
Research Article
Austin Chem Eng. 2016; 3(4): 1037.
Silver(I) Catalysis for Oxidation of L-Glutamine By Cerium(IV) in Perchlorate Solutions: Kinetics and Mechanistic Approach
Fawzy A1,2* and Al-Jahdali BA2
1Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
2Chemistry Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia
*Corresponding author: Ahmed Fawzy, Associate Professor, Chemistry Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia; Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
Received: July 20, 2016; Accepted: August 05, 2016; Published: August 08, 2016
Abstract
The influence of silver(I) catalyst on the oxidation of L-glutamine (Gln) by cerium(IV) in perchlorate solutions was studied spectrophotometrically. The study was carried out at a constant ionic strength of 1.0 mod dm-3 and a temperature of 25oC. In both uncatalyzed and Ag(I)-catalyzed paths, the reactions exhibited first order kinetics with respect to [Ce(IV)] and [Ag(I)], and less than unit order with respect to [Gln]. The reactions exhibited negative fractional-first order kinetics with respect to [H+]. Increasing both ionic strength and dielectric constant increased the oxidation rates. Addition of cerium(III) ion as a reaction product did not affect the rates. The rate of Ag(I)-catalyzed oxidation was found to be about seven times higher than that of uncatalyzed one. Ce(OH)3+ was suggested to be the kinetically active species of cerium(IV) under the experimental conditions. Probable mechanistic schemes for both uncatalysed and catalysed reactions are proposed. In both paths, the final oxidation products of L-glutamine are identified as formyl propanamide, ammonium ion, and carbon dioxide. The rate-law expressions consistent with the reactions mechanisms are derived. The activation parameters are evaluated and discussed.
Keywords: Kinetics; Mechanism; L-Glutamine; Cerium(IV); Oxidation; Silver(I)
Introduction
L-Glutamine is a non-essential a-amino acid that is employed in the biosynthesis of proteins. Glutamine plays an essential role in a variety of biochemical functions [1-3]. In human blood, glutamine is the most abundant free amino acid. Production of glutamine in the body often slows down with age and does not generate sufficient quantities. It also plays a decisive role in keeping a balanced acidbase ratio. Glutamine can be converted to glucose in the kidneys, without effecting glucagon or insulin levels. There are also indications that glutamine can reduce the demand for sugar and alcohol [2,3]. Generally, due to the biological importance of amino acids, the kinetics and mechanistic studies of their oxidation by a variety of oxidants have received considerable attention [4-24].
Cerium(IV) has been widely employed as an oxidant in mechanistic studies in acid media especially in sulphuric acid solutions [25-36]. Cerium(IV) is less stable in aqueous nitric and perchloric acid solutions [37-41]. The oxidant has rarely been employed in perchloric acid medium probably due to the presence of dimers and polymers of cerium(IV) in such medium [39-41]. Identification of kinetically active Ce(IV) species [26,27] is a problem during studies of the kinetics and mechanisms of cerium(IV) oxidation in aqueous sulphuric acid solutions using different types of organic and inorganic substrates. In aqueous sulphuric acid solutions, cerium(IV) can exist as a mixture of different types of sulphate species such as Ce(SO4)2+, Ce(SO4)2, HCe(SO4)3 - and H3Ce(SO4)4 - [25-30].
A Literature survey revealed no work has been done on the kinetics and mechanism of the oxidation of L-glutmine by cerium(IV) in the absence or presence of a catalyst. This observation prompted us to investigate the title reactions. We aim to investigate the selectivity of L-glutamine towards cerium(IV) in perchlorate solutions, to understand the active species of the oxidant in this medium, to check the catalytic activity of Ag(I) towards L-glutamine oxidation, and to elucidate plausible oxidation mechanisms.
A Literature survey revealed no work has been done on the kinetics and mechanism of the oxidation of L-glutmine by cerium(IV) in the absence or presence of a catalyst. This observation prompted us to investigate the title reactions. We aim to investigate the selectivity of L-glutamine towards cerium(IV) in perchlorate solutions, to understand the active species of the oxidant in this medium, to check the catalytic activity of Ag(I) towards L-glutamine oxidation, and to elucidate plausible oxidation mechanisms.
Experimental and Methods
Materials
The stock solution of L-glutamine was prepared by dissolving the required amount of the sample (E. Merck) in double-distilled water. A solution of cerium(IV) was freshly prepared by dissolving ceric ammonium sulphate (S.D. Fine Chem.) in a 1.0 mol dm-3 sulfuric acid solution, then diluting with double-distilled water and kept overnight. The concentration of cerium(IV) solution was determined as reported earlier [42]. Cerium(III) solution was also prepared by dissolving cerium(III) acetate sample (S.D. Fine Chem.) in doubledistilled water. Sodium perchlorate and acetic acid were used to vary the ionic strength and dielectric constant of the medium, respectively.
