
Review Article
Austin Chem Eng. 2015; 2(2): 1018.
Engineering Yeast for Cellulosic Ethanol Production
Tzi-Yuan Wang
Biodiversity Research Center, Taiwan
*Corresponding author: MTzi-Yuan Wang, Biodiversity Research Center, Academia Sinica, Taipei, 115, Taiwan
Received: August 11, 2015; Accepted: September 28, 2015; Published: October 06, 2015
Abstract
Biofuel, the alternative energy, aims to greatly reduce the carbon emissions on earth. Cellulosic ethanol is expected to replace the first generation biofuel made by agriculture crops, such as corn. Lignocelluloses degradation and fermentation efficiencies are the main limitations. Thus, the objective of consolidated bioprocessing is to engineer a “super” yeast with multiple cellulolytic enzymes, multi-sugars consumption, thermo-tolerance, toxintolerance and efficient ethanol production. The super yeast needs to be engineered to be suitable glycosylation, highly secretable, deficient protease, stress-tolerance and ethanol assimilation for cellulosic ethanol. Omics analysis, adaptive laboratory evolution and genome editing tools would accelerate yeast engineering not only for biofuel, but also applications in other biosynthetic areas.
Keywords: Biomass; Omics; Yeast; Fermentation; Cellulosic ethanol; Synthetic biology; Genome editing
Abbreviations
EU: European Union; CBP: Consolidated Bioprocessing; C5: Pentose; C6: Hexose; GH: Glycosyl Hydrolase; CRISPR: Clustered, Regularly Interspaced, Short Palindromic Repeats; Cas9: CRISPRAssociated Protein 9; SHF: Separate Hydrolysis and Fermentation; SSF: Simultaneous Saccharification and Fermentation; SSCF: Simultaneous Saccharification and Co-Fermentation
Introduction
Alternative fuels are important materials in battling ongoing green-house effects [1,2]. The EU passed a statement that asked car manufacturers to reduce the carbon emissions of their products. Since the 1970’s, bio-ethanol has successfully been produced commercially using agriculture cultivates (especially in corn); benefits the green energy production in several food-supply countries, such as Brazil & USA. However, it enlarged the subsistence problem in other fooddeprived countries [3]. Therefore, cellulosic ethanol produced by feed stocks became the new generation biofuel. Cellulosic ethanol from non-grain plant materials is used as one of the green energy to replace the fossil fuel which causes net carbon emissions and seriously affects climate change for decades.
The basic bioprocess for cellulosic ethanol is feed stocks degradation, sugar utilization and then fermentation (Figure 1). Feedstocks are composed by the main component of lignocelluloses, including celluloses, hemicelluloses, and lignin’s. These polysaccharides could be degraded into sugars by kinds of cellulolytic enzymes. After lignocelluloses degradation, 1-2% C5 & C6 sugar mixtures (D-xylose, L-arabinose, glucose, lactose, etc) are hydrolyzed with the inhibitory compounds (acid/base, furfural, etc), formed during biomass pretreatment process. Yeast utilized these carbon sources can be further fermented and isolated in higher temperature for ethanol products.
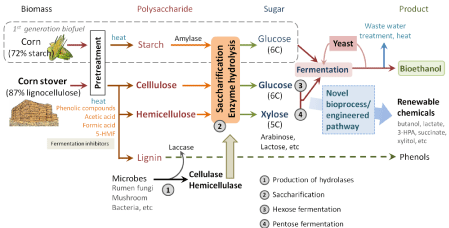
Figure 1: How to make cellulosic ethanol. There are typical hydrolysis and fermentation strategies: separate hydrolysis and fermentation (SHF with four-steps
sequential process: (1) production of hydrolase +(2) saccharification +(3) hexose fermentation +(4)pentose fermentation), Simultaneous Saccharification and
Fermentation (SSF with three step process: (1)+(2+3)+(4)), simultaneous saccharification and co-fermentation (SSCF with two step process: (1)+(2+3+4)), and
Consolidated Bioprocessing (CBP with one-step process: (1+2+3+4)).
