
Research Article
Austin Chem Eng. 2016; 3(4): 1040.
Kinetics and Mechanism of Oxidation of Fluorenone Hydrazone by Permanganate Ion in Different Acidic Media
Fawzy A1,2*, Ahmed SA1,2*, Altass HM1, Althagafi II1, Zaafarany IA1 and Khairou KS1
1Chemistry Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia
2Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
*Corresponding author: Ahmed Fawzy, Associate Professor, Chemistry Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia; Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt Saleh A Ahmed, Professor, Chemistry Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia; Chemistry Department, Faculty of Science, Assiut University, Assiut, Egypt
Received: August 23, 2016; Accepted: September 07, 2016; Published: September 09, 2016
Abstract
The kinetics of permanganate oxidation of Fluorenone Hydrazone (FH) in both perchloric and sulfuric acid solutions was studied spectrophotometrically at a constant ionic strength of 1.5 mol dm-3 and at 20oC. In both acids, the reactions showed a first order dependence with respect to permanganate ion concentration, whereas the orders with respect to fluorenone hydrazone concentration were less than unity. The orders with respect to both perchloric and sulfuric acid concentrations were found to be fractional-second. Variation of either ionic strength or dielectric constant of the medium had no any significant effect on the oxidation rates. The reactions mechanism describing the kinetic data was proposed. In both acids, the main oxidation products of fluorenone hydrazone as confirmed by GC/MS analysis and FT-IR spectroscopy as the corresponding ketone (9H-fluorenone). Under comparable experimental conditions, the oxidation rate of fluorenone hydrazone in perchloric acid was slightly lower than that in sulfuric acid. The activation parameters of the second order rate constants have been evaluated and discussed.
Keywords: Fluorenone hydrazone; Oxidation; Permanganate; Kinetics; Mechanism
Introduction
Oxidation reactions are very important in organic synthesis. Among the important oxidizing agents, permanganate ion is widely used in the oxidation of many organic compounds in neutral, alkaline and acidic media [1-11]. The mechanism of oxidation reactions by permanganate ion is governed by pH of the medium [12]. During oxidation by permanganate, it is evident that the Mn(VII) in permanganate is reduced to various oxidation states neutral, alkaline and acidic media. In acid media, permanganate ion (MnO4 -) can exist in several different forms, HMnO4, H2MnO4 +, HMnO3, and Mn2O7 depending on the nature of the reluctant. The oxidant has been assigned with an inner-sphere and an outer-sphere mechanism pathways in their redox reactions [13,14].
Fluorene and its derivatives (FLs) are a unique class of polycyclic aromatic hydrocarbons (PAHs) which exist in the fossil fuels, petrogenic sources burning of gasoline [15,16]. Recently, studies on the exhaust emitting of different types of reformulated diesel fuels showed presence of fluorene as a precedence compound and isomers of methyl fluorene as a hesitant compound in the exhaust [17]. The fluorene unit is regularly employed in the growth of an assortment of visual devices with latent application as dye-sensitized solar cells [18], polymer light-emitting diodes [19,20] and other electro emissive materials [21]. In addition, fluorene based systems possess sole photo physical properties such as high fluorescent quantum yield, huge photo stability, and excellent hole-transporting properties [22,23]. Furthermore, fluorene is one of the highest plentiful polycyclic aromatic hydrocarbons (PAHs) in the surroundings due to its high volatility. Established to be a neurotoxicant through mouthful of air, it was also recognized as a contributive PAH to food contagion. Fluorene compounds with intrinsic rigid structures have been attracting much consideration as organic functional materials because of their promising physical and chemicals properties such as glass transition temperatures, good solubility and their amorphous nature, which make them very talented as an move toward for optic electric materials [24,25]. In addition, hydrazone derivatives were found to be biologically important class of compounds [26]. Hydrazone derivatives were found in natural and synthetic products of biological interest [27]. Literature studies revealed that, hydrazones and the different substituted derivatives showed a broad spectrum of biological activities. Furthermore, fluorenone hydrazones are used as precursors for the synthesis of photochromic di and tetrahydroindolizines [28-30] and more recently as efficient corrosion inhibitors [31].