Kinetic measurements
The kinetic runs for uncatalyzed and catalysed reactions were followed under conditions in which [Gln] >> [Ce(IV)]. The progress of the reactions was followed by monitoring the decay of cerium(IV) absorbance as a function of time at its absorption maximum, λ = 315nm, where the other reactions constituents were not significantly absorbed at this wavelength. The absorbance measurements were conducted on a temperature-controlled Shimadzu UV-VIS-NIR-3600 double-beam spectrophotometer.
The observed first order rate constants of uncatalyzed and catalyzed reactions (kU and kC) were calculated using non-linear leastsquares fitting to ln Abs. - time plots. The observed rate constants were the mean values of at least two kinetic run. The rate constants were reproducible to within 2-3%. Whereas the reaction between cerium(IV) and L-glutamine in perchlorate solutions proceeded with a slow rate in the absence of Ag(I) catalyst, the catalyzed reaction is thought to carried out in a parallel path with the contributions from both uncatalyzed and catalyzed paths. Therefore, the total rate constant (kT) is equal to kU + kC.
Results
Stoichiometry and product analysis
Different mixtures of the reactions with an excess of cerium(IV) concentration at [H+] = 0.5 mol dm-3 and at I = 1.0 mol dm-3, have been equilibrated for about 24h at room temperature. The stoichiometry, determined spectrophotometrically and by titration, indicated the consumption of two Ce(IV) ions per one molecule of L-glutamine yielding the final products as in the following equation,
H2N (CO) CH2 –CH2 – CH (NH2) COOH + 2Ce(IV) + H2O = H2N (CO) CH2 –CH2 – CHO + 2Ce(III) + NH4 + + CO2 + H+
The above stoichiometric equation is consistent with the results of product analysis. The products were identified as the corresponding aldehyde (formyl propanamide) by spot test [43], ammonia by Nessler’s reagent [44] and carbon dioxide by lime water. The product, formyl propanamide was also identified by 2,4-dinitophenyhydrazine [44].
Spectral changes
The spectral changes during the oxidation of L-glutamine by cerium(IV) in perchlorate solutions in the absence and presence of Ag(I) catalyst are shown in Figure 1a and 1b, respectively. In both cases, the spectra indicate gradual disappearance of the Ce(IV) band at its absorption maximum as a result of its reduction to Ce(III).
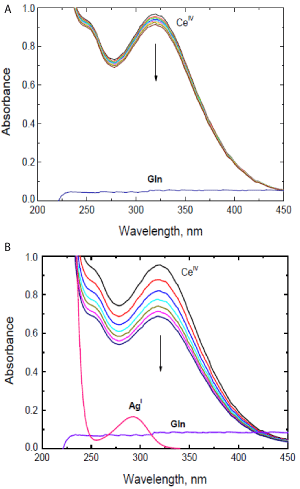
Figure 1: Spectral changes during: (a) uncatalyzed, and (b) Ag(I)-catalyzed oxidations of L-glutamine by cerium(IV) in perchlorate solutions. [Ce(IV)] =
2.0×10-4, [Gln] = 8.0×10-3, [H+] = 0.5, [Ag(I)] = 6.0×10-5 and I = 1.0 at 25 °C. Scan time intervals = 1 min.
Order of reactions
The order of uncatalyzed and catalyzed reactions with respect to the reactants was determined from the slopes of the plots of log kU and log kC versus log(concentration) by varying the concentrations of L-glutamine, perchloric acid, and Ag(I) catalyst, in turn, while keeping all other conditions constant.
The concentration of the oxidant, cerium(IV), was varied in both uncatalyzed and catalyzed reactions in the range of 0.5×10-4 to 5.0×10-4 mol dm-3, keeping all other variables constant. Plots of ln(absorbance) versus time were linear up to about two-half lives of the reaction completion. Furthermore, an increase in the initial
oxidant concentration did not alter the oxidation rates of L-glutamine as listed in Table 1. These results indicate that the order of reactions with respect to [Ce(IV)] is one.
