
Research Article
Austin Chem Eng. 2016; 3(1): 1026.
Kinetics and Mechanism of Oxidation of Vanillin by Permanganate in Neutral Medium and the Effect of Different Transition Metal Ion Catalysts
Fawzy A1,2*, Zaafarany IA2, Althagafi I2, Alfahemi J2 and Morad M2
1Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
2Chemistry Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia
*Corresponding author: Fawzy A, Associate Professor, Chemistry Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia; Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
Received: March 25, 2016; Accepted: April 01, 2016; Published: April 04, 2016
Abstract
The kinetics of oxidation of vanillin (VAN) by permanganate ion in neutral medium has been investigated spectrophtometrically. The stoichiometry of the reaction was found to be 3:2 (VAN: MnO4 -). The oxidation reaction exhibited a first order dependence in [MnO4 -] and less than unit order dependence with respect to vanillin concentration. Addition of small amounts of Ag(I), Co(II) and Ru(III) catalysts increased the oxidation rate and the catalytic efficiency increased in the order: Ru(III) > Ag(I) > Co(II). The proposed oxidation mechanism involves formation of a 1:1 intermediate complex between vanillin and permanganate ion in pre-equilibrium step. The final oxidation product of vanillin was identified by both spectral and chemical analysis as vanillic acid. The appropriate rate law was deduced. The reaction constants involved in the different steps of the mechanism were evaluated. The activation parameters associated with the rate constant of the slow step of the proposed mechanism along with the thermodynamic quantities of the equilibrium constant have been evaluated and discussed.
Keywords: Vanillin; Permanganate; Neutral medium; Oxidation; Kinetics; Mechanism
Introduction
Vanillin is a phenolic aldehyde, which is an organic compound including aldehyde, hydroxyl and ether. It is the primary component of the extract of the vanilla bean. Vanillin is a very popular flavouring reagent in the food industry and is widely used in the synthesis of drugs such as Aldomet, L-Dopa (I) and Trimethaprin 2A. It has been also used as a chemical intermediate in the production of pharmaceuticals and other fine chemicals. Furthermore, it is used in the preparation of perfume and as a catalyst in various polymerization reactions. If the hydroxyl group in vanillin is protected, it undergoes oxidation to vanillic acid [1]. Kinetics of oxidation of vanillin has been studied previously in alkaline media by few reagents, such as hexacyanoferrate(III) [2], diperiodatoargentate(III) [3], bismuth(V) [4], diperiodatonickelate(IV) [5] and periodate catalyzed by ruthenium(III) [6], and in acid media by cerium(IV) [7].
Permanganate ion is an efficient oxidant in acid, neutral and alkaline media [8-14] which still remains as one of the most important, eco-friendly and powerful multi-electron oxidants employed in the kinetic studies [15]. The mechanism of oxidation by this multivalent oxidant depends not only on the substrate but also on the medium used for the study. During oxidation by permanganate ion, it is evident that the Mn(VII) species in permanganate is reduced to various oxidation states in different media
Transition metal ions, M(Y), where M is the metal and Y is its valance, have been widely employed as homogenous catalysts for oxidation of organic and inorganic substrates by reaction pathways such as formation of complexes with the reactants, oxidation of a substrate, or the formation of free radicals [16-28]. The mechanistic study of the catalyzed reactions is considered an important research field due to the role played by metals in biological systems.
The present study deals with the oxidative behavior of permanganate ion with vanillin in neutral medium and study the catalytic effect of some metal ions with different valences, namely Ag(I), Co(II) and Ru(III). We aim in the present to establish the optimum conditions affecting such oxidation, to examine the catalytic activity of the investigated metal ions, and finally to elucidate a plausible oxidation mechanism on the basis of the obtained kinetic and spectral results.
Experimental
Materials
All reagents used in this investigation were from Merck or Sigma. A stock solution of vanillin was prepared afresh by dissolving the appropriate amount of the sample (S.D. Fine Chem.) in the required volume of distilled water. Solution of potassium permanganate was prepared and standardized as reported earlier [29]. Other chemicals were of analytical grade and their solutions were prepared by dissolving requisite amounts of the samples in double-distilled water.