Although the kinetics of oxidation of fluorenone hydrazone by permanganate ion in alkaline medium has been studied previously [32], the present title studied the kinetics and mechanism of oxidation of this important organic compound with permanganate ion in two different acidic media, namely, perchloric and sulfuric acids. The objectives of the present work are to establish the most favorable conditions affecting oxidation of such compound, to investigate the effect of the acidic medium used on the oxidation kinetics and finally to elucidate a plausible oxidation mechanism.
Experimental
Materials
The chemicals used in the present work were of Aldrich grades.
Fluorenone hydrazone was prepared according to the described procedures with some modifications [33,34]. The synthesized fluorenone hydrazone was confirmed by both spectroscopic and analytical tools. All solvents used were of spectroscopic grade and used without further purifications. The solvents used were checked for the absence of absorbing or any fluorescent impurities. A fresh solution of potassium permanganate was prepared and standardized as reported earlier [35]. Sodium perchlorate and sodium sulfate were used to vary the ionic strength of the reactions media in both perchloric and sulfuric acid solutions, respectively.
Kinetic measurements
All kinetic runs were followed under pseudo-first order conditions where fluorenone hydrazone was existed in a large excess over that of permanganate. Initiation of the reactions were done by mixing the previously thermostatted solutions of both permanganate and fluorenone hydrazone that also contained the required amounts of the acid, NaClO4 or Na2SO4. The courses of the reactions were followed by monitoring the decay in the absorbance of permanganate as a function of time at its absorption maximum (λ = 526 nm), whereas the other constituents of the reaction mixtures did not absorb considerably at this wavelength. The absorption measurements were done in a temperature-controlled Shimadzu UV-VIS-NIR-3600 double-beam spectrophotometer. Application of Beer’s law was verified for permanganate concentrations at λ = 526 nm, and the molar extinction coefficient was found to be e = 2234 ± 43 dm3 mol-1 cm-1. The orders of the reactions with respect to the reactants were determined from the slopes of the log kobs versus log (concentration) plots by varying the concentrations of the substrate and acids, in turn, while keeping other conditions constant.
Results
Reaction stoichiometry and product analysis
Reaction mixtures containing various amounts of permanganate ion and fluorenone hydrazone at constant [H+], ionic strength, and temperature were allowed to react for 24 h for completion of the oxidation reactions. The unconsumed [permanganate] was determined spectrophotometrically at 526 nm. The results indicate expenditure of four permanganate ions for five molecule of fluorenone hydrazone substrate to yield the oxidation products as shown in the following equation,
The above stoichiometric equation is consistent with the results of products analysis as confirmed by the head-space GC/MS. Further assignment of the oxidation products was done by the help of FT-IR spectra as described elsewhere [32].
Spectral changes
The spectral scans during the oxidation of fluorenone hydrazone by permanganate ion in both perchloric and sulfuric acid solutions are shown in Figure 1 (a) and (b), respectively. In both acids, there was gradual disappearance of permanganate band at its absorption maximum (λ = 526nm).
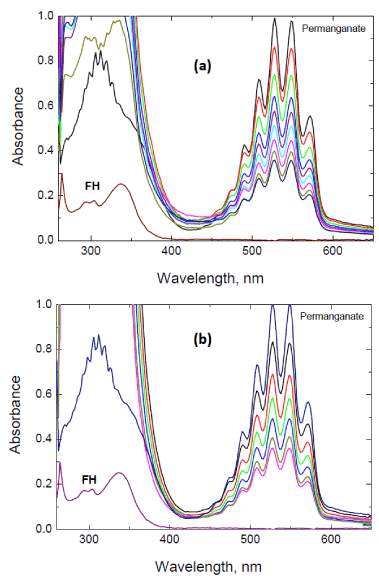
Figure 1: Spectral changes during oxidation of fluorenone hydrazone by
permanganate ion in: (a) perchloric, and (b) sulfuric acid solutions. [FH] = 1.0
x 10-2, [MnO4
-] = 4.0 x 10-4, [H+] = 0.8 and I = 1.5 mol dm-3 at 20°C. Scanning
time intervals = 1.0 min.