104 [Ce(IV)]
(mol dm-3)
103 [Gln]
(mol dm-3)
[H+]
(mol dm-3)
105 [Ag(I)]
(mol dm-3)
I
(mol dm-3)
105 kU
(s-1)
105 kC
(s-1)
0.5
8.0
0.5
6.0
1.0
17.6
119.8
1.0
8.0
0.5
6.0
1.0
16.5
121.0
2.0
8.0
0.5
6.0
1.0
17.3
122.4
3.0
8.0
0.5
6.0
1.0
17.7
121.7
4.0
8.0
0.5
6.0
1.0
15.9
120.0
5.0
8.0
0.5
6.0
1.0
17.8
122.6
2.0
2.0
0.5
6.0
1.0
5.6
43.1
2.0
4.0
0.5
6.0
1.0
9.5
73.7
2.0
6.0
0.5
6.0
1.0
13.0
97.4
2.0
8.0
0.5
6.0
1.0
17.3
122.4
2.0
10.0
0.5
6.0
1.0
21.7
148.0
2.0
12.0
0.5
6.0
1.0
24.9
171.2
2.0
8.0
0.1
6.0
1.0
41.7
214.1
2.0
8.0
0.3
6.0
1.0
25.2
159.3
2.0
8.0
0.5
6.0
1.0
17.3
122.4
2.0
8.0
0.7
6.0
1.0
13.4
95.0
2.0
8.0
0.9
6.0
1.0
10.2
77.1
2.0
8.0
1.0
6.0
1.0
8.9
68.4
2.0
8.0
0.5
2.0
1.0
17.3
39.6
2.0
8.0
0.5
4.0
1.0
17.3
80.4
2.0
8.0
0.5
6.0
1.0
17.3
122.4
2.0
8.0
0.5
8.0
1.0
17.3
159.7
2.0
8.0
0.5
10.0
1.0
17.3
195.1
2.0
8.0
0.5
12.0
1.0
17.3
229.7
2.0
8.0
0.5
6.0
1.0
17.3
122.4
2.0
8.0
0.5
6.0
1.4
19.8
129.7
2.0
8.0
0.5
6.0
1.8
23.1
136.1
2.0
8.0
0.5
6.0
2.2
25.9
143.2
2.0
8.0
0.5
6.0
2.6
28.2
149.7
2.0
8.0
0.5
6.0
3.0
30.4
154.0
Experimental error ±3%.
Table 1: Effect of variation of [Ce(IV)], [Gln], [H+], [Ag(I)] and I on the observed first order rate constants in the uncatalyzed and Ag(I)-catalyzed oxidations of L-glutamine by cerium(IV) in perchlorate solutions at 25 °C.
The observed rate constants were determined at different initial concentrations of the reductant L-glutamine while other variables were kept constant. Increasing [Gln] increases the rates of the reactions as listed in Table 1. Plots of the observed first order rate constants versus [Gln] are linear with positive intercepts as shown in Figure 2, confirming less than unit order kinetics on [Gln].
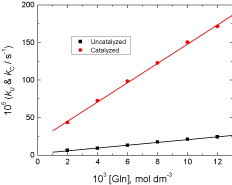
Figure 2: Effect of [Gln] on the observed first order rate constants in the uncatalyzed and Ag(I)-catalyzed oxidations of L-glutamine by cerium(IV) in
perchlorate solutions. [Ce(IV)] = 2.0×10-4, [H+] = 0.5, [Ag(I)] = 6.0×10-5 and I = 1.0 mol dm-3 at 25 °C.
In order to study the effect of hydrogen ion concentration on the rates of the reactions, kinetic runs were carried out by varying the perchloric acid concentration (0.1 – 1.0 mol dm–3) keeping the concentrations of all other reactants constant. It was observed that the rates of the reactions decrease with increasing [H+] in both cases (Table 1). Plots of log kU and log kC versus [H+] were linear with negative slopes of less than unity (Figure 3) confirming negative fractional-first order dependences with respect to [H+].
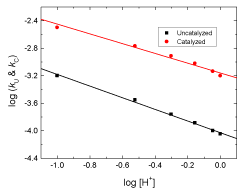
Figure 3: Plots of log kU and log kC versus log [H+] in the uncatalyzed and Ag(I)-catalyzed oxidations of L-glutamine by cerium(IV) in perchlorate solutions. [Ce(IV)] = 2.0×10-4, [Gln] = 8.0 ×10-3, [Ag(I)] = 6.0×10-5 and I = 1.0 mol dm-3 at 25 °C.
To evaluate the reaction orders with respect to the concentrations of Ag(I) catalyst, the reaction rate was measured at various [Ag(I)], (2.0–12.0) × 10,-5 mol dm-3, while other variables were kept constant. The reaction rate increased as [Ag(I)] increased (Table 1). The order with respect to Ag(I) was found to be less than unity, as determined from the plot of log kC versus log [Ag(I)], as shown in Figure. 4.
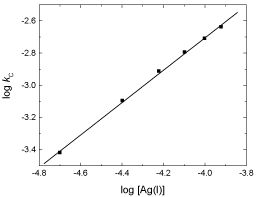
Figure 4: Plot of log kC versus log [Ag(I)] in the Ag(I)-catalyzed oxidations of L-glutamine by cerium(IV) in perchlorate solutions. [Ce(IV)] = 2.0×10-4, [Gln] = 8.0×10-3, [H+] = 0.5 and I = 1.0 mol dm-3 at 25 °C.
Effect of ionic strength and dielectric constant
The effect of ionic strength on the rates of uncatalyzed and catalyzed reactions was studied by varying the ionic strength in the range of 1.0-3.0 mol dm–3 using sodium perchlorate while maintaining the concentrations of all other reactants constant, and at constant pH and temperature. Increasing the ionic strength of the medium increased the rates of reactions and the Debye–Huckel plots were linear with positive slopes (Figure 5a).