Kinetic measurements
Kinetic runs were performed under pseudo-first order conditions with a large excess of vanillin over permanganate. The reactions temperature (25°C) was controlled within ±0.1°C. The progress of the reaction was followed by monitoring the decrease in the absorbance of permanganate ion, as a function of time, at λ = 526 nm, its absorption maximum, on a thermostatted Shimadzu UV-VIS-NIR-3600 doublebeam spectrophotometer.
First order plots, ln(absorbance) – time plots, were straight lines for more than two half-lives completion of the reaction, and the observed first order rate constants (kobs) were calculated as the slopes of such plots. Average values of at least three kinetic measurements of the rate constant were taken. The rate constants were reproducible to within 2-3%. The reaction orders with respect to the reactants were determined from the plots of log kobs versus log (conc.). Some kinetic runs were performed under purified nitrogen and compared with those taken under air, and the results were the same. Thus, dissolved oxygen did not affect the oxidation rate.
Results
Stoichiometry and product analysis
Different sets of reaction mixtures containing varying ratios of permanganate to vanillin were mixed in neutral medium at constant temperature then were kept for about 24 hours. Estimation of the remaining permanganate concentrations was performed spectrophotometrically. The results confirm that the stoichiometry is 3:2 (vanillin: permanganate) which holds by the following equation 1,
equation 1:
The above stoichiometric equation is consistent with the results of product analysis. The oxidation product of vanillin was identified as the corresponding carboxylic acid (vanillic acid) by both spectral and chemical analyses as reported [30,31]. Similar oxidation product of vanillin has been also reported earlier [2,3].
Spectral changes
Spectral changes during the oxidation of vanillin by permanganate ion in neutral medium are shown in Figure 1. The main characteristic feature observed from the figure was the gradual disappearance of permanganate band at λ = 526 nm. Also there was growth of two absorption bands at wavelengths above λ = 580 and at λ = 430 nm with appearance of two isosbestic points at wavelengths of about 576 and 485 nm during the reaction course.
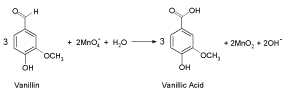
Figure 1: Spectral changes during the oxidation of vanillin by permanganate
ion in neutral medium. [VAN] = 0.01, [MnO4
-] = 4.0 x 10-4 mol dm-3 at 25°C.
Effect of Permanganate concentration on the oxidation rate
The effect of permanganate concentration on the rate of reaction was studied by varying its concentration in the range of 2.0 x 10-4 to 14.0 x 10-4 mol dm-3 at fixed vanillin concentration and temperature. The order with respect to [MnO4 -] was found to be unity, as plots of ln(absorbance) versus time were linear up to about two half-lives of the reaction completion. The first order dependence of the reaction on [MnO4 -] was also confirmed by the non-variation of the observed first order rate constant (kobs) at various [MnO4 -] while keeping others constant as listed in Table 1.
104 [MnO4-]
(mol dm-3)
102 [MAPF]
(mol dm-3)
105 [Ag(I)]
(mol dm-3)
105 [Co(II)]
(mol dm-3)
105 [Ru(III)]
(mol dm-3)
105 kobs
(s-1)
2.0
1.0
37.2
4.0
1.0
36.9
6.0
1.0
38.1
8.0
1.0
37.4
10.0
1.0
36.8
12.0
1.0
35.9
14.0
1.0
37.4
4.0
0.4
17.2
4.0
0.6
23.6
4.0
0.8
29.4
4.0
1.0
36.9
4.0
1.2
44.9
4.0
1.4
51.2
4.0
1.6
54.7
4.0
1.0
2.0
52 .1
4.0
1.0
4.0
6 7.9
4.0
1.0
6.0
84 .3
4.0
1.0
8.0
100 .2
4.0
1.0
10.0
11 0.7
4.0
1.0
12.0
12 4.6
4.0
1.0
14.0
136 .0
4.0
1.0
2.0
4 2.9
4.0
1.0
4.0
49.7
4.0
1.0
6.0
59.7
4.0
1.0
8.0
7 1.0
4.0
1.0
10.0
81 .2
4.0
1.0
12.0
88 .3
4.0
1.0
14.0
93 .0
4.0
1.0
2.0
58.9
4.0
1.0
4.0
79.4
4.0
1.0
6.0
99.7
4.0
1.0
8.0
120 .2
4.0
1.0
10.0
137 .4
4.0
1.0
12.0
152 .0
4.0
1.0
14.0
169 .1
Experimental error ± 3%.