Effect of permanganate ion concentration
Permanganate ion oxidant was varied in the concentration range (2.0 - 10.0) x 10-4 mol dm-3 while the rest of the reactant concentrations were kept constant. Variation of the initial concentration of permanganate showed almost no influence on the pseudo-first order rate constants as listed in Table 1, indicating first order dependence with respect to permanganate ion concentration.
Effect of fluorenone hydrazone concentration
The pseudo-first order rate constants were measured at different fluorenone hydrazone concentrations while keeping others constant. Plots of kobs versus [FH] in both perchloric and sulfuric acid solutions were found to be linear with positive intercepts as shown in Figure 2 confirming less than unit order dependences with respect to fluorenone hydrazone concentration.
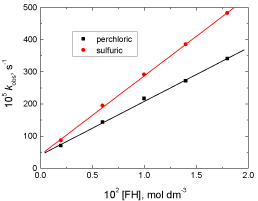
Figure 2: Plots of the pseudo-first order rate constants (kobs) versus [FH] in
the oxidation of fluorenone hydrazone by permanganate ion in: (a) perchloric
and, (b) sulfuric acid solutions. [MnO4
-] = 4.0 x 10-4, [H+] = 0.8 and I = 1.5 mol
dm-3 at 20°C.
Effect of acids concentration
The influence of both perchloric and sulfuric acids on the oxidation rates was investigated by varying the hydrogen ion concentration in the range: 0.2 – 1.4 mol dm-3, keeping all other reactants concentrations constant. Plots of log kobs versus log [H+] were found to be linear with slopes of 1.66 and 1.62 in perchloric and sulfuric acids, respectively as illustrated in Figure 3, suggesting that the orders of the reactions with respect to [acid] were fractionalsecond.
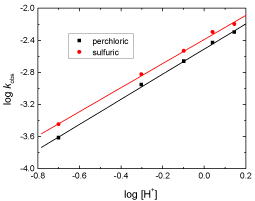
Figure 3: Plots of log kobs versus log [H+] in the oxidation of fluorenone
hydrazone by permanganate ion in: (a) perchloric and, (b) sulfuric acid
solutions. [MnO4
-] = 4.0 x 10-4, [FH] = 1.0 x 10-2 and I = 1.5 mol dm-3 at 20°C.
Effect of ionic strength and dielectric constant
The effect of ionic strength was studied by varying the concentration of NaClO4 and Na2SO4 in perchloric and sulfuric acids media, respectively, at constant concentrations of permanganate, fluorenone hydrazone and acids. It was found that increasing ionic strength did not affect the oxidation rates as listed in Table 1.
104 [MnO4-]
102 [FH]
[H+]
I
105 kobs (s-1)
(mol dm-3)
(mol dm-3)
(mol dm-3)
(mol dm-3)
Perchloric
Sulfuric
2
1
0.8
1.5
214
286.2
4
1
0.8
1.5
216.3
291
6
1
0.8
1.5
213.9
295.4
8
1
0.8
1.5
217.4
290.1
10
1
0.8
1.5
220.3
289.6
4
0.2
0.8
1.5
69.9
86.3
4
0.6
0.8
1.5
143.2
194.7
4
1
0.8
1.5
216.3
291
4
1.4
0.8
1.5
271
385.4
4
1.8
0.8
1.5
340.9
482.2
4
1
0.2
1.5
27.9
33
4
1
0.5
1.5
113.1
149.4
4
1
0.8
1.5
216.3
291
4
1
1.1
1.5
318
390.8
4
1
1.4
1.5
433.2
510.6
4
1
0.8
1.5
216.3
291
4
1
0.8
1.8
221
302.4
4
1
0.8
2.2
224.7
295.5
4
1
0.8
2.6
213.9
289.4
4
1
0.8
3
219.1
294
Experimental error ± 3%.
