
Research Article
Austin J Chem Eng. 2015;2(1): 1014.
Experimental Investigation of Biogas Production from Wastewater Sludge
Mousa H1,2*, Al-Muhtaseb A¹, Abu Qdais H³ and Abd Alaa ¹R²
¹Petroleum and Chemical Engineering Department, Sultan Qaboos University, Oman
²Department of Chemical engineering, Jordan University of Science and Technology, Jordan
³Department of Civil Engineering, Jordan University of Science and Technology, Jordan
*Corresponding author: Hasan Abdellatif Hasan, Department of Petroleum and Chemical Engineering, Sultan Qaboos University, P.O. Box 33, P.C. 123, Muscat- Oman
Received: December 28, 2014; Accepted: March 18, 2015; Published: April 27, 2015
Abstract
The paper has information about experimental investigation of biogas production from wastewater sludge. The effect of incubation temperature, volatile suspended solids contents, and pressure on the amount of the biogas produced was studied in 2L plastic flasks. The experiments were repeated in 25L vessel using the above conditions showed that the CH4 content increased with time reaching a maximum value in 16 days then decreased. No H2S gas was detected indicating that biogas produced from wastewater sludge is environmental friendly source of energy. The results show the optimum conditions under which digesters should be operated.
Keywords: Biogas; Methane; Sludge; Wastewater treatment; Anaerobic digestion; Volatile suspended solids
Introduction
Anaerobic Digestion (AD) of organic waste has been recognized as a cost-effective and environmental friendly process to convert organic solid waste into biogas which can be used to produce heat, electricity and fuel [1]. In this digestion process the biodegradable organic wastes are converted to simple molecules by anaerobic bacteria producing a combustible biogas containing about 50–75% methane and 25–50% carbon dioxide [2]. Several feedstock have been used to produce biogas using anaerobic digestion, such as biomass waste [3,4], algae [5,6], food waste [7,8], municipal solid waste [9-16]. Waste Activated Sludge (WAS) is a highly putrescible residue generated from wastewater treatment plants. Activated sludge contains abundant organic matters, bacterial pathogens, nutrients, and high water content. Therefore sludge handling is a big problem in itself from environmental and economic point of view since it accounts for 50% of the operating costs of wastewater treatment plants [17]. Therefore, anaerobic digestion is considered to be an interesting option for WAS treatment as it can allow sludge stabilization to reduce odors and pathogens, sludge decrement, and biogas recovery [18].
Elango et al. [19] investigated the production of biogas from domestic sewage in Chennai metropolitan city using anaerobic digestion process at 26 and 36°C for 25 days. Biogas generation was enhanced by the addition of domestic sewage to MSW. The maximum biogas production (0.36 m³/kg VS added-day), the maximum reduction of Total Solids (TS) (87.6%), VS (88.1%) and Chemical Oxygen Demand (COD) (89.3%) occurred at a feeding rate of 2.9 kg of VS/m³-day.Kalloum et al. [20] studied biogas production from the wastewater treatment plant in Adrar city, Algeria. The diluted sludge with a content of 16 g/l of Total Solids (TS) was fermented under anaerobic conditions during 33 days. The biogas produced was 280.31 Nml with a yield of 30 Nml of biogas/mg of COD removed. The biogas production increased for 26 days then started to decrease. During the first 5 days the pH dropped from 7.0 to 6.3 due to formation of Volatile Fatty Acids (VFA). After this period pH increases to 6.9 and remains unchanged.
Several attempts have been done to enhance biogas production. Feng et al. [21] enhanced the anaerobic digestion of wasted activated sludge, from municipal wastewater treatment plant in Dalian, China, by the addition of Zero Valent Iron (ZVI) as a reducing material. The production of VFAs was enhanced by 37.3% with ZVI during the hydrolysis and the acidification steps. After the digestion for 20 days, the methane productivity at ZVI of 20 g/L increased by 43.5%, and the sludge reduction ratio increased by 12.2%.Wang et al. [22] enhanced the anaerobic digestion of wasted activated sludge, from wastewater treatment plant in Brisbane, Australia, by 26% using combined Free Nitrous Acid (FNA) and heat pre-treatment. Debowski et al. [6] studied the effect of Magneto-Active Filling (MAF) on the effectiveness of methane fermentation of dairy wastewaters. It has been found that MAF incorporation into the technological system significantly improved effectiveness of biogas production, increased methane concentration and lowered content of hydrogen sulfide in gaseous metabolites of fermentative bacteria. A significant increase was also observed in the effectiveness of COD removal from dairy wastewaters.