Table 1: Effects of concentration variation of permanganate, vanillin and metal ion catalysts on the observed first order rate constant (kobs) in the oxidation of vanillin by permanganate ion in neutral medium at 25°C
Effect of Vanillin concentration on the oxidation rate
The observed first order rate constant was determined at different initial concentrations of the reductant vanillin keeping other conditions constant. It was found that the rate of reaction increased with increasing the concentration of vanillin as listed in Table 2. A plot of kobs versus [VAN] was found to be linear with a positive intercept on the kobs axis as shown in Figure 2 suggesting that the order with respect to [VAN] was less than unity.
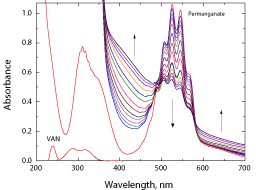
Figure 2: A plot of kobs versus [VAN] in the oxidation of vanillin by
permanganate ion in neutral medium. [MnO4
-] = 4.0 x 10-4 mol dm-3 at 25°C.
Constant
Temperature (K)
293
298
303
308
313
103 k (s-1)
1.80
2.12
2.44
2.63
2.77
K (dm3 mol-1)
2.02
2.18
2.32
2.53
2.86
Experimental error ±3%
Table 2: Values of the rate constant of the slow step (k) and the equilibrium constant (K) at different temperatures in the oxidation of vanillin by permanganate ion in neutral medium.
Effect of metal ion catalysts
The reaction rate was measured at various Ag(I), Co(II) and Ru(III) concentrations (2.0 – 14.0 x 10-5 mol dm-3) at constant other variables. The results showed that the oxidation rate was increased with increasing metal ion concentration and the order of catalytic efficiency was: Ru(III) > Ag(I) > Co(II) as shown in Figure 3.
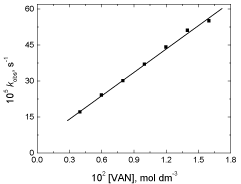
Figure 3: Effect of variation of Ag(I), Co(III) and Ru(III) concentrations on
the observed first order rate constant (kobs) in the oxidation of vanillin by
permanganate ion in neutral medium. [VAN] = 0.01, [MnO4
-] = 4.0 x 10-4 mol
dm-3 at 25oC.
Effect of temperature
The reaction rate was measured at five different temperatures namely, 288, 293, 298, 303 and 308 K under varying vanillin concentration. The results indicated that raising temperature enhanced the oxidation rate. The activation parameters of the rate constant of the slow step (k) along with thermodynamic quantities of the equilibrium constant involved in the reaction mechanism have been evaluated and listed in Tables 3 and 4.
Test for free radical intermediate
Known quantity of acrylonitrile was added to the reaction mixture and was kept in an inert atmosphere for about 4 hours at room temperature. When the reaction mixture was diluted with methanol, a heavy white precipitate was formed suggesting that there was a participation of free radical in the present oxidation reaction. When the experiment was repeated in the absence of the vanillin under similar conditions, the test was negative. This indicates that the reaction was routed through a free radical path.