An effective saccharification-fermentation biomass process for ethanol production is needed for the biorefinery. Among the kind of bioprocesses, Consolidated Bioprocessing (CBP) is often considered as the preferential process because it has the potential for high efficiency and low cost [4]. However, the rate-limiting step in the conversion of cellulose to fuels is feedstock degradation, especially in hydrolysis of cellulose, hemicelluloses & lignin to sugars. A great deal of effort has gone into the development of methods for conversion of cellulose to sugars through glycosyl hydrolase digestion [5-15]. Good degradation enzymes are always needed to increase the lignocelluloses hydrolysis in the biorefinery process [7,16-19]. Numerous cellulolytic enzyme cocktails, such as Cellic series of enzymes (Novozymes) and Accellerase series of enzymes (Genencor), were already applied for biomass hydrolysis in biorefinery [5,20-23].
Another improvement for CBP is to use efficient fermentation microbes, mainly yeast, which can carry out cellulase/hemicellulase production, hydrolysis, and fermentation to convert pentose sugars and glucose into ethanol [7,24-27]. To simplify the bioprocess for feed stocks conversion via CBP, for example, several good cell factories for both enzyme production and ethanol production were investigated, especially in yeast. Yeast has been a preferable host for fermentation since nineteen century when industrial factories used yeast to produce wine & beer. For example, Saccharomyces cerevisiae is the most common fermentation yeast and could tolerate higher alcohol percentage during fermentation [28]. However, thermosensitivity, over-glycosylation and secretion ability of yeast are still the limitations for CBP application and need to be addressed.
Yeast strain improvement for CBP
Several key approaches for both cellulase expression and ethanol production by consolidated bioprocessing were investigated [6,29- 31]. Improving the efficiency of lignocellulosic breakdown requires engineering of yeast secretory pathway from system-wide metabolic analysis and DNA constructs for enhanced cellulase gene expression (Figure 2). Also, yeast exhibit high tolerance to severe stress due to inhibitory compounds [6]. Such improvements could be applied to industrial cellulosic ethanol as follows:
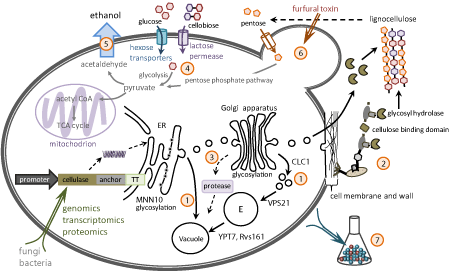
Figure 2: Strategies for CBP yeast engineering. (1) deglycosylation and secretion improvement; (2) cell surface engineering; (3) protease deficient strain; (4)
multiple carbon utilization; (5) Increase ethanol production; (6) tolerance adaptation; (7) immobilization and high cell density.
Deglycosylation and secretion improvement for better enzyme activity: Increasing the extracellular cellulases in S. cerevisiae directly influences the efficiency of biomass breakdown. Both overand under-glycosylation may alter the enzyme activity of cellulases in S. cerevisiae [32-34]. For example, knockout of the inherent glycosylation-related MNN10 gene had a more than 6-folds increase in extracellular exocellulase activity [34]. In addition, blocking Golgito- endosome transport may force S. cerevisiae to export cellulases. Wang et al. (2013) showed yeast with deficient VPS21 genes could increase extracellular cellulases activity by 6-fold and reduce the bioprocess time. Improving extracellular protein trafficking and gene expression can also accelerate the biomass degradation [6,34-37].
Cell surface engineering for better digestion: To degrade the feed stocks into sugars efficiently, feedstocks need to directly interact with cellulases/hemicellulases/ligninases. Therefore, artificial cellulosome is an alternative way to improve cellulose degradation [38-41]. For example, both clostridium and rumen fungi have CBM10 domain and cellulosome structure genes [13,42], which could be adapted for cell surface engineering in yeast [39,43].
Protease deficient strain for enzyme stability: Foreign proteins expressed in yeast are usually degraded by ubiquitin proteasome [44]. Thus, even though yeast could over-express cellulase proteins, the functional cellulase may not be exported out of the cell for cellulose breakdown. One needs to delete such proteases to prevent the heterologous cellulase degradation in engineered yeast [45-47]. For example, the proteins in K. lactis could be degraded because of its proteases [48]. Selected mutants or deficient proteases strain could be applied for heterologous proteins without degradation [49].