The objective of this study is to investigate the potential production of biogas from the sludge produced at the wastewater treatment plants and to investigate the effect of various operating parameters such as pH, VSS concentration and reactor pressure and temperature on the biogas production rate.
Materials and Methods
Experiment procedure
Two sets of experiments were performed, the first set was performed in a 25L vessel, and the second set was performed in 2L plastic bottles both to mimic batch bioreactors. Details of these two sets of experiments are discussed below:
Set 1: The second part of the experiments was carried out in 2L plastic bottles filled by 1 L sludge. The purpose of this part is to study the effect of sludge concentration, temperature, and pressure on biogas production. Conditions at which maximum biogas production obtained were used for set 2 experiment carried out in a 25L reactor. The bottles were hooked with a hollow pipe sealed with rubber tube which can be closed to prevent gas escape from the bottles. When sampling is needed, a syringe is inserted through the rubber tube which is then opened allowing the gas to flow into the syringe. The withdrawn gas sample was then sent to the gas analyzer. The sludge as received contains 6% VSS. To study the effect of sludge concentration on biogas produced, the sludge was diluted with distilled water to obtain sludge of Volatile Suspended Solids (VSS) concentrations of 1%, 2%, and 4%.The effect of pressure was investigated by preparing 3 groups of bottles. In the first group the gas in the bottles was analyzed and then it was released till the pressure in the bottles reaches atmospheric pressure. In the second group, the bottles were left without any interference till the end of the incubation period (9 weeks) when agas sample was taken for analysis. In the third group, a sample from the gas was taken for analysis and the bottles were immediately sealed keeping the bottles pressurized. The reason for the third group is to determine biogas production with time under pressure and compare its results to that of group 1 conducted under atmospheric pressure condition. The effect of temperature on methane generation was studied by carrying out the same tests under constant temperature of 35°C and 25°C. Three bottles for each test were prepared to assure the reproducibility of the results. The data presented is the average of the three readings. The initial weight of the bottles and their weight after sampling were recorded. Knowing the difference in the weight and the concentration of CH4 gas enables calculating it mass (see results and discussion below, section 3.1).
Set 2: The anaerobic digestion was carried out in a 25L vessel containing a heating element and a thermostat to control the temperature of the vessel at 35°C. The vessel contains a pressure gauge, a gas sampling point at the top of the vessel and a drainage valve at the bottom for liquid sample withdrawal. The vessel is filled with 23L sludge and gas samples were taken periodically every 2 days for 44 days for analysis. The pressure was read directly from the pressure gauge. The gas samples were withdrawn by a syringe and analyzed for its CH4, CO2, and H2S contents. Every time a gas sample was withdrawn, the pressure was released until it reaches atmospheric pressure. Knowing the pressure of the vessel, the temperature, and methane gas content its amount can be calculated assuming that the gas is ideal (see results and discussion section, section 3.2).
Sludge characterization
The Volatile Suspended Solids (VSS) and the Total Suspended Solids (TSS) of the sludge were determined following the standard methods for the examination of water and wastewater [23]. The pH was measured using portable pH meter (pH 3310 IDS, WTW, Germany). The Chemical Oxygen Demand (COD), the PO4-P, and the NH4-N were determined using photometer photo lab (photoLabS12, WTW, Germany). Heavy metals including Fe, Mn, Zn, and Cu as well as other metals such as Na and K were measured using atomic absorption spectrophotometer (Varian SpectrAA-10 and SpectrAA-20, Australia). The alkalinity was determined using the potentiometric method. The sludge characteristics are presented in Table 1.
Parameter
Concentration
PO4-P
22.2 mg L-1
NH4-N
2.2 mg L-1
COD
262 mg L-1
pH
7.5
DO
0.8 mg L-1
TDS
790 mg L-1
TSS
2.60wt%
alkalinity
526 mg L-1
Cd
0.13 mg L-1
Ni
1.44 mg L-1
Mn
0.084 mg L-1
Fe
1.74 mg L-1
Pb
1.38 mg L-1
CU
0.05 mg L-1
Zn
0.418 mg L-1
Table 1: Characteristics of the sludge produced by the wastewater treatment plant.