Table 1: Effect of variation of [MnO4 -], [FH], [H+] and I on the pseudo-first order rate constants in the oxidations of fluorenone hydrazone by permanganate ion in perchloric and sulfuric acid solutions at 20°C.
The effect dielectric constant (D) was also studied by varying the acetic acid - water content in the reactions mixtures with all other conditions being kept constant. The results revealed that the rate constants did not significantly affected by the decrease in dielectric constant of the solvent mixture; i.e. increase in acetic acid content.
Effect of temperature
The rates of the reactions were carried out at five different temperatures between 288 and 308K at constant concentrations of the reactants and other conditions being constant. The results indicate that the rate constants increased with rise in temperature. The activation parameters of the second order rate constant (k2) are calculated using Eyring and Arrhenius plots and are listed in Table 2.
Acid
DS? , lom J1-K1-
DH? , lom Jk1-
DG?392 , lom Jk1-
Ea?, kJ mol-1
Perchloric
-88.41
51.07
76.98
54.18
Sulfuric
-91.23
49.24
75.97
51.79
Table 2: parameters of the second order rate constant (k2) in the oxidation of fluorenone hydrazone by permanganate ion in perchloric and sulfuric acid solutions. [MnO4 -] = 4.0 x 10-4, [FH] = 1.0 x 10-2, [H+] = 0.8 and I = 1.5 mol dm-3.
Polymerization study
To check the existence of free radicals in the present reactions, the reactions mixtures were mixed with known quantities of acrylonitrile and kept for about 6 hours in an inert atmosphere. On diluting the mixtures with methanol, heavy white precipitates of polymers were formed, indicating the intervention of free radicals in the reactions. Blank experiments with either fluorenone hydrazone or permanganate alone with acrylonitrile did not induce polymerization under the same condition as those induced for the reactions mixtures.
Discussion
It has been reported [1,36] that reduction of permanganate in acidic media goes to either Mn(II) or Mn(IV), where the reduction potential of the Mn(VII)/Mn(II) couple is 1.51 V and of the Mn(VII)/ Mn(IV) couple is 1.695 V according to the equations: MnO4 - + 8H+ + 5e = Mn2+ + 4H2O. Manganese(VII) (MnO4 -) is reduced to Mn(II) during oxidation processes via many manganese intermediate species having different oxidation states such as Mn(VI), Mn(V), Mn(IV) and Mn(III). The appearance of these intermediate oxidation states depends upon various reaction conditions, types of substrate and their stability. The manganese chemistry involved in these multistep redox reactions is an important source of information because the manganese intermediates are relatively easy to identify when they have a sufficiently long lifetimes, and the oxidation states of the intermediate species permit useful conclusions about possible reaction mechanisms, including the nature of the intermediates [1].
In the present work, the fractional-second order dependences of the reactions in [H+] in both acids suggest that both fluorenone hydrazone substrate and permanganate oxidant [37-41] are subjected to protonation, i.e., the protonated forms of both reactants will be considered as the kinetically reactive species in the rate-determining step as defined by the equilibria, (2) and (3) in Scheme 1. On the other hand, the linearity of the plots of 1/kobs versus 1/[FH] (Figure 4) is considered as a kinetic evidence in favor of possible formation of an intermediate complex between oxidant and substrate, similar to the well-known Michaelis–Menten mechanism for enzyme– substrate reactions [42]. The failure of spectrophotometric detection of such intermediate complexes may be interpreted by either a lower concentration of the reactants used and, hence, the expected lower absorbitivity of the formed complex and/or the fast subsequent decomposition of the intermediates in comparison with their formation. The negligible effect of the ionic strength on the rates indicates that the reactions are between an ion, protonated substrate (FH+), and a neutral molecules, acid permanganate (HMnO4) [43,44].