Discussion
Permanganate ion in various media provides excellent results when used in oxidation processes. In the permanganate ion, manganese has an oxidation state of VII. It is stable in neutral or slightly alkaline media, but, in a strongly alkaline medium, [15] it disproportionates or reacts with hydroxide ion to form manganese(V) (hypomanganate) or manganese(VI) (manganate). Consequently, at high pH values, it is sometimes difficult to ascertain whether an oxidation is proceeding via a one- or a two-electron process. Manganese(VII) is reduced to Mn(II) during oxidation processes via many manganese species having different oxidation states such as Mn(VI), Mn(V), Mn(IV) and Mn(III). The appearance of these intermediate oxidation states depends upon various reaction conditions and the type of substrate. In neutral or slightly alkaline solutions, permanganate used as a powerful oxidizing agent (Eo = +1.23 V) according to the following equation:
MnO4 - + 2H2O + 3e = MnO2 + 4OH-
Many investigators [8-12,32-35] suggested that most of the oxidation reactions by permanganate ion especially in neutral and alkaline media proceed through intermediate complex formation between oxidant and substrate. The formation of manganate(VI) and/or hypomanganate(V) short-lived intermediates may be confirmed by the change in the color of the solution mixture as the reaction proceeded from purple-pink, Mn(VII), to blue, Mn(V), to green, Mn(VI). As the reaction proceeds, a yellow turbidity slowly develops and on prolonged standing, the solution turns to colorless with a brown colloidal precipitate, MnIVO2. The failure to detect Mn(V), absence of an absorption maximum aroundλ = 700 nm, may be interpreted by its extreme short lifetime and undergoing a rapid disproportionation [32,36].
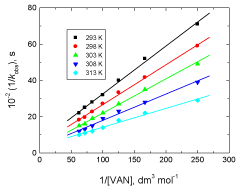
The present reaction between vanillin and permanganate ion in neutral medium has a stoichiometry of 3:2 (VAN:MnO4 - ) with a firstorder dependence on [MnO4 -] and apparent less than unit order in [VAN]. The less than unit order with respect to vanillin concentration suggests formation of an intermediate complex between vanillin and permanganate ion in a pre-equilibrium step. Spectral evidence for complex formation was obtained from the UV–V is spectra (Figure 1) where the growth of new band after a wavelength of 600 nm corresponds to the manganate(VI) intermediate species. Also, appearance of two isosbestic points suggests that an equilibrium is established between MnO4 - and MnVIO4 2- [37,38]. Another support for complex formation is the kinetic evidence as the plot of 1/kobs versus 1/[VAN] was found to be linear with a positive intercept on 1/[VAN] axis as shown in Figure 4, similar to the well-known Michaelis– Menten mechanism for enzyme–substrate reactions [39]. Based on the experimental results, permanganate ion is suggested to react with one mole of vanillin in a pre-equilibrium step to give an intermediate complex (C). The cleavage of such complex leads to the formation of a free radical derived from vanillin and an intermediate Mn(VI) species. Such intermediate is rapidly attacked by manganate(VI) ion to yield the final oxidation product of vanillin (vanillic acid) and Mn(V) intermediate species. In a further fast step, the intermediate Mn(V) being very active and unstable reacts with another mole of vanillin to give rise to vanillic acid and an intermediate Mn(III) species. This step is further followed by a reaction between the third mole of vanillin and another mole of permanganate to give vanillic acid and Mn(V) intermediate species. Finally, knowing the fact that the species Mn(V) is very unstable, it will attack the intermediate Mn(III) leading to formation of MnIVO2 as the final oxidation product of permanganate, satisfying the observed reaction stoichiometry. The proposed mechanism is illustrated in Scheme 1.
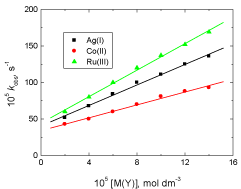
Figure 4: Plots of 1/kobs versus 1/[VAN] in the oxidation of vanillin by
permanganate ion in neutral medium at different temperatures. [MnO4
-] = 4.0
x 10-4 mol dm-3.
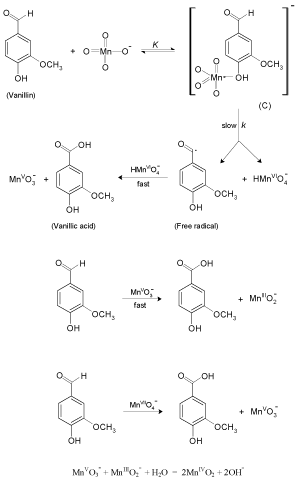
Scheme 1: Mechanism of oxidation of vanillin by permanganate ion in neutral medium.