Gas sampling
The biogas samples were analyzed using a portable gas analyzer (EAGLE, RKI Instruments, Inc. USA) that determines the CH4, CO2, and H2S content of the biogas. The analyzer includes PID (Photo Ionization Detector) capability. Conversion factors for a variety of common gases are programmed into the PID sensor and LEL/PPM catalytic sensor.
Results and Discussion
Results of set 1 (plastic bottles)
Group # 1: Table 2 shows measured concentration of CH4 (CCH4) in the biogas produced for the various VSS% tested. The mass of CH4 produced (MCH4) was calculated from the following equation assuming that the gas is ideal
1%VSS
2% VSS
4% VSS
6%VSS
25°C
35°C
25°C
35°C
25°C
35°C
25°C
35°C
Week
Weight loss/week (g week-1)
Concentration mg L-1
Weight loss/week (g week-1)
Concentration (mg L-1)
Weight loss/week (g week-1)
Concentration (mg L-1)
Weight loss/week (g week-1)
Concentration (mg L-1)
Weight loss/week (g week-1)
Concentration (mg L-1)
Weight loss/week (g week-1)
Concentration (mg L-1)
Weight loss/week (g week-1)
Concentration (mg L-1)
Weight loss/week (g week-1)
Concentration (mg L-1)
1
0.85
350
0.50
450
0.60
1800
0.90
2300
0.85
1000
0.80
1450
0.50
740
0.40
400
2
1.10
1750
1.75
1300
0.40
1550
0.60
3900
0.55
1300
0.75
2850
1.20
1250
0.90
1350
3
0.15
190
0.25
35
0.50
2500
0.70
2200
0.50
1450
0.60
2250
0.40
1300
0.70
2250
4
0.40
1050
0.60
630
0.35
2550
0.35
2500
0.45
1400
0.35
1200
0.45
1550
0.55
1850
5
0.13
150
0.55
1050
0.65
2700
0.60
2250
0.60
1650
0.85
1700
0.50
1550
0.80
1600
6
0.17
200
0.50
500
0.50
2300
0.65
2000
0.45
2000
0.55
1100
0.50
1700
0.55
1420
7
0.25
340
0.30
450
0.25
3100
0.60
1850
0.45
2300
0.40
940
0.40
1450
0.55
1300
8
0.60
3700
0.60
1900
0.60
2450
0.50
850
0.54
2000
0.60
1150
9
0.65
3100
0.18
1500
0.35
2100
0.19
670
0.51
1900
0.17
900
Table 2: Measured weight loss and CH4 concentration (CCH4) from gas samples for group #1 (2L bottles) (The initial VSS weight is 6 g).
Where: m is the mass in grams of the measured biogas released from the bottles determined by weighing the bottles before and after sampling, Mw is the molar mass of the biogas, Pa is the atmospheric pressure, and R is the international gas constant. The factor 1000 appears in the equation is a conversion factor to get the mass in grams.
The cumulative mass of CH4 produced, presented as MC = S(M
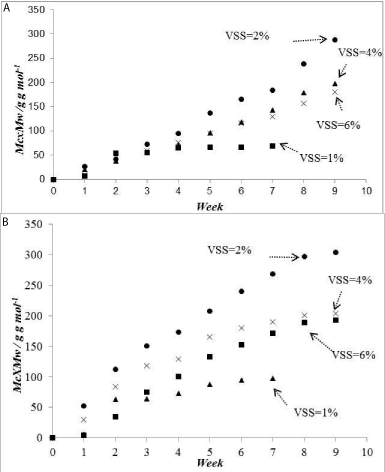
Figure 1: Cumulative mass of CH4 produced at (a) T = 25°Cand (b) T = 35°C.
(2L bottles).
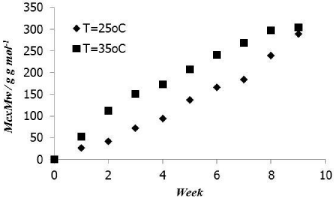
Figure 2: Cumulative mass of methane gas produced (Mc) × Mw for 2 VSS%.