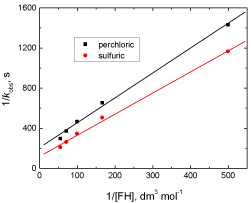
Figure 4: Plot of 1/kobs versus 1/[FH] in the oxidation of fluorenone hydrazone
by permanganate ion in both perchloric and sulfuric acid solutions. [FH] = 1.0
x 10-2, [MnO4
-] = 4.0 x 10-4, [H+] = 0.8, and I = 1.5 mol dm-3 at 20°C.
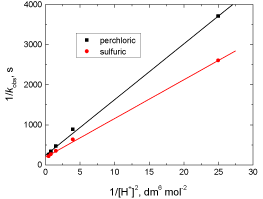
Figure 5: Plot of 1/kobs versus 1/[H]2 in the oxidation of fluorenone hydrazone
by permanganate ion in both perchloric and sulfuric acid solutions. [MnO4
-] =
4.0 x 10-4 and I = 1.5 mol dm-3 at 20°C.
In view of the above arguments, the following reactions mechanism can be proposed, which involves attack of the powerful oxidant, acid permanganate, on the protonated fluorenone hydrazone leading to the formation a complex (C) prior to the rate-controlling step. The cleavage of such complex leads to the formation of a free radical intermediate derived from fluorenone hydrazone substrate and manganate(VI) intermediate. The substrate radical is rapidly attacked by manganate(VI) species to yield the corresponding diazo derivative and hypomanganate(V) intermediate. In a further fast step, the intermediate Mn(V) being very active and unstable reacts with the diazo derivative to gives the corresponding ketone (9H-fluorenone) as the final oxidation product of fluorenone hydrazone and manganese(III) intermediate. This step is followed by other fast reactions between other three moles of fluorenone hydrazone and three moles of permanganate to give 9H-fluorenone product and Mn(III) intermediate species. The last step is rapidly attack of the four formed Mn(III) intermediate species on another mole of fluorenone hydrazone to give the last 9H-fluorenone product and the final oxidation product of permanganate, Mn(II), satisfying the obtained stoichiometry. The proposed mechanism is illustrated in Scheme 1.
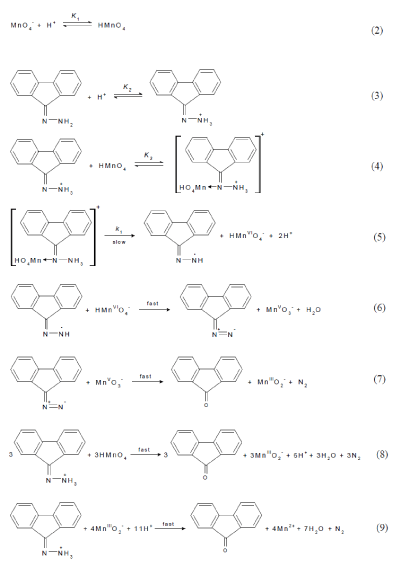
scheme 1: Mechanism of oxidation of fluorenone hydrazone by permanganate ion in acidic media.
The activation parameters listed in Table 2 may be interpreted as follows. The obtained negative values of DS≠ suggest that the reactions point towards the inner-sphere pathway [45]. The positive values of both DH≠ and DG≠ confirm endothermic formation of the intermediate complexes and their non-spontaneities, respectively.
According to the proposed mechanistic Scheme 1, the oxidation rate can be expressed by the following rate law:
,[HMnO4] = K1[MnO4 -][H+] (11)
Also,
,[FH+] = K2[FH][H+] (12)
,[C] = K3[FH+][HMnO4] (13)
Substituting Eqs. (11) and (12) into Eq. (13) leads to:
[C] = K1K2K3[FH][MnO4 -][H+]2 (14)
Substituting Eq. (14) into Eq. (10) leads to:
Rate = k1K1K2K3[FH][MnO4-][H+]2 (15)
The total concentration of MnO4 - is given by:
[MnO4 -]T = [MnO4 -]F + [HMnO4] + [C] (16)
where ‘T’ and ‘F’ refer to the total and free concentrations.