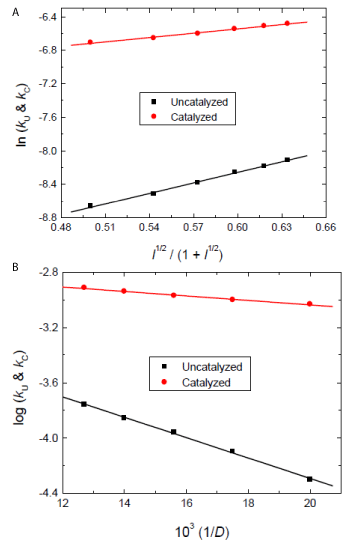
Figure 5: Effect of a) ionic strength, and b) dielectric constant of the reaction medium on the rate constants in the uncatalyzed and Ag(I)-catalyzed
oxidations of L-glutamine by cerium(IV) in perchlorate solutions. [Ce(IV)] = 2.0×10-4, [Gln] = 8.0×10-3, [H+] = 0.5, [Ag(I)] = 8.0×10-5 and I = 1.0 mol dm-3 at 25 °C.
In order to determine the effect of the dielectric constant (D) of the medium on the reaction rates, the reactions were studied at different solvent compositions (vol%) of acetic acid and water. At different compositions, D of the medium was calculated using the D values for water (78.5) and acetic acid (6.15) at 25 °C. The rate constants clearly decreased as D of the solvent decreased (i.e., increasing acetic acid content). Plots of log kU and log kC versus 1/D were linear with negative slopes (Figure 5b).
Effect of initially added product
The effect of added cerium(III) was studied over the concentration range (0.5 – 5.0) ×10-4 mol dm-3 at fixed concentrations of other reactants. Addition of Ce(III) did not affect significantly the rates of both uncatalysed and catalysed reactions.
Effect of temperature
The rates of the reactions were carried out at five different temperatures (288 - 308 K) at constant concentrations of the reactants and all other conditions being constant. The results indicate that the rate constants increased with the rise in temperature. The activation parameters of the second order rate constant (k’) are calculated using Arrhenius Eyring plots and are listed in Table 2.
Reaction
DS≠ , Jmol-1K-1
DH≠ , kJ mol-1
DG≠298 , kJ mol-1
Ea≠, kJ mol-1
Uncatalyzed
-94.08
49.12
77.15
52.11
Catalyzed
-112.14
37.7
71.11
39.02
Experimental error = ±3.0.
Table 2: Activation parameters of k’ in the uncatalyzed and Ag(I)-catalyzed oxidations of L-glutamine by cerium(IV) in perchlorate solutions. [Ce(IV)] = 2.0×10-4, [Gln] = 6.0×10-3, [H+] = 0.5, [Ag(I)] = 8.0×10-5 and I = 1.0 mol dm-3.
Test for free radical intermediates
Known quantities of acrylonitrile scavenger were added to samples of the reactions mixtures that had been kept in inert atmosphere for about 6h. White precipitates were formed upon diluting the reactions mixtures with methanol indicating free radicals intervention in the reactions. When the experiments were repeated in the absence of L-glutamine under similar conditions, the tests were negative. These tests indicated that the reactions proceeded through free radical paths.
Discussion
It was reported earlier [45-47] the active species of Ce(IV) in perchloric acid medium was suggested to be either Ce4+, Ce(OH)3+ and Ce(OH)2 2+ or (Ce–O–Ce)6+ and (HOCe–O–CeOH)4+. However, it showed from the spectrophotometric studies [48] that Ce4+ is proposed to the predominant species at [H+] = 1.0 mol dm-3 up to [Ce(IV)] = 1.5 x 10-3 mol dm-3, whereas the other species are more predominant at [H+] < 0.8 mol dm-3. Under the present experimental conditions of low [H+] and deceasing rates of reactions with the increase in [H+], Ce(OH)3+ may be considered as the active species of Ce(IV) according to the equilibrium,
Also, it is reported [22,24,49,50] that in acid solutions, amino acids exist in zwitterions and predominantly tend to protonate at higher acid concentrations according to the following equilibria,
Where R-CH(NH2)COOH and R-CH(+NH3)COOH denote the amino acid and its protonated form.
Mechanism of uncatalyzed oxidation
The reaction between L-glutamine and cerium(IV) in perchlorate solutions in the absence of silver(I) catalyst has a stoichiometry of 1:2, i.e., one mole of Gln requires two moles of Ce(IV). The reaction exhibits a first order dependence with respect to [Ce(IV)], less than unit order with respect to [Gln] and negative fractional order in [H+]. The less than unit order with respect to L-glutamine concentration may be interpreted by formation of an intermediate complex (C1) between the protonated L-glutamine (Gln+) and the kinetically active species of cerium(IV), Ce(OH)3+. Increasing the oxidation rate with increasing both ionic strength and dielectric constant supports that the reaction was between two similarly charged ions [51,52]. Formation of an intermediate complex is proved kinetically by the non-zero intercept of the plot of 1/kU versus 1/[Gln] (Figure 6). This kinetic result favours the possible formation of an intermediate complex between the oxidant and substrate [53]. Furthermore, complex formation was supported by the obtained negative value of the entropy of activation (Table 2), which corresponds to the decrease in the degrees of freedom accompanying the transition from the initial state to the transition state [54,55]. The positive values of both DH≠ and DG≠ indicate endothermic formation of the intermediary complex and its non-spontaneity, respectively.