Group # 2: In this group of experiments, no gas samples were withdrawn and analyzed till the end of the experiment (9 weeks). The results for the experiments performed at 25°C and at 35°C are shown in Table 3. As can be seen the concentration of CH4 at 35°C is higher than that at 25°C similar to that observed for group #1. However the percent mass loss is larger at 25°C compared to that at 35°C. This can be explained as follows: at 35°C more biogas and hence more CO2 is produced initially compared to that at 25°C. Since the gas was not released from the bottle, the produced CO2 gas dissolves in the water producing HCO3 - reducing the pH and that in turn reduces the activity of the bacteria. Since the reduction in the pH value is faster at 35°C compared to that at 25°C the weight loss at 25°C is larger. This result recommends that bioreactors should operate at atmospheric pressure and the produced biogas should be removed as soon as it forms. To confirm the above result, weight loss for group #2 where the bottles were under pressure to those of group #1 where the pressure in the bottles was atmospheric is presented in Table 4. The results confirm the findings mentioned above that releasing the pressure or withdrawing the gas as it forms gives better gas production.
% VSS content
25°C
35°C
CH4 concentration (mg L-1)
% Weight Loss
CH4 Concentration (mg L-1)
% Weight Loss
1%
336
4.2
450
10.8
2%
840
81.7
1820
72.5
4%
800
80.0
1480
67.5
6%
828
79.2
1125
70.8
Table 3: Measured CH4 concentration (CCH4), % weight loss after 9 weeks at 25°C and 35°C for group #2 (2L bottles) (The initial VSS weight is 6 g).
group #1
group #2
%VSS content
25°C
35°C
25°C
35°C
1
-
-
4.2
10.8
2
75.0
86.3
81.7
72.5
4
80.0
83.2
80.0
67.5
6
83.3
87.0
79.2
70.8
Table 4: Comparison between weight loss of the sample in group #1 (atmospheric pressure) and group #2 (under pressure) after 9 weeks. (The initial VSS weight is 6 g).
Group # 3: In this set of experiments, gas samples were withdrawn for analysis and the bottles were then immediately closed keeping the contents of the bottles under pressure. The results are presented in Table 5 where it can be seen that the conversion at 25°C is higher compared to that at 35°C confirming the results obtained for group # 2. The results also show that conversion at VSS content of 2% is larger than those at VSS content of 6% as was observed in the previous sets of experiments. Comparing the results of group # 3 where the bottle contents were under pressure to those of group #1 where the contents were under atmospheric pressure is also shown in Table 5. It can be seen that group #3 gives lower biogas conversion compared to those of group #1 which confirms the results obtained previously from group #2.
Group #1
Group #3
Week
2%
6%
2%
6%
åMc
25°C
å Mc
35°C
å Mc
25°C
å Mc
35°C
å Mc
25°C
å Mc
35°C
å Mc
25°C
å Mc
35°C
1
10.0
15.0
8.33
6.66
20.8
10.8
12.5
11.7
2
16.7
25.0
28.3
21.7
30.8
20
23.3
20.8
3
25.0
36.7
35.0
33.3
44.2
30
35
31.7
4
30.8
42.5
42.5
42.5
55
43.3
49.2
44.2
5
41.7
52.5
50.8
55.8
75
65
65
65
6
50.0
63.3
59.2
65.0
85.8
76.7
75.8
79.2
7
54.2
73.3
65.8
74.2
-
-
-
-
8
64.2
83.3
74.8
84.2
-
-
-
-
9
75.0
86.3
83.3
87.0
-
-
-
-
Table 5: A comparison between the cumulative mass × Mw(Mc) obtained at 25°C and 35°C for group #1 (atmospheric pressure) and group #3 (under pressure). (The initial VSS weight is 6 g).