According to the mechanistic Scheme 1, the following rate law expression was deduced (See Appendix 1),
Under pseudo-first order conditions, the rate-law can be expressed as,
Comparing Eqs (1) and (2), and with rearrangement the following relationship is obtained,
According to Eq. (3), the plots of 1/kobs versus 1/[VAN] (at different temperatures) were found to be straight lines with positive intercepts on 1/kobs axes (Figure 4) satisfying the proposed mechanism. From the intercepts and slopes of such plots, the rate constant of the slow step, k, and the formation constant of the intermediate complex, K, at different temperatures were determined and were listed in Table 2.
The obtained activation parameters listed in Table 3 can be discussed as follows. The large negative values of ΔS# suggest formation of a compacted intermediate complex of inner-sphere nature [40]. The values of ΔH≠ and ΔS≠ are both favorable for electron transfer processes. On the other hand, the positive values of both ΔH≠ and ΔG≠ indicate endothermic formation of the complex and its non-spontaneity, respectively.
DS?
J mol-1K-1
DH?
kJ mol-1
DG? 298
kJ mol-1
Ea?
kJ mol-1
-97.15
17.77
46.72
18.63
Experimental error ±4%
Table 3: Activation parameters of k in the oxidation of vanillin by permanganate ion in neutral medium.
DHo
kJ mol-1
DGo 298
kJ mol-1
DSo
J mol-1K-1
12.80
-1.93
49.43
Experimental error ±5%
Table 4: Thermodynamic parameters associated with K in the oxidation of vanillin by permanganate ion in neutral medium.
Inspection of the effect of metal ion catalysts, a critical literature survey on salt effect shows that a generalized theory of the influence of salts in reaction velocity is not yet available. Many authors [41,42] have interpreted specific effects of metal cations in terms of bridging which facilitates electron transfer in redox systems, while Wahl [43,44] and his co-workers have interpreted specific effects in terms of complex formation. It was found that metal complexes can be more active than the free ligands, i.e. vanillin, and can exhibit bioactivities which are not shown by the free ligands.
Conclusion
The kinetics and mechanistic study of oxidation of vanillin by permanganate ion in neutral medium was investigated. Addition of small amounts of Ag(I), Co(II) and Ru(III) catalysts increased the oxidation rate and the catalytic efficiency increased in the order: Ru(III) > Ag(I) > Co(II). The final oxidation product of vanillin was identified by both spectral and chemical analysis as vanillic acid. The activation parameters along with the thermodynamic quantities have been evaluated and discussed
Appendix
https://austinpublishinggroup.com/chemical-engineering/ fulltext/ace-v3-id1026-appendix.docx
References
- Yuan L, Huiling L, Yan L. Comparative study of the electro-catalytic oxidation and mechanism of nitrophenols at Bi-doped lead dioxide. Appl Catal B Environm. 2008; 84: 297-302.
- Jose TP, Nandibewoor ST, Tuwar SM. Kinetics and mechanism of oxidation of vanillin by hexacyanoferrate(III) in aqueous alkaline medium. J Solution Chem. 2006; 35: 51-62.
- Deepak SM, Chimatadar SA, Nandibewoor ST. Oxidation of vanillin by a new oxidant diperiodatoargentate(III) in aqueous alkaline medium. Ind Eng Chem Res. 2007; 46: 1459-1464.
- Mishra P. Kinetics and mechanisms of oxidation of 4-hydroxy-3-methoxy benzaldehyde (vanillin) by Bi(V) in aqueous alkaline medium. Int J Pharm Tech Res. 2009; 1: 1234-1240.
- Kathari C, Pol P, Nandibewoor ST. The kinetics and mechanism of oxidation of vanillin by diperiodatonickelate(IV) in aqueous alkaline medium. Turk J Chem. 2002; 26: 229-236.
- Patil DG, Magdum PA, Nandibewoor ST. Mechanistic Investigations of uncatalyzed and ruthenium(III) catalyzed oxidation of vanillin by periodate in aqueous alkaline medium. J Solution Chem. 2015; 44: 1205-1223.
