gattar milling project, Egyptian after 4 cycle leaching/adsorption. For this purpose, 10 L of barren solution (10ppmU) was adjusted to pH 3.5 for precipitation of Fe2+ as shown in table 4, before being run through 3. 0 g of MOCMT at a flow rate of 1.0 mL/min. (Figure 10), shows the obtained results based on the optimum factors of the experimental adsorption process. From the plotted curve of barren volume versus uranium in barren, the adsorption efficiency reached 82%. The low adsorption efficiency may be due to adsorption of some interfering elements with uranium.
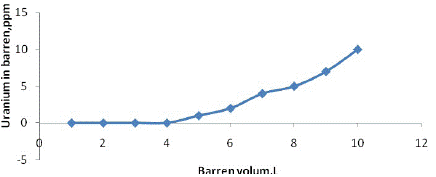
Figure 10: Adsorption of uranium barren solution from Gattar onto MOCMT.
Element
Al
Si
Fe
Mn
Na
K
U
Mg
Ca
Cu
Cons, ppm
10
60
2100
380
135
125
10
175
95
16
After ppt
9
29
350
210
340
90
9
95
70
15
Table 4: Chemical analysis of elements associated uranium in GII pilot plant barren liquors before and after PPt.
Uranium (VI) was eluted from the loaded modified (MOCMT) using 0.4 M H2SO4. The purpose of the adsorption-desorption cycle tests was to decide if MOCMT could be reused. The adsorption efficiency dropped as the cycle number increased, as can be shown in Figure 11. The drop was caused by a reduction in the number of effective adsorption sites. First, the uranium adsorbed on MOCMT could not be entirely desorbed by H2SO4 solution; second, the chemical adsorption of the inner-sphere surface complex was not totally reversible. Despite this, after five cycles, the adsorption efficiency fallen from 92 to 79%.

Figure 11: Reusability performance of MOCMT during 5 cycle.
Conclusion
MnO2 was deposited over the modified talc surface, resulting in a novel manganese dioxide coated modified talc (MOCMT) adsorbent. The (MOCMT) was originally evaluated as an adsorbent for removing uranium from effluent solution. On the adsorption, reusability, and adsorption mechanisms, the effects of pH, ionic strength, and initial uranium concentration and mixing time were shown. When compared to the published values, the theoretical adsorption capacity of (MOCMT) computed as 38.8 mg/g, was competitive. The MOCMT was successfully employed to extract uranium from barren solution produced during the uranium milling plant's after four-cycle leaching/adsorption process.
Author Statements
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Zhu KR, Chen CL, Xu MWC, Chen K, Tan X, Wakeel M, et al. In situ carbothermal reduction synthesis of Fe nanocrystals embedded into N-doped carbon nanospheres for highly efficient U(VI) adsorption and reduction. Chem Eng J. 2018; 331: 395-405.
- Anirudhan TS, Bringle CD, Rijith S. Removal of uranium (VI) from aqueous solutions and nuclear industry effluents using humic acid-immobilized zirconium-pillared clay. J Environ Radioact. 2010; 101: 267-76.
- Ma F, Gui Y, Liu P, Xue Y, Song W. Functional fibrous materials-based adsorbents for uranium adsorption and environmental remediation. Chem Eng J. 2020; 390: 124597.
- Das S, Pandey AK, Athawale A, Kumar V, Bhardwaj YK, Sabharwal S, et al. Chemical aspects of uranium recovery from seawater by amidoximated electron-beam-grafted polypropylene membranes. Desalination. 2008; 232: 243-53.
- Zhang A, Uchiyama G, Asakura T. pH Effect on the uranium adsorption from seawater by a macroporous fibrous polymeric material containing amidoxime chelating functional group. React Funct Polym. 2005; 63: 143-53.
- Zhang A, Asakura T, Uchiyama G. The adsorption mechanism of uranium(VI) from seawater on a macroporous fibrous polymeric adsorbent containing amidoxime chelating functional group. React Funct Polym. 2003; 57: 67-76.
- Mellah A, Chegrouche S, Barkat M. The removal of uranium (VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci. 2006; 296: 434-41.
- Sakaguchi T, Nakajima A. Recovery of Uranium by Immobilized Polyhydroxyanthraquinone. Sep Sci Technol. 1986; 21: 519-34.
- Benedict B, Pigford TH, Levi HW. Nuclear chemical engineering. New York: McGraw-Hill; 1981.