Results of set2 (25L vessel)
The measured CH4 concentration (CCH4), CO2volume percent, and the measured gauge pressure of the vessel (Pg), are shown in Table 6. The pressure and the concentration allow calculating the mass of CH4 produced (MCH4) assuming that the gas is ideal
Day
CCH4(mg L-1)
Pg (bar)
MCH4(g)
CO2 (vol% )
Day
CCH4(mg L-1)
Pg (bar)
MCH4(g)
CO2 (vol% )
2
6750
1.20
15.7
3.18
24
7600
0.40
5.9
5
4
8000
1.00
15.5
4.36
26
7370
0.39
5.6
5
6
8950
0.71
12.3
4.74
28
7150
0.37
5.1
5
8
8900
0.70
12.1
5
30
6780
0.35
4.6
5
10
8750
0.60
10.2
5
32
6560
0.33
4.2
5
12
8900
0.50
8.6
5
34
6420
0.31
3.9
5
14
9750
0.48
8.5
5
36
6250
0.30
3.6
5
16
9350
0.51
9.2
5
38
5860
0.29
3.3
5
18
8910
0.48
8.3
5
40
5700
0.27
3.0
5
20
8500
0.45
7.4
5
42
5450
0.26
2.7
5
22
8050
0.43
6.7
5
44
5250
0.25
2.5
5
Table 6: Measured CH4 concentration (CCH4), gauge pressure (Pg), and CO2volume (%) in the 25 L vessel. The VSS content is 2wt%. H2S concentration = 0.
Where: the subscript “a” and “v” indicate the ambient and the vessel conditions respectively, Vv is the volume of the space in the vessel above the liquid which is equal to 2L, P is the absolute pressure, and T is the absolute temperature. The factor 1000 appears in the equation is a conversion factor to get the mass in grams. Figure 3 shows the cumulative mass of CH4 gas produced versus time. As can be seen the rate is fast at the beginning then decreases and eventually becomes zero. The variation of pH and CH4 concentration are plotted in Figure 4. It can be seen that the biodegradation process was fast since the amount of CH4 reaches 6750 ppm within 2 days. The CH4 content of the biogas continues to increase and reaches its maximum value within 16 days then starts to decline slowly. At this time the bacteria enters the decay phase, when the rate of its death exceeds that of its reproduction [22]. The pH values in Figure 4 drops from 7.5 to 7.1 within the first 8 days and then increases again to 7.5. The reason for this is that Volatile Fatty Acids (VFA) is produced by the acidogenic bacteria causing the pH to drop. At the same time methanogenic bacteria produces CH4 gas from the produced VFA causing the pH to rise. The experimental results indicate that within the first 8 days the rate of VFA production is faster than its consumption to produce CH4gas which causes the pH to drop. However after 8 days the rate of CH4 production exceeds that of VFA resulting in an increase in the pH value till the two rates become equal causing the pH value to remain constant [20]. Moreover the reaction of CO2, which is soluble in water, with the hydroxide ions results in the formation of HCO3 -, which tends to restore the neutrality of the process pH. The above results are in accordance with those of [20]. Table 6 also shows that pressure values are high at the beginning then it drops gradually. This confirms that biogas production rate is fast at the beginning then drops gradually. The amount of CO2 also increases with time until it reaches the maximum detection limit of the instrument (5% Vol.). The analysis shows that no H2S was detected in the biogas produced (or the concentration is below the detection limit of the instrument). This may be attributed to the low sulfate concentration [25] since the rate of SRB growth is much faster than that of Methane Producing Bacteria (MPB) [26]. The undetected H2S concentration under such fermentation conditions indicates that sludge is an environmentally friendly source of energy.
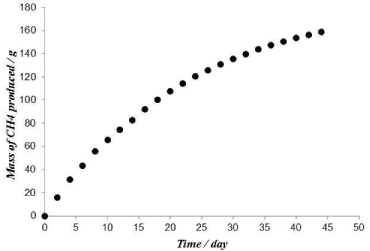
Figure 3: Calculated cumulative mass of CH4 produced in the 25 L vessel
versus time at 35°C and VSS content of 2%.
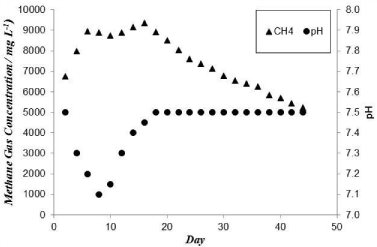
Figure 4: Variation of CH4 concentration and pH with time at 35°C and VSS
content of 2% in the 25 L vessel.