Multiple carbon utilization for CBP: More and better yeast sugar transporters could uptake sugar for efficient fermentation [30,50]. One way is to find out the yeast strains that could uptake various sugar, such as Kluyveromyces marxianus [51], Pichia anomala [52]. The other way is to engineer sugar pathway genes into yeast [29,53-55]. For example, C5 sugar pathway in S. cerevisiae could uptake xylose and glucose at once [29,56-58]; the same for C6 sugar pathway in Pichia stipitis [59]. Such recombinant yeasts could directly utilize these sugars for ethanol fermentation.
Increase ethanol production: Yeast ethanol assimilation is another mutant engineering method for CBP. Ethanol production is the final step to evaluate the efficiency of CBP bioprocess [54,60]. To increase ethanol production, for example, the Aspartic Protease gene (Asp) of Neurospora crassa expressed in industrial ethanolproducing yeast could increase both growth rate and viable yeast counts; also the recombinant strain of S. cerevisiae exhibits a higher ethanol yield [61]. Other approaches for a promising producer include mutagenesis and/or adaptive strains selected from feedstocks [62]. However, most wild yeasts also consume lots of ethanol, which reduce the production yield. Ethanol assimilation is one possible way to prevent yeast consume ethanol. Evolutionary adaptation or mutagenesis could select for such evolved yeast strains for high-yield ethanol production.
Tolerance adaptation for better yeast: Most yeast cannot have higher conversion ratio of ethanol due to thermo-intolerance [51], oxidative stress [63], and/or toxin-intolerance [51,52,64,65]. Therefore, adaptive evolution helps to select evolved strains that can grow at higher temperature and survive under quite amount of furfural or acid to reduce the energy cost during bioprocessing.
Immobilization and high cell density for efficient process: The other way to increase the efficiency is cell immobilization [66] and high cell density [67]. Biochemical engineers already have matured the techniques for efficient bioprocess. These methods enlarge the contact area with feedstocks and significantly reduce the fermentation time.
In short, an ideal yeast strain for CBP can secrete good glycosyl hydrolases for feedstock degradation, consume multiple sugars and tolerate high ethanol concentrations to optimize ethanol yields under harsh environment (high temperature and toxic conditions). Therefore, cellulosic ethanol bioprocess could be the economic scaleup for the industrial biorefinery in the near future.
Future direction
Yeast for cellulosic ethanol production is the main CBP for industrial application, not only because of its well-known fermentation efficiency, but also the simple genetic manipulation process. New bio-techniques recently accelerate yeast engineering to achieve cost-effective cellulosic ethanol for energy usage.
Omics approach reveals novel enzymes and fermentation engineering: Microbial strategies for degrading lignocellulose are diverse, but we know the enzymes involved in these processes are limited. Recent advances in genomics, metagenomics, transcriptomics, and secretomics offer a cost-effective approach to identify kinds of efficient cellulases and production from microbes [15,16,42,68-73]. Another approach is to identify efficient secretion and fermentation through metabolomes and transcriptome profiling of yeast, such as S. cerevisiae, P. stipitis, K. marxianus, Zymomonas mobilis [71,74,75]. By taking advantage of available ’Omics tools, this strategy is likely to succeed in generating CBP strains in the near future.
Genome editing accelerates yeast engineering: The CRISPRCas9 system makes genome editing easier in diploid, which has already succeeded in animals [76], plants [77] and microbes [78]. This new technology enables researchers to knock-out and/or mutate multiple genes at once in a yeast genome [79]. Designed yeast can utilize a set of genes or integrate new pathway genes faster than before. For example, a construction kit in T. reesei was established for high throughput generation of gene knock-outs [80]. Theoretically, the CRISPR-Cas9 system could engineer 5-10 pathway genes into yeast at once. Suitable CBP platform for cellulosic ethanol production will be realized soon through these genome editing tools.