- Satapathy PK, Baral DK, Aswar AS, Mohanty P. Kinetics and mechanism of oxidation of vanillin by cerium(IV) in aqueous perchlorate medium. Indian J Chem Tech. 2013; 20: 271–275.
- Fawzy A, Ashour SS, Musleh MA. Kinetics and mechanism of oxidation of L-histidine by permanganate ions in sulfuric acid medium. Int J Chem Kinet. 2014; 46: 370-381.
- Perez Benito JF, Mata Perez F, Brillas E. Permanganate oxidation of glycine: Kinetics, catalytic effects, and mechanisms. Can J Chem. 1987; 65: 2329-2337.
- Fawzy A, Ashour SS, Musleh MA. Base-catalyzed oxidation of L-asparagine by alkaline permanganate and the effect of alkali-metal ion catalysts: Kinetics and mechanistic approach. React Kinet Mech Catal. 2014; 111: 443-460.
- Kini AK, Farokhi SA, Nandibewoor ST. A comparative study of ruthenium(III) catalysed oxidation of L-leucine and L-isoleucine by alkaline permanganate. A kinetic and mechanistic approach. Transition Met Chem. 2002; 27: 532-540.
- Halligudi LL, Desai SM, Mavalangi AK, Nandibewoor ST. Kinetics of the oxidative degradation of rac-serine by aqueous alkaline permanganate. Monatsh Chem. 2000; 131: 321–332.
- Verma RS, Reddy JM, Shastry VR. Kinetic study of homogeneous acid-catalyzed oxidation of certain amino-acids by potassium permanganate in moderately concentrated acidic media. J Chem Soc Perkin Trans. 1976; 124: 469-473.
- Mohanty B, Behera J, Acharya S, Mohanty P, Pantaik AK. Metal ion catalyzed oxidation of L-lysine by alkaline permanganate Ion-A kinetic and mechanistic approach. Chem Sci Trans. 2013; 2: 51-60.
- Cotton FA, Wilkinson G. Advanced Inorganic Chemistry, p. 747, John Wiley and Sons, New York. 1980.
- Fawzy A. Kinetics and mechanism of uncatalyzed and ruthenium(III)-catalyzed oxidation of formamidine derivative by hexacyanoferrate(III) in aqueous alkaline medium. J Chem Sci. 2016: In press.
- Fawzy A, Zaafarany IA, Yarkandi N, Al-Bonayan A, Almallah Z. Kinetic and mechanism of oxidation of methylaminopyrazole formamidine by alkaline hexacyanoferrate(III) and the effect of divalent transition metal ions. Sci J Chem. 2016; 1: 1-8.
- Fawzy A. Kinetics and mechanistic approach to the oxidative behavior of biological anticancer platinum(IV) complex towards L-asparagine in acid medium and the effect of copper(II) catalyst. Int J Chem Kinet. 2014; 47: 1-12.
- Fawzy A, Zaafarany IA. Mechanistic investigation of copper(II)-catalyzed oxidation of L-asparagine by hexachloroplatinate(IV) in aqueous alkaline medium: a kinetic approach. J Multidisc Eng Sci Technol. 2015; 2: 1038-1045.
- Asghar BH, Altass HM, Fawzy A. Transition metal ions-catalyzed oxidation of L-asparagine by platinum(IV) in acid medium: a kinetic and mechanistic study. Transition Met Chem. 2015; 40: 587–594.
- Kelson EP, Phengsy PP. Kinetic study of 2-propanol and benzyl alcohol oxidation by alkaline hexacyanoferrate(III) catalysed by a terpyridyl ruthenium complex. Int J Chem Kinet. 2000; 32: 760–770.
- Asiri AM, Khan SA. Palladium(II) complexes of NS donor ligands derived from steroidal thiosemicarbazones as antibacterial agents. Molecules. 2010; 15: 4784-4791.