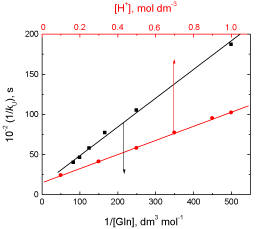
Figure 6: Plots of 1/kU versus 1/[Gln] and [H+] in the uncatalyzed oxidation of L-glutamine by cerium(IV) in perchlorate solutions. [Ce(IV)] = 2.0×10-4 and I = 1.0 mol dm-3 at 25 °C.
The oxidation reaction is proposed to be a one-electron process in the rate-determining step, which leads to decomposition of the intermediate complex forming an intermediate radical and Ce(III) ion. The formation of a free radical is evident through the acrylonitrile polymerization test. The rate was not affected by Ce(III), suggesting that the reaction step involving decomposition of the intermediate complex into L-glutamine free radical and Ce(III) ion may not be a reversible process. The rate-determining step should be irreversible, which is generally the case for one electron oxidants [56]. Next, decarboxylation of the free radical occurred, forming a new radical intermediate and the latter was rapidly attacked by another Ce(OH)3+ species to yield the final oxidation products. The suggested mechanism was illustrated in Scheme 1.
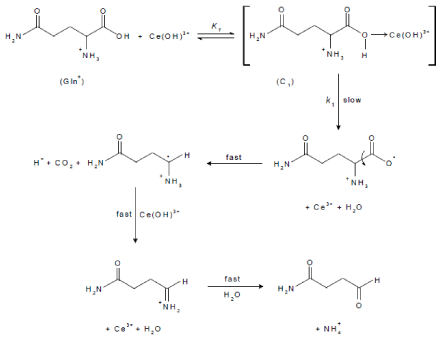
scheme 1: Mechanism of uncatalyzed oxidation of L-glutamine by cerium(IV) in perchlorate solution.
The suggested mechanism leads to the following rate law expression (Appendix A),
Rate=
Under pseudo-first order condition, the rate-law can be expressed by Eq. (2),
Rate=
Comparing Eqs. (1) and (2), the following relationship is obtained,
The rate law (3) is consistent with all the observed orders with respect to different species, which can be verified by rearranging to the following equations,
According to Eqs. (4) and (5), the plots of 1/kU versus 1/[Gln] at constant [H+], and 1/kU versus [H+] at constant [Gln] should be linear with positive intercepts and are found to be so as shown in Figure 6. From the slopes and intercepts of such plots, the values of k1, K1 and KOH are calculated to be 7.2 x 10-4 s-1, 1.8 x 103 dm3 mol–1 and 22.2 x 10-3, respectively.
Mechanism of silver(I)-catalyzed oxidation
The kinetics of the oxidation of L-glutamine by cerium(IV) in perchlorate solutions in the presence of small amounts of silver(I) catalyst is similar to that for the uncatalyzed reaction. In addition, the reaction shows a first order dependence with respect to silver(I) concentration. The less than unit order for the concentration of Gln suggests formation of a complex between the protonated glutamine and Ag(I) catalyst prior to reaction with the oxidant. Complex formation was also proved kinetically by non-zero intercepts of the plots of [Ag(I)]/kC versus 1/[Gln] (Figure 7). The complex reacts in a slow step with Ce(OH)3+ to give rise to the intermediate radical and Ce(III) ion with regeneration of the catalyst. This is followed by decarboxylation of Gln free radical, forming a new radical intermediate which is rapidly attacked by another Ce(OH)3+ species to yield the final oxidation products as illustrated in Scheme 2.
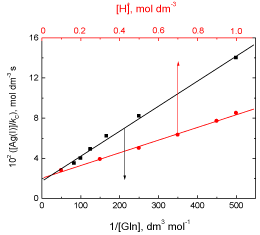
Figure 7: Plots of [Ag(I)]/kC versus 1/[Gln] and [H+] in the Ag(I)-catalyzed oxidation of L-glutamine by cerium(IV) in perchlorate solutions. [Ce(IV)] =2.0×10-4 and I = 1.0 mol dm-3 at 25 °C.
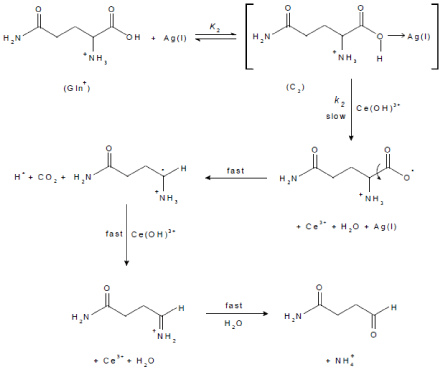
scheme 2: Mechanism of Ag(I)-catalyzed oxidation of L-glutamine by cerium(IV) in perchlorate solution.