Therefore,
[MnO4 -]T = [MnO4 -]F + K1[MnO4 -][H+] + K1K2K3[FH][MnO4 -] [H+]2 (17)
[H+]T = [H+]F (19)
Similarly,
[FH]T = [FH]F (20)
Substituting Eqs. (18) - (20) into Eq. (15) (and omitting ‘T’ and ‘F’ subscripts) leads to:
Under pseudo-first order condition, the rate-law can be expressed by equation (A.11):
Comparing Eqs. (21) and (22), we get the following relationship:
With rearrangement of Eq. (23), the following equation is obtained:
According to Eq. (24), other conditions being constant, the plots of 1/kobs versus 1/[FH] at constant [H+], and 1/kobs versus 1/[H]2 at constant [FH] are expected to be linear and are found to be so as shown in Figures 4 and 5, respectively.
Conclusion
The kinetics of oxidation of fluorenone hydrazone by permanganate ion in both perchloric and sulfuric acid solutions has been studied. The main oxidation product of fluorenone hydrazone was identified in both cases as the corresponding ketone (9H-fluorenone). Under comparable experimental conditions, the oxidation rate of fluorenone hydrazone in perchloric acid was slightly lower than that in sulfuric acid. The activation parameters of the second order rate constants have been evaluated and discussed.
References
- Stewart R. Oxidation in Organic Chemistry, Part A (ed.) Wiberg KB, New York, Academic Press. 1965.
- Jose TP, Nandibewoor ST, Tuwar SM. Mechanism of oxidation of L-histidine by heptavalent manganese in alkaline medium. E-J Chem. 2005; 2: 75-85.
- Fawzy A, Ashour S, Musleh MA. Base-catalyzed oxidation of L-asparagine by alkaline permanganate and the effect of alkali-metal ion catalysts: kinetics and mechanistic approach. React Kinet Mech Catal. 2014; 111: 443-460.
- Fawzy A, Shaaban MR. Kinetic and mechanistic investigations on the oxidation of N’-heteroaryl unsymmetrical formamidines by permanganate in aqueous alkaline medium. Transition Met Chem. 2014; 39: 379-386.
- Fawzy A, Zaafarany IA, Alfahemi J, Tirkistani FA. Base-catalyzed oxidation of aminotriazole derivative by permanganate ion in aqueous alkaline medium: a kinetic study. Int J Inn Res Sci Eng Tech. 2015; 4: 6802-6814.
- Asghar BH, Fawzy A. Kinetic, mechanistic, and spectroscopic studies of permanganate oxidation of azinylformamidines in acidic medium, with autocatalytic behavior of manganese(II). J Saudi Chem Soc. 2016; 20: 561-569.
- Fawzy A, Ashour SS, Musleh MA. Kinetics and mechanism of oxidation of L-histidine by permanganate ions in sulfuric acid medium. Int J Chem Kinet. 2014; 46: 370-381.
- Ahmed GA, Fawzy A, Hassan RM. Spectrophotometric evidence for the formation of short-lived hypomanganate(V) and manganate(VI) transient species during the oxidation of K-carrageenan by alkaline permanganate. Carbohydr Res. 2007; 342: 1382-1386.
- Zaafarany IA, Fawzy A, Ahmed GA, Ibrahim SA, Hassan RM, Takagi HD. Further evidence for detection of short-lived transient hypomanganate(V) and manganate(VI) intermediates during oxidation of some sulfated polysaccharides by alkaline permanganate using conventional spectrophotometeric techniques. Carbohydr Res. 2010; 345: 1588-1593.
- Hassan RM, Fawzy A, Alarifi A, Ahmed GA, Zaafarany IA, Takagi HD. Base-catalyzed oxidation of some sulfated macromolecules: kinetics and mechanism of formation of intermediate complexes of short-lived manganate (VI) and/or hypomanganate (V) during oxidation of iota- and lambda-carrageenan polysaccharides by alkaline permanganate. J Mol Catal A. 2011; 335: 38-45.
- Hassan RM, Dahy A, Ibrahim S, Zaafarany IA, Fawzy A. Oxidation of some macromolecules. Kinetics and mechanism of oxidation of methyl cellulose polysaccharide by permanganate ion in acid perchlorate solutions. Ind Eng Chem. Res. 2012; 51: 5424–5432.