Adaptive laboratory evolution for ideal CBP yeast: Environmental adaptation could easily change yeast phenotypes into thermo-tolerance and toxin-tolerance [81-83]. Such mutants selected by adaptive laboratory evolution could also reduce the energy wastes during fermentation. Thus, ideal CBP yeast could be constructed to fulfill all the needs for cellulosic ethanol production.
Conclusion
Ideal yeast is necessary for CBP, which new biotechnologies make it easier to create a suitable cell factory for cellulosic ethanol production. Yeast with secret able, high activity of glycosyl hydrolases and efficient, high-yield ethanol production will be essential not only to make cheaper cellulosic ethanol, but also to initiate new cell factory in agriculture and biomedical applications.
References
- Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature. 2012; 488: 294-303.
- Goldemberg J. Ethanol for a sustainable energy future. Science. 2007; 315: 808-810.
- Tenenbaum DJ. Food vs. fuel: diversion of crops could cause more hunger. Environ Health Perspect. 2008; 116: A254-257.
- Olson DG, McBride JE, Shaw AJ, Lynd LR. Recent progress in consolidated bioprocessing. Curr Opin Biotechnol. 2012; 23: 396-405.
- Banerjee G, Car S, Scott-Craig JS, Borrusch MS, Walton JD. Rapid optimization of enzyme mixtures for deconstruction of diverse pretreatment/biomass feedstock combinations. Biotechnology for biofuels. 2010; 3: 22.
- Hasunuma T, Ishii J, Kondo A. Rational design and evolutional fine tuning of Saccharomyces cerevisiae for biomass breakdown. Curr Opin Chem Biol. 2015; 29: 1-9.
- Inoue H, Decker SR, Taylor LE, Yano S, Sawayama S. Identification and characterization of core cellulolytic enzymes from Talaromyces cellulolyticus (formerly Acremonium cellulolyticus) critical for hydrolysis of lignocellulosic biomass. Biotechnology for biofuels. 2014; 7: 151.
- Katahira S, Mizuike A, Fukuda H, Kondo A. Ethanol fermentation from lignocellulosic hydrolysate by a recombinant xylose- and cellooligosaccharide-assimilating yeast strain. Appl Microbiol Biotechnol. 2006; 72: 1136-1143.
- Park JI, Steen EJ, Burd H, Evans SS, Redding-Johnson AM, Batth T, et al. A thermophilic ionic liquid-tolerant cellulase cocktail for the production of cellulosic biofuels. PLoS One. 2012; 7: e37010.
- Song L, Siguier B, Dumon C, Bozonnet S, O'Donohue MJ. Engineering better biomass-degrading ability into a GH11 xylanase using a directed evolution strategy. Biotechnology for biofuels. 2012; 5: 3.
- Strobel GA. Methods of discovery and techniques to study endophytic fungi producing fuel-related hydrocarbons. Nat Prod Rep. 2014; 31: 259-272.
- Walton J, Banerjee G, Car S. GENPLAT: an automated platform for biomass enzyme discovery and cocktail optimization. J Vis Exp. 2011.
- Wang TY, Chen HL, Li WH, Sung HM, Shih MC. Omics Applications to Biofuel Research. In: Hou CT, Shaw JF, editors. Biocatalysis and Biomolecular Engineering. New York: John Wiley & Sons, Inc. 2010; 265-276.
- Yamada R, Yoshie T, Sakai S, Wakai S, Asai-Nakashima N, Okazaki F, et al. Effective saccharification of kraft pulp by using a cellulase cocktail prepared from genetically engineered Aspergillus oryzae. Bioscience, biotechnology, and biochemistry. 2015; 79: 1034-1037.
- Yan X, Geng A, Zhang J, Wei Y, Zhang L, Qian C, et al. Discovery of (hemi-) cellulase genes in a metagenomic library from a biogas digester using 454 pyrosequencing. Appl Microbiol Biotechnol. 2013; 97: 8173-8182.
- Del Pozo MV, Fernandez-Arrojo L, Gil-Martinez J, Montesinos A, Chernikova TN, Nechitaylo TY, et al. Microbial beta-glucosidases from cow rumen metagenome enhance the saccharification of lignocellulose in combination with commercial cellulase cocktail. Biotechnology for biofuels. 2012; 5: 73.