An alternative reaction mechanism [27,57] for metal ion-catalyzed oxidation may be proposed. It involves formation of an intermediary complex between the metal ion catalyst, Ag(I), and the amino acid (C2), that on further interaction with Ce(IV) in the rate-determining step yields another complex (C3) of a higher valence silver ion, Ag(II), and Ce(III) ion. Such a complex rapidly decomposes giving rise to the intermediate radical with regeneration of the catalyst Ag(I), followed by subsequent fast steps to yield the final oxidation products as illustrated in Scheme 3.
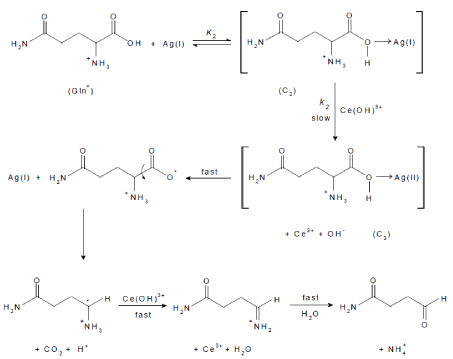
scheme 3: An alternative mechanism of Ag(I)-catalyzed oxidation of L-glutamine by cerium(IV) in perchlorate solution.
In a similar manner to the uncatalyzed reaction, the proposed mechanism leads to the following rate law expression (Appendix B),
Rate=
Under pseudo-first order condition, the rate-law can be expressed by Eq. (7),
Rate=
Comparing Eqs. (6) and (7), the following relationship is obtained,
Rate=
The rate law (8) is consistent with all the observed orders with respect to different species, which can be verified by rearranging to the following equations,
According to Eqs. (9) and (10), plots of [Ag(I)]/kC versus 1/[Gln] at constant [H+], and [Ag(I)]/kC versus [H+] at constant [Gln] should be linear with positive intercepts on [Ag(I)]/kC axes and are found to be so as shown in Figure 7. From the slopes and intercepts of such plots, the values of K2 and KOH are calculated to be 70.8 dm3 mol-1 and 23.2 x 10-3, respectively. Also, the value of k2 is evaluated form the slope or intercept of any plot to be 381.6 dm3 mol-1 s-1.
Conclusion
The catalytic effect of silver(I) ion on the oxidation of L-glutamine by cerium(IV) in perchlorate solutions was studied. The rate of Ag(I)- catalyzed oxidation was found to be about seven times higher than that of uncatalyzed one. Ce(OH)3+ was suggested to be the kinetically active species of cerium(IV). In both paths, the final oxidation products of L-glutamine are identified as formyl propanamide, ammonium ion, and carbon dioxide.
Appendix
https://austinpublishinggroup.com/chemical-engineering/ fulltext/ace-v3-id1037-Appendix.docx
References
- Bowtell JL, Gelly K, Jackman ML, Patel A, Simeoni M, Rennie MJ. Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. J Appl Physiol. 1999; 86: 1770-1777.
- Prada PO, Hirabara SM, de Souza CT, Schenka AA, Zecchin HG, Vassallo J, et al. L-glutamine supplementation induces insulin resistance in adipose tissue and improves insulin signalling in liver and muscle with diet-induced obesity. Diabetologia. 2007; 50: 149-159.
- Sapolsky R. Biology and Human Behavior: The Neurological Origins of Individuality, 2nd edition. The Teaching Company. 2005; 19-20.
- Devasingh CNP. Kinetics and mechanism of oxidation of glutamine and serine by peroxomonosulfate in the absence and presence of acetaldehyde and propionaldehyde. Indian J Chem. A. 1988; 498-503.
- Kumar A, Jha MK, Jha AK. Kinetics and mechanism of oxidation of glutamic acid with BI(V) phosphato complex. Oriental J. Chem. 2012; 28: 901-906.
- Khan ASP, Khan A, Asiri AM. Mechanistic investigation of osmium(VIII) catalyzed oxidation of glutamic acid with sodium salt of N-chloro 4-methylbenzenesulfonamide in aqueous media: a practical approach. Synth React Inorg Met-Org Nano-Met Chem. 2016; 46: 10-16.
- Puttaswamy, Vaz N. Kinetics of oxidation of acidic amino acids by sodium N-bromobenzenesulphonamide in acid medium: A mechanistic approach. Proc. Indian Acad. Sci. (Chem. Sci.). 2001; 113: 325-332.
- Singh R, Tamta DK, Joshi SK, Chandra N, Kandpal ND. Oxidation of amino acids by manganese (III) in aqueous sulphuric acid. J Chem Pharm Res. 2011; 3: 529-535.
- Devra V, Jain S, Sharma PD. Kinetics and mechanism of oxidation of glycine, alanine, and threonine by fluoride coordinated bismuth(V) in aqueous HClO4–HF medium. Int J Chem Kinet. 1994; 26: 577-585.
- Criado S, Marioli JM, Allegretti PE, Furlong J, Rodríguez Nieto FJ, Mártire DO, et al. Oxidation of di- and tripeptides of tyrosine and valine mediated by singlet molecular oxygen, phosphate radicals and sulfate radicals. J Photochem Photobiol B. 2001; 65: 74-84.