- Yusan SD, Akyil S. Sorption of uranium (VI) from aqueous solutions by akaganeite. J Hazard Mater. 2008; 160: 388-95.
- Mellah A, Silem A, Boualia A, Kada R. Adsorption of organic matter from a wet phosphoric acid using activated carbon: equilibrium study. Chem Eng Process. 1992; 31: 191-4.
- Saleem M, Afzal M, Qadeer R, Hanif J. Selective Adsorption of Uranium on Activated Charcoal from Electrolytic Aqueous Solutions. Sep Sci Technol. 1992; 27: 239-53.
- Morsy AMA, Hussein AEM. Adsorption of uranium from crude phosphoric acid using activated carbon. J Radioanal Nucl Chem. 2011; 288: 341-6.
- Zou W, Zhao L, Han R. Adsorption characteristics of uranyl ions by manganese oxide coated sand in batch mode. J Radioanal Nucl Chem. 2011; 288: 239-49.
- Olmez Aytas S, Akyil S, Eral M. J Radioanal Nucl Chem. 2004; 260: 119-25.
- Donat R, Esen K, Cetisli H, Aytas S. Adsorption of uranium (VI) onto Ulva sp.-sepiolite composite. J Radioanal Nucl Chem. 2009; 279: 253-61.
- Cheira MF, Mira HI, Sakr AK, Mohamed SA. Adsorption of U (VI) from acid solution on a low-cost sorbent: equilibrium, kinetic, and thermodynamic assessments. Nucl Sci Tech. 2019; 30: 156.
- Merikhy A, Heydari A, Eskandari H, Ghahraman-Rozegar F. Carbonized spent bleaching earth as a low-cost adsorbent: a facile revalorization strategy via response surface methodology. Chem Eng Process Process Intensif. 2020; 158: 108167.
- Shameli K, Ahmad MB, Zargar M, Wan M, Yunus ZW, Ibrahim NA, et al. Int J Nanomedicine. 2011; 6: 271-84.
- Luo H-M, Zhang F, Yang PB. Preparation and electrochemical properties of CPAC/Mn3O4 nanocomposite electrode. J Mater Sci Mater Electron. 2013; 24: 601-6.
- Rhim JW, Hong SI, Park HM, Perry KW PKW. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J Agric Food Chem. 2006; 54: 5814-22.
- Krishnan Bama, Mahalingam S. Facile synthesis and antimicrobial activity of manganese oxide/bentonite nanocomposites. Res Chem Intermed. 2017; 43: 2351-65.
- Michot LJ, Villieras F, François M, Yvon J, le Dred R, Cases JM. The Structural Microscopic Hydrophilicity of Talc. Langmuir. 1994; 10: 3765-73.
- Wallqvist V, Claesson PM, Swerin A, Schoelkopf J, Gane PAC. Interaction forces between talc and hydrophobic particles probed by AFM. Colloids Surf A Physicochem Eng Aspects. 2006; 277: 183-90.
- Castillo LA, Barbosa SE, Capiati NJ. Influence of talc genesis and particle surface on the crystallization kinetics of polypropylene/talc composites. J Appl Polym Sci. 2012; 126: 1763-72.
- Wang Y, Lin H, Lin Z, Yuan Y. Application And Prospect Of Talc As Heavy Metal Passivation Agent. IOP Conf Ser.: Mater Sci Eng. 2018; 392: 032029.
- Chauhan VS, Bhardwaj NK, Chakrabarti SK. Effect of particle size of magnesium silicate filler on physical properties of paper. Can J Chem Eng. 2013; 91: 855-61.
- Xu Y. Min Metall. 2015:75-7 + 80.
- Sprynskyy M, Kowalkowski T, Tutu H, Cukrowska EM, Buszewski B. Adsorption performance of talc for uranium removal from aqueous solution. Chem Eng J. 2011; 171: 1185-93.
- Xu Y, Ruan T, Zhang J, Li P. Contemporary chemicals; 2014; 1959-61.
- Sani HA, Ahmad MB, Saleh TA. Synthesis of zinc oxide/talc nanocomposite for enhanced lead adsorption from aqueous solutions. RSC Adv. 2016; 6: 108819-27.
- Kalantari K, Ahmad MB, Masoumi HR, Shameli K, Basri M, Khandanlou R. Rapid adsorption of heavy metals by Fe3O4/talc nanocomposite and optimization study using response surface methodology. Int J Mol Sci. 2014; 15: 12913-27.