- Prade RA. Xylanases: from biology to biotechnology. Biotechnol Genet Eng Rev. 1996; 13: 101-131.
- Sydenham R, Zheng Y, Riemens A, Tsang A, Powlowski J, Storms R. Cloning and enzymatic characterization of four thermostable fungal endo-1,4-β-xylanases. Appl Microbiol Biotechnol. 2014; 98: 3613-3628.
- Tiwari P, Misra BN, Sangwan NS. β-Glucosidases from the fungus trichoderma: an efficient cellulase machinery in biotechnological applications. BioMed research international. 2013; 203735.
- Bhattacharya A, Pletschke BI. Review of the enzymatic machinery of Halothermothrix orenii with special reference to industrial applications. Enzyme Microb Technol. 2014; 55: 159-169.
- Ju X, Bowden M, Engelhard M, Zhang X. Investigating commercial cellulase performances toward specific biomass recalcitrance factors using reference substrates. Appl Microbiol Biotechnol. 2014; 98: 4409-4420.
- Kawai T, Nakazawa H, Ida N, Okada H, Tani S, Sumitani J, et al. Analysis of the saccharification capability of high-functional cellulase JN11 for various pretreated biomasses through a comparison with commercially available counterparts. Journal of industrial microbiology & biotechnology. 2012; 39: 1741-1749.
- Rajan K, Carrier DJ. Characterization of Rice Straw Prehydrolyzates and Their Effect on the Hydrolysis of Model Substrates Using a Commercial -Cellulase, beta-Glucosidase and Cellulase Cocktail. ACS sustainable chemistry & engineering. 2014; 2: 2124-2130.
- Kim SR, Park YC, Jin YS, Seo JH. Strain engineering of Saccharomyces cerevisiae for enhanced xylose metabolism. Biotechnol Adv. 2013; 31: 851-861.
- Kumari R, Pramanik K. Improved bioethanol production using fusants of Saccharomyces cerevisiae and xylose-fermenting yeasts. Appl Biochem Biotechnol. 2012; 167: 873-884.
- Wei N, Quarterman J, Kim SR, Cate JH, Jin YS. Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast. Nat Commun. 2013; 4: 2580.
- Yanase H, Miyawaki H, Sakurai M, Kawakami A, Matsumoto M, Haga K, et al. Ethanol production from wood hydrolysate using genetically engineered Zymomonas mobilis. Appl Microbiol Biotechnol. 2012; 94: 1667-1678.
- Hu XH, Wang MH, Tan T, Li JR, Yang H, Leach L, et al. Genetic dissection of ethanol tolerance in the budding yeast Saccharomyces cerevisiae. Genetics. 2007; 175: 1479-1487.
- Khattab SM, Saimura M, Kodaki T. Boost in bioethanol production using recombinant Saccharomyces cerevisiae with mutated strictly NADPH-dependent xylose reductase and NADP(+)-dependent xylitol dehydrogenase. J Biotechnol. 2013; 165: 153-156.
- Kim SR, Ha SJ, Wei N, Oh EJ, Jin YS. Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol. Trends Biotechnol. 2012; 30: 274-282.
- Laluce C, Schenberg AC, Gallardo JC, Coradello LF, Pombeiro-Sponchiado SR. Advances and developments in strategies to improve strains of Saccharomyces cerevisiae and processes to obtain the lignocellulosic ethanol--a review. Applied biochemistry and biotechnology. 2012; 166: 1908-1926.
- Beckham GT, Dai Z, Matthews JF, Momany M, Payne CM, Adney WS, et al. Harnessing glycosylation to improve cellulase activity. Curr Opin Biotechnol. 2012; 23: 338-345.
- Montesino R, García R, Quintero O, Cremata JA. Variation in N-linked oligosaccharide structures on heterologous proteins secreted by the methylotrophic yeast Pichia pastoris. Protein Expr Purif. 1998; 14: 197-207.