- Sanjeevagowda TP, Mahantesh AA, Abdulazizkhan LH. Oxidative deamination and decarboxylation of L-asparagine by the aqueous alkaline diperiodato-nickelate(IV) complex. J Solution Chem. 2008; 37: 1795-1808.
- Khalid MAA. Oxidative kinetics of amino acids by peroxydisulfate: Effect of dielectric constant, Arab J Sci Eng. 2007; 33: 199-210.
- Asghar BH, Altass HM, Fawzy A. Copper(II) catalysis for oxidation of L-tryptophan by hexacyanoferrate(III) in alkaline medium: a kinetic and mechanistic approach. J Saudi Chem Soc in press. 2015.
- Fawzy A, Ashour SS, Musleh MA, Hassan RM, Asghar BH. Kinetics and mechanistic approach to the chromic acid oxidation of L-tryptophan with a spectral detection of chromium(III) product. J Saudi Chem Soc. 2016; 20: 450-458.
- Fawzy A. Palladium(II)-catalyzed oxidation of L-tryptophan by hexacyanoferrate(III) in perchloric acid medium: a kinetic and mechanistic approach. J Chem Sci. 2016; 128: 247-256.
- Fawzy A. Influence of copper(II) catalyst on the oxidation of L-histidine by platinum(IV) in alkaline medium: a kinetic and mechanistic study. Transition Met Chem. 2014; 39: 567-576.
- Fawzy A. Kinetics and mechanistic approach to the oxidative behavior of biological anticancer platinum(IV) complex towards L-asparagine in acid medium and the effect of copper(II) catalyst. Int J Chem Kinet. 2015; 47: 1-12.
- Fawzy A, Asghar BH. Kinetics and mechanism of uncatalyzed and silver(I)-catalyzed oxidation of L-histidine by hexachloroplatinate(IV) in acid medium. Transition Met Chem. 2015; 40: 287-295.
- Asghar BH, Altass HM, Fawzy A. Transition metal ions-catalyzed oxidation of L-asparagine by platinum(IV) in acid medium: a kinetic and mechanistic study. Transition Met Chem. 2015; 40: 587–594.
- Fawzy A, Zaafarany IA. Kinetic and mechanistic investigation on the zirconium(IV)-catalyzed oxidation of L-histidine by hexachloroplatinate(IV) in acid medium. Chem Sci Rev Lett. 2015; 4: 608-618.
- Fawzy A, Zaafarany IA. Mechanistic investigation of copper(II)-catalyzed oxidation of L-asparagine by hexachloroplatinate(IV) in aqueous alkaline medium: a kinetic approach. J Multidisc Eng Sci Technol. 2015; 2: 1038-1045.
- Asghar BH, Altass HM, Fawzy A. Silver(I)-catalysis of oxidative deamination and decarboxylation of L-asparagine and L-histidine by platinum(IV) in perchloric acid solutions: a comparative kinetics study. J Env Chem Eng. 2016; 4: 617-623.
- Fawzy A, Ashour SS, Musleh MA. Base-catalyzed oxidation of L-asparagine by alkaline permanganate and the effect of alkali-metal ion catalysts: Kinetics and mechanistic approach. React Kinet Mech Catal. 2014; 111: 443-460.
- Fawzy A, Ashour SS, Musleh MA. Kinetics and mechanism of oxidation of L-histidine by permanganate ions in sulfuric acid medium. Int J Chem Kinet. 2014; 46: 370-381.
- Richardson WH. Ceric ion oxidation of organic compounds. In: Wiberg KB (ed) Oxidation of organic chemistry, Part A, p 244, Academic Press, London. 1965.
- Adari KK, Nowduri A, Parvataneni V. Kinetics and mechanism of oxidation of L-cystine by cerium(IV) in sulphuric acid medium. Acta. Chim. Slov. 2008; 55: 425-429.
- Sumathi T, Shanmugasundaram P, Chandramohan G. A kinetic and mechanistic study on the silver(I) catalyzed oxidation of L-Serine by cerium(IV) in sulfuric acid medium. J Saudi Chem Soc. 2013; 17: 227-235.
- Sumathi T, Shanmugasundaram P, Chandramohan G. A kinetic and mechanistic study on the silver(I)-catalyzed oxidation of L-alanine by cerium (IV) in sulfuric acid medium. Arab. J. Chem. 2011; 4: 427-435.
- Sumathi T, Shanmugasundaram P, Chandramohan G. A kinetic and mechanistic study on the oxidation of L-methionine and N-acetyl L-methionine by cerium(IV) in sulfuric acid medium. Arab J Chem. 2011.
- Thabaj KA, Chimatadar SA, Nandibewoor ST. Mechanistic study of oxidation of palladium(II) by cerium(IV) in aqueous acid. Transition Met. Chem. 2006; 31: 186-193.