- Moradihamedani P, Kalantari K, Abdullah AH, Morad NA. High efficient removal of lead(II) and nickel(II) from aqueous solution by novel polysulfone/Fe 3 O 4 –talc nanocomposite mixed matrix membrane. Desalin Water Treat. 2016; 57: 28900-9.
- Zou W, Han R, Chen Z, Shi J. Hongmin Lb. J Chem Eng Data. 2006; 51: 534-41.
- Han R, Zou W, Zhang Z, Shi J, Yang J. J Hazard Mater. 2006; B137: 384-95.
- Zou W, Zhao L, Han R. Sep sci Eng Chin J. Chem Eng. 2009; 17: 585-93.
- Zou W, Han R, Chen Z, Jinghua Z, Shi J. Kinetic study of adsorption of Cu(II) and Pb(II) from aqueous solutions using manganese oxide coated zeolite in batch mode. Colloids Surf A Physicochem Eng Aspects. 2006; 279: 238-46.
- Maliyekkal SM, Philip L, Pradeep T. As(III) removal from drinking water using manganese oxide-coated-alumina: performance evaluation and mechanistic details of surface binding. Chem Eng J. 2009; 153: 101-7.
- Al-degs YS, Tutunju MF, Shawabkeh RA. The Feasibility of Using Diatomite and Mn–Diatomite for Remediation of Pb 2+ , Cu 2+ , and Cd 2+ from Water. Sep Sci Technol. 2000; 35: 2299-310.
- Eren E. Removal of copper ions by modified Unye clay, Turkey. J Hazard Mater. 2008; 159: 235-44.
- Eren E, Afsin B, Onal Y. Removal of lead ions by acid activated and manganese oxide-coated bentonite. J Hazard Mater. 2009; 161: 677-85.
- Han R, Zou W, Wang Y, Zhu L. Removal of uranium(VI) from aqueous solutions by manganese oxide coated zeolite: discussion of adsorption isotherms and pH effect. J Environ Radioact. 2007; 93: 127-43.
- Zou W, Zhao L, Han R. Adsorption characteristics of uranyl ions by manganese oxide coated sand in batch mode. J Radioanal Nucl Chem. 2011; 288: 239-49.
- Taffarel SR, Rubio J. Removal of Mn2+ from aqueous solution by manganese oxide coated zeolite. Miner Eng. 2010; 23: 1131-8.
- Nagar MS, Abdou AA, Ghazala RAS. Mediterr J Chem. 2018; 7: 105-14.
- Yousef LA, Bakry AR, Ahmad AA. Uranium(VI) recovery from acidic leach liquor using manganese oxide coated zeolite (MOCZ) modified with amine. J Radioanal Nucl Chem. 2020; 324: 409-21.
- Tao W, Lv R, Tao Q. Facile Preparation of Novel Manganese Dioxide Modified Nanofiber and Its Uranium Adsorption Performance. J Appl Math Phys. 2021; 09: 1837-52.
- Zeyni ARSOY, Bahri ERSOY, Atilla EVCIN, Mehmet G, ICDUYGU. Physicochem Probl Miner Process. 2017; 53: 288-306.
- Mathew KJ, Mason B, Morales ME, Narayann UI. J Radioanal Nucl Chem. 2009; 282: 939.
- Marczenko Z, Balcerzak M. Uranium. Separation, pre-concentration and spectrophotometry in inorganic analysis. Elsevier. 2000; 10: 446-55.
- Mahdavi M, Ahmad MB, Haron MJ, Gharayebi Y, Shameli K, Nadi B. Fabrication and Characterization of SiO2/(3-Aminopropyl)triethoxysilane-Coated Magnetite Nanoparticles for Lead(II) Removal from Aqueous Solution. J Inorg Organomet Polym Mater. 2013; 23: 599-607.
- Ferrah N, Abderrahim O, Didi MA, Villemin D. J Radioanal Nucl Chem. 2011; 289: 721.
- Wu FC, Tseng RL, Juang RS. J Hazard Mater. 2001; 3894: 00340-X.
- Ding L, Deng HP, Wu C, Han X. Affecting factors, equilibrium, kinetics and thermodynamics of bromide removal from aqueous solutions by MIEX resin. Chem Eng. 2012; 181-182: 360-70.