Conclusion
The experimental investigation performed in this research showed that the sludge produced from the wastewater treatment plant is an environmental friendly potential source of biogas. The following conclusions can be withdrawn from the results obtained in this study:
- Amount of biogas produced from sludge and its CH4 content depends on the Volatile Suspended Solids (VSS) concentration. The highest amount of biogas production was achieved at 2% VSS content.
- Biogas production and methane concentration at 35°C was higher than that at 25°C.
- Bioreactor pressure negatively affects biogas production and its methane content. It is recommended to withdraw the produced biogas as soon as it forms.
Acknowledgment
The authors wish to thank the Scientific Research Deanship at Jordan University of Science and Technology for funding this research.
Nomenclature
CCH4 [mgL-1] Concentration of methane gas
Mc [g] Cumulative mass of methane gas produced
MW [g gmol-1] Molar mass of the biogas
P [Pa] Absolute pressure
R [N.mkmol-1 K-1] International gas constant
T [K] Absolute temperature
References
- Sanderson K. Lignocellulose: A chewy problem. Nature. 2011; 474: 12-14.
- Menon V, Rao M. Trends in bioconversion of lignocellulose: biofuels, platform chemicals & biorefinery concept. Prog Energy Combust Sci. 2012; 38: 522–550.
- Dashtban M, Schraft H, Qin W. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int J Biol Sci. 2009; 5: 578-595.
- Wan C, Li Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol Adv. 2012; 30: 1447-1457.
- Narayanaswamy N, Dheeran P, Verma S, Kumar S. Pretreatment Techniques for Biofuels and Biorefineries. Heidelberg, Berlin, Germany: Springer-Verlag. 2013.
- Schmid J, Stahl U, Meyer V. Physiology and Genetics. In: Anke T, Weber D, editors. Genetic and Metabolic Engineering in Filamentous Fungi. Heidelberg, Berlin, Germany: Springer-Verlag. 2009; 377–392.
- Fernandez-Fueyo E, Ruiz-Dueñas FJ, Ferreira P, Floudas D, Hibbett DS, Canessa P, et al. Comparative genomics of Ceriporiopsis subvermispora and Phanerochaete chrysosporium provide insight into selective ligninolysis. Proc Natl Acad Sci USA. 2012; 109: 5458–5463.
- Mahajan S, Master ER. Proteomic characterization of lignocellulose-degrading enzymes secreted by Phanerochaete carnosa grown on spruce and microcrystalline cellulose. Appl Microbiol Biotechnol. 2010; 86: 1903-1914.
- Sato S, Feltus FA, Iyer P, Tien M. The first genome-level transcriptome of the wood-degrading fungus Phanerochaete chrysosporium grown on red oak. Curr Genet. 2009; 55: 273-286.
- Bak JS. Extracellular breakdown of lignocellulosic biomass by Dichomitus squalens: peroxidation-based platform and homeostatic regulation. Biotechnol Lett. 2015; 37: 349-358.
- Tian C, Beeson WT, Iavarone AT, Sun J, Marletta MA, Cate JH, et al. Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa. Proc Natl Acad Sci USA. 2009; 106: 22157-22162.
- Vanden Wymelenberg A, Gaskell J, Mozuch M, Sabat G, Ralph J, Skyba O, et al. Comparative transcriptome and secretome analysis of wood decay fungi Postia placenta and Phanerochaete chrysosporium. Appl Environ Microbiol. 2010; 76: 3599–3610.
- Bak JS. Lignocellulose depolymerization occurs via an environmentally adapted metabolic cascades in the wood-rotting basidiomycete Phanerochaete chrysosporium. Microbiologyopen. 2014.
- Martinez D, Challacombe J, Morgenstern I, Hibbett D, Schmoll M, Kubicek CP, et al. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Natl Acad Sci USA. 2009; 106: 1954-1959.
- Bak JS. Electron beam irradiation enhances the digestibility and fermentation yield of water-soaked lignocellulosic biomass. Biotechnol Rep. 2014; 4: 30–33.
- Bak JS. Process evaluation of electron beam irradiation-based biodegradation relevant to lignocellulose bioconversion. Springerplus. 2014; 3: 487.
- Bak JS. Downstream optimization of fungal-based simultaneous saccharification and fermentation relevant to lignocellulosic ethanol production. SpringerPlus. 2015; 4: 47.