- Fawzy A. Oxidation of alginate and pectate biopolymers by cerium(IV) in perchloric and sulfuric acid solutions: A comparative kinetic and mechanistic study. Carbohydr Polym. 2016; 138: 356-364.
- Fawzy A. Kinetic and mechanistic aspects of oxidation of aminotriazole formamidine by cerium(IV) in aqueous perchloric and sulfuric acid solutions: a comparative study. J Solution Chem. 2016; 45: 246-264.
- Melichercik M, Treindl L. Kinetics and mechanism of the oxidation of acrolein, crotonaldehyde, and methacrolein with cerium(IY) sulfate. Chem. Zvesti. 1981; 35: 153-163.
- Das AK, Islam M, Bayen R. Studies on kinetics and mechanism of oxidation of D-sorbitol and D-mannitol by cerium(IV) in aqueous micellar sulfuric acid media. Int J Chem Kinet. 2008; 40: 445-453.
- Das AK. Kinetic and mechanistic aspects of metal ion catalysis in cerium(IV) oxidation. Coord. Chem. Rev. 2001; 213: 307-325.
- Chimatadar SA, Madawale SV, Nandibewoor ST. Mechanistic study of iodide catalysed oxidation of L-glutamic acid by cerium(IV) in aqueous sulphuric acid medium. Transition Met. Chem. 2007; 32: 634-641.
- Datt, N, Nagori RR, Mehrotra RN. Kinetics and mechanisms of oxidations by metal ions. Part VI. Oxidation of a-hydroxy acids by cerium(IV) in aqueous nitric acid. Can J Chem. 1986; 64: 19-23.
- McCurdy, Jr WH, Guilbault GG. Catalysts for cerium(IV) oxidimetry: determination of mixtures of mercury(I) and mercury(II). Anal Chem. 1960; 32: 647–650.
- Mishra SK, Gupta YK. Kinetics of oxidation of antimony(III) by cerium(IV) in media containing perchloric acid. J Chem Soc A. 1970; 260-264.
- Yadav MB, Derva V, Rani A. Kinetics and mechanism of uncatalyzed and silver(I) catalyzed oxidation of lysine by cerium(IV) in acid perchlorate medium. J Indian Chem Soc. 2009; 86: 600-604.
- Singh B, Gaur CPS, Singh RB, Saxena BBL. Kinetics of Ag(I)-catalyzed oxidation of glycine by Ce(IV) in aqueous HClO4 media. J. Indian. Chem. Soc. LIX. 1982; 59-65.
- Hardwick TJ, Robertson E. Ionic species in ceric perchlorate solutions. Can J Chem. 1951; 29: 818-828.
- Feigl F. Spot tests in organic analysis, 195 pp. Elsevier, New York. 1975.
- Vogel AI. Text book of practical organic chemistry, third ed., ELBS Longman, London, 1973; 332 and 679.
- Sherill MS, King CB, Spooner RC. The oxidation potential of cerous-ceric perchlorates. J. Am. Chem. Soc. 1943; 65: 170-179.
- Heidt LJ, Smith ME. Quantum yields of the photochemical reduction of ceric ions by water and evidence for the dimerization of ceric ions. J Am Chem Soc. 1948; 70: 2476-2481.
- King EL, Pandow ML. The spectra of cerium(IV) in perchloric acid. Evidence for polymeric species. J Am Chem Soc. 1952; 74: 1966-1969.
- Offner HG, Skoog DA. Hydrolysis constant of quadrivalent cerium from spectrometric measurements. Anal. Chem. 1966; 38: 1520-1521.
- Badi SS, Tuwar SM. Oxidation of clindamycin phosphate by cerium(IV) in perchloric acid medium – A kinetic and mechanistic approach. Arab J Chem In press. 2013.
- Martell AE, Smith RM. In: Critical stability constants. Vol I, Plenum Press, New York. 1974; 321.
- Frost AA, Person RG. Kinetics and mechanism, Wiley Eastern, New Delhi. 1970; 147.
- Laidler K. Chemical kinetics. McGraw-Hill, New York. 1965.
- Michaelis L. and Menten M.L. The kinetics of invertase action. Biochemistry Z. 1913; 49: 333-369.
- Hicks KW, Toppen DL, Linck RG. Inner-sphere electron-transfer reactions of vanadium(II) with azidoamine complexes of cobalt(III). Inorg Chem. 1972; 11: 310.
- Weissberger A. In Investigation of rates and mechanism of reactions in techniques of chemistry, John Wiley & Sons (New York: Interscience Publication). 1974; 421.
- Leal JM, Domingo PL, Garcla B, Ibeas S. Alkali metal ion catalysis of the oxidation of L-ascorbic acid by hexacyanoferrate(III) in strongly acidic media. J. Chem. Soc. Faraday Trans. 1993; 89: 3571–3577.
- Mathur S, Yadav MB, Devra V. Kinetics and mechanism of uncatalyzed and Ag(I) catalysed oxidation of hydroxylysine by cerium(IV) in acid medium. J Phys Chem Biophys. 2013; 3: 128-132.