- Wang TY, Huang CJ, Chen HL, Ho PC, Ke HM, Cho HY, et al. Systematic screening of glycosylation- and trafficking-associated gene knockouts in Saccharomyces cerevisiae identifies mutants with improved heterologous exocellulase activity and host secretion. BMC biotechnology. 2013; 13: 71.
- Gardner JG, Keating DH. Requirement of the type II secretion system for utilization of cellulosic substrates by Cellvibrio japonicus. Appl Environ Microbiol. 2010; 76: 5079-5087.
- Rodriguez-Limas WA, Tannenbaum V, Tyo KE. Blocking endocytotic mechanisms to improve heterologous protein titers in Saccharomyces cerevisiae. Biotechnology and bioengineering. 2015; 112: 376-385.
- Tang H, Hou J, Shen Y, Xu L, Yang H, Fang X, et al. High β-glucosidase secretion in Saccharomyces cerevisiae improves the efficiency of cellulase hydrolysis and ethanol production in simultaneous saccharification and fermentation. Journal of microbiology and biotechnology. 2013; 23: 1577-1585.
- Hong W, Zhang J, Feng Y, Mohr G, Lambowitz AM, Cui GZ, et al. The contribution of cellulosomal scaffoldins to cellulose hydrolysis by Clostridium thermocellum analyzed by using thermotargetrons. Biotechnology for biofuels. 2014; 7: 80.
- Huang GL, Anderson TD, Clubb RT. Engineering microbial surfaces to degrade lignocellulosic biomass. Bioengineered. 2014; 5: 96-106.
- Inokuma K, Hasunuma T, Kondo A. Efficient yeast cell-surface display of exo- and endo-cellulase using the SED1 anchoring region and its original promoter. Biotechnol Biofuels. 2014; 7: 8.
- Wen F, Sun J, Zhao H. Yeast surface display of trifunctional minicellulosomes for simultaneous saccharification and fermentation of cellulose to ethanol. Applied and environmental microbiology. 2010; 76: 1251-1260.
- Wang TY, Chen HL, Lu MY, Chen YC, Sung HM, Mao CT, et al. Functional characterization of cellulases identified from the cow rumen fungus Neocallimastix patriciarum W5 by transcriptomic and secretomic analyses. Biotechnology for biofuels. 2011; 4: 24.
- Tanaka T, Kondo A. Cell surface engineering of industrial microorganisms for biorefining applications. Biotechnol Adv. 2015.
- Will TJ, McWatters MK, McQuade KL. Exploring the ubiquitin-proteasome protein degradation pathway in yeast. Biochemistry and molecular biology education: a bimonthly publication of the International Union of Biochemistry and Molecular Biology. 2006; 34: 444-446.
- Idiris A, Tohda H, Bi KW, Isoai A, Kumagai H, Giga-Hama Y. Enhanced productivity of protease-sensitive heterologous proteins by disruption of multiple protease genes in the fission yeast Schizosaccharomyces pombe. Appl Microbiol Biotechnol. 2006; 73: 404-420.
- Sanglard D, Togni G, de Viragh PA, Monod M. Disruption of the gene encoding the secreted acid protease (ACP) in the yeast Candida tropicalis. FEMS Microbiol Lett. 1992; 74: 149-156.
- Suzuki K, Ichikawa K, Jigami Y. Yeast mutants with enhanced ability to secrete human lysozyme: isolation and identification of a protease-deficient mutant. Molecular & general genetics: MGG. 1989; 219: 58-64.
- Ganatra MB, Vainauskas S, Hong JM, Taylor TE, Denson JP, Esposito D, et al. A set of aspartyl protease-deficient strains for improved expression of heterologous proteins in Kluyveromyces lactis. FEMS yeast research. 2011; 11: 168-178.
- Curto P, Lufrano D, Pinto C, Custodio V, Gomes AC, Trejo SA, et al. Establishing the yeast Kluyveromyces lactis as an expression host for production of the saposin-like domain of the aspartic protease cirsin. Applied and environmental microbiology. 2014; 80: 86-96.