- Jose TP, Angadi MA, Salunke MS, Tuwar SM. Oxidative study of gabapentin by alkaline hexacyanoferrate(III) in room temperature in presence of catalytic amount of Ru(III). A mechanistic approach. J Mol Struct. 2008; 892: 121–124.
- Sharanabasamma K, Angadi MA, Salunke MS, Tuwar SM. Osmium(VIII) catalysed oxidative cleavage of pyrrolidine ring in L-proline by hexacyanoferrate(III) in alkaline media. Ind Eng Chem Res. 2009; 48: 10381-10386.
- Goel A, Sharma S. Mechanistic study of the oxidation of L-phenylalanine by hexacyanoferrate(III) catalyzed by iridium(III) in aqueous alkaline medium, Transition Met Chem. 2010; 35: 549-554.
- Devra V, Yadav MB. Kinetics and mechanism of osmium(VIII) catalyzed oxidation of valine by hexacyanoferrate in alkaline medium. Rassian J Chem. 2012; 5: 67-73.
- Upadhyay SK, Agrawal MC. Kinetics of Os(VIII)-catalyzed alkaline hexacyanoferrate(III) oxidation of some a-amino acids in presence of excess of ferricyanide. Ind J Chem. 1977; 15A: 709-712.
- Jose TP, Nandibewoor ST, Tuwar SM. Osmium(VIII) catalyzed oxidation of a sulfur containing amino acid – A kinetic and mechanistic approach. J Sulfur Chem. 2006; 27: 25-36.
- Vogel IA. A Text Book of Quantitative Inorganic Analysis. 4th edn, p 352. ELBS, Longman. 1978.
- Feigl F. Spot Tests in Organic Analysis, p 195. Elsevier, New York. 1975.
- Vogel AI. Text Book of Practical Organic Chemistry Including Quantitative Organic Analysis, 3rd edn p 332. ELBS, Longman. 1973.
- Ahmed GA, Fawzy A, Hassan RM. Spectrophotometric evidence for the formation of short lived hypomanganate(V) and manganate(VI) transient species during the oxidation of K-carrageenan by alkaline permanganate. Carbohydr Res. 2007; 342: 1382-1386.
- Mahesh RT, Bellakki MB, Nandibewoor ST. Kinetics and mechanism of oxidation of L-proline by heptavalent manganese: a free radical intervention and decarboxylation. J Chem Res. 2005; 1: 13-17.
- Jose TP, Nandibewoor ST, Tuwar SM. Mechanism of oxidation of L-histidine by heptavalent manganese in alkaline medium. E-J Chem. 2005; 2: 75–85.
- Halligudi LL, Desai SM, Mavalangi AK, Nandibewoor ST. Free radical intervention, deamination and decarboxylation in the ruthenium(III)-catalysed oxidation of L-arginine by alkaline permanganate—a kinetic study. Transition Met Chem. 2001; 26: 28–35.
- Zimmerman CL. Ph D. Thesis University of Chicago. 1949.
- Laider KJ. Chemical Kinetics. p 51. McGraw-Hill, New York. 1965.
- Entelis SG, Tiger RP. Reaction Kinetics in the Liquid Phase. Wiley, New York. 1976.
- Michaelis L, Menten ML. The kinetics of invertase action. Biochem Z. 1913; 49: 333–369.
- Hicks KW, Toppen DL, Linck RG. Inner-sphere electron-transfer reactions of vanadium(II) with azidoamine complexes of cobalt(III). Inorg Chem. 1972; 11: 310-315.
- Duke FR, Parchen RF. The Kinetics of the Ce(IV)-Ce(III) Exchange Reaction in Perchloric Acid. J Am Chem Soc. 1956; 78:1540-1543.
- Taube H, Myers H. Evidence for a Bridged Activated Complex for Electron Transfer Reactions. J Am Chem Soc. 1956; 76: 2103-2111.
- Wahl AC. Rapid electron-transfer isotopic-exchange reactions. Z Electrochem. 1960; 64: 90-93.
- Sheppard JC, Wahl AC. Kinetics of the manganate-permanganate exchange reaction. J Am Chem Soc. 1957; 79: 1020-1024.