- Chandel AK, Singh OV, Rao LV, Chandrasekhar G, Narasu ML. Bioconversion of novel substrate Saccharum spontaneum, a weedy material, into ethanol by Pichia stipitis NCIM3498. Bioresour Technol. 2011; 102: 1709-1714.
- Chang JJ, Ho CY, Ho FJ, Tsai TY, Ke HM, Wang CH, et al. PGASO: A synthetic biology tool for engineering a cellulolytic yeast. Biotechnol Biofuels. 2012; 5: 53.
- Zha Y, Hossain AH, Tobola F, Sedee N, Havekes M, Punt PJ. Pichia anomala 29X: a resistant strain for lignocellulosic biomass hydrolysate fermentation. FEMS Yeast Res. 2013; 13: 609-617.
- Kato H, Matsuda F, Yamada R, Nagata K, Shirai T, Hasunuma T, et al. Cocktail d-integration of xylose assimilation genes for efficient ethanol production from xylose in Saccharomyces cerevisiae. J Biosci Bioeng. 2013; 116: 333-336.
- Matsushika A, Inoue H, Murakami K, Takimura O, Sawayama S. Bioethanol production performance of five recombinant strains of laboratory and industrial xylose-fermenting Saccharomyces cerevisiae. Bioresour Technol. 2009; 100: 2392-2398.
- Roca C, Nielsen J, Olsson L. Metabolic engineering of ammonium assimilation in xylose-fermenting Saccharomyces cerevisiae improves ethanol production. Appl Environ Microbiol. 2003; 69: 4732-4736.
- Ho NW, Chen Z, Brainard AP, Sedlak M. Successful design and development of genetically engineered Saccharomyces yeasts for effective cofermentation of glucose and xylose from cellulosic biomass to fuel ethanol. Advances in biochemical engineering/biotechnology. 1999; 65: 163-192.
- Hughes SR, Sterner DE, Bischoff KM, Hector RE, Dowd PF, Qureshi N, et al. Engineered Saccharomyces cerevisiae strain for improved xylose utilization with a three-plasmid SUMO yeast expression system. Plasmid. 2009; 61: 22-38.
- Sedlak M, Ho NW. Production of ethanol from cellulosic biomass hydrolysates using genetically engineered Saccharomyces yeast capable of cofermenting glucose and xylose. Appl Biochem Biotechnol. 2004; 113-116: 403-416.
- Biswas R, Uellendahl H, Ahring BK. Conversion of C6 and C5 sugars in undetoxified wet exploded bagasse hydrolysates using Scheffersomyces (Pichia) stipitis CBS6054. AMB Express. 2013; 3: 42.
- Goncalves FA, Santos ES, de Macedo GR. Alcoholic fermentation of Saccharomyces cerevisiae, Pichia stipitis and Zymomonas mobilis in the presence of inhibitory compounds and seawater. Journal of basic microbiology. 2015; 55: 695-708.
- Guo ZP, Qiu CY, Zhang L, Ding ZY, Wang ZX, Shi GY. Expression of aspartic protease from Neurospora crassa in industrial ethanol-producing yeast and its application in ethanol production. Enzyme Microb Technol. 2011; 48: 148-154.
- Tao N, Gao Y, Liu Y. Isolation and characterization of a Pichia anomala strain: a promising candidate for bioethanol production. Braz J Microbiol. 2011; 42: 668-675.
- Spencer J, Phister TG, Smart KA, Greetham D. Tolerance of pentose utilising yeast to hydrogen peroxide-induced oxidative stress. BMC Res Notes. 2014; 7: 151.
- Liu ZL. Molecular mechanisms of yeast tolerance and in situ detoxification of lignocellulose hydrolysates. Appl Microbiol Biotechnol. 2011; 90: 809-825.
- Liu ZL, Slininger PJ, Dien BS, Berhow MA, Kurtzman CP, Gorsich SW. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. Journal of industrial microbiology & biotechnology. 2004; 31: 345-352.
- Liu YK, Yang CA, Chen WC, Wei YH. Producing bioethanol from cellulosic hydrolyzate via co-immobilized cultivation strategy. J Biosci Bioeng. 2012; 114: 198-203.
- Sarks C, Jin M, Sato TK, Balan V, Dale BE. Studying the rapid bioconversion of lignocellulosic sugars into ethanol using high cell density fermentations with cell recycle. Biotechnol Biofuels. 2014; 7: 73.
- Hori C, Gaskell J, Igarashi K, Samejima M, Hibbett D, Henrissat B, et al. Genomewide analysis of polysaccharides degrading enzymes in 11 white- and brown-rot Polyporales provides insight into mechanisms of wood decay. Mycologia. 2013; 105: 1412-1427.
- Kaur B, Sharma M, Soni R, Oberoi HS, Chadha BS. Proteome-based profiling of hypercellulase-producing strains developed through interspecific protoplast fusion between Aspergillus nidulans and Aspergillus tubingensis. Appl Biochem Biotechnol. 2013; 169: 393-407.
- Nagendran S, Hallen-Adams HE, Paper JM, Aslam N, Walton JD. Reduced genomic potential for secreted plant cell-wall-degrading enzymes in the ectomycorrhizal fungus Amanita bisporigera, based on the secretome of Trichoderma reesei. Fungal genetics and biology: FG & B. 2009; 46: 427-435.
- Rubin EM. Genomics of cellulosic biofuels. Nature. 2008; 454: 841-845.
- Wei H, Wang W, Alahuhta M, Vander Wall T, Baker JO, Taylor LE, et al. Engineering towards a complete heterologous cellulase secretome in Yarrowia lipolytica reveals its potential for consolidated bioprocessing. Biotechnology for biofuels. 2014; 7: 148.
- Wei H, Wang W, Yarbrough JM, Baker JO, Laurens L, Van Wychen S, et al. Genomic, proteomic, and biochemical analyses of oleaginous Mucor circinelloides: evaluating its capability in utilizing cellulolytic substrates for lipid production. PloS one. 2013; 8: e71068.
- Feng X, Zhao H. Investigating host dependence of xylose utilization in recombinant Saccharomyces cerevisiae strains using RNA-seq analysis. Biotechnol Biofuels. 2013; 6: 96.
- He MX, Wu B, Shui ZX, Hu QC, Wang WG, Tan FR, et al. Transcriptome profiling of Zymomonas mobilis under ethanol stress. Biotechnology for biofuels. 2012; 5: 75.
- Peng J, Zhou Y, Zhu S, Wei W. High-throughput screens in mammalian cells using the CRISPR-Cas9 system. FEBS J. 2015; 282: 2089-2096.
- Belhaj K, Chaparro-Garcia A, Kamoun S, Patron NJ, Nekrasov V. Editing plant genomes with CRISPR/Cas9. Curr Opin Biotechnol. 2015; 32: 76-84.
- DiCarlo JE, Norville JE, Mali P, Rios X, Aach J, Church GM. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013; 41: 4336-4343.
- Hughes SR, Cox EJ, Bang SS, Pinkelman RJ, Lopez-Nunez JC, Saha BC, et al. Process for Assembly and Transformation into Saccharomyces cerevisiae of a Synthetic Yeast Artificial Chromosome Containing a Multigene Cassette to Express Enzymes That Enhance Xylose Utilization Designed for an Automated Platform. Journal of laboratory automation. 2015.
- Schuster A, Bruno KS, Collett JR, Baker SE, Seiboth B, Kubicek CP, et al. A versatile toolkit for high throughput functional genomics with Trichoderma reesei. Biotechnol Biofuels. 2012; 5: 1.
- Heer D, Sauer U. Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain. Microb Biotechnol. 2008; 1: 497-506.
- Mitsumasu K, Liu ZS, Tang YQ, Akamatsu T, Taguchi H, Kida K. Development of industrial yeast strain with improved acid- and thermo-tolerance through evolution under continuous fermentation conditions followed by haploidization and mating. Journal of bioscience and bioengineering. 2014; 118: 689-695.
- Tilloy V, Ortiz-Julien A, Dequin S. Reduction of ethanol yield and improvement of glycerol formation by adaptive evolution of the wine yeast Saccharomyces cerevisiae under hyperosmotic conditions. Applied and environmental microbiology. 2014; 80: 2623-2632.