- Gardner KA, Kuehnert LL, Mayer JM. Hydrogen atom abstraction by permanganate:? oxidations of arylalkanes in organic solvents. Inorg. Chem. 1997; 36: 2069-2078.
- Perez-Benito JF. Permanganate oxidation of α-amino acids: kinetic correlations for the nonautocatalytic and autocatalytic reaction pathways. J Phys Chem. 2011; 115: 9876–9885.
- Babatunde OA. A study of the kinetics and mechanism of oxidation L-ascorbic acid by permanganate ion in acidic medium. World J Chem. 2008; 3: 27–31.
- Thormann T, Rogojerov M, Jordanov B, Thulstrup EW. Vibrational polarization spectroscopy of fluorene: alignment in stretched polymers and nematic liquid crystals. J Mol Str. 1999; 509: 93-99.
- Irwin RJ, Service NP. Environmental contaminants encyclopedia fluorene entry. 1997.
- Borras E, Tortajada-Genaro LA, Vazquez M, Zielinska B. Polycyclic aromatic hydrocarbon exhaust emissions from different reformulated diesel fuels and engine operating conditions. Atmospheric Environment. 2009; 43: 5944-5952.
- Li X, Lü H, Wang S, Guo J, Li J. Sensitizers of dye-sensitized solar cells, Prog. Chem. 2011; 23: 569-588.
- Ma Z, Ding J, Cheng Y, Xie Z, Wang L, Jing X, Wang F. Synthesis and characterization of red light-emitting electrophosphorescent polymers with different triplet energy main chain. Polymer. 2011; 52: 2189-2197.
- Wang HY, Qian Q, Lin KH, Peng B, Huang W, Liu F, et al. Stable and good color purity white light-emitting devices based on random fluorene/spirofluorene copolymers doped with iridium complex. J Polym Sci. 2012; 50: 180-1883.
- Yang XH, Wu FI, Neher D, Chien CH, Shu CF. Polyfluorene-based semiconductors combined with various periodic table elements for organic electronics. Chem. Mater. 2008; 20: 1629-1635.
- Kucherak OA, Didier P, Mély Y, Klymchenko AS. Fluorene Analogues of Prodan with Superior Fluorescence Brightness and Solvatochromism. J Phys Chem. Lett. 2010; 1: 616-620.
- Cheng YJ, Yang SH, Hsu CS. Synthesis of conjugated polymers for organic solar cell applications, Chem. Rev. 2009; 109: 5868-5923.
- Xing X, Zhang L, Liu R, Li S, Qu B, Chen Z. A deep-blue emitter with electron transporting property to improve charge balance for organic light-emitting device. ACS Appl. Mater. Interf. 2012; 4: 2877-2883.
- Pina J de, Melo JSS, Egkert A, Scherf U. Unusual photophysical properties of conjugated, alternating indigo–fluorene copolymers. J Mater Chem A. 2015; 3: 6373-6381.
- Jin L, Chen J, Song B, Chen Z, Yang S, Li Q, Hu D, Xu R. Synthesis, structure, and bioactivity of N'-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)- 2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg Med Chem Lett. 2006; 16: 5036-5040.
- Maria J. Synthesis and spectral characterisation of hydrazone based 14-membered octaaza macrocyclic Ni(II) complexes. J Chem. Pharm. Res. 2012; 4: 986-988.
- Ahmed SA, Abdel-Wahab AA, Dürr H. CRC Handbook of organic photochemistry and photobiology, Horspool WM, Lenci F edn, CRC press, New York, 2nd edn, Chapter 96. 2003; p 1.
- Ahmed SA, Hartmann Th, Dürr H. Photochromism of dihydroindolizines: Part VIII. First holographic image recording based on di- & tetrahydroindolizines photochromes. J Photochem Photobiol. 2008; 200: 50-56.
- Ahmed SA, Pozzo JL. Photochromism of dihydroindolizines Part IX. First attempts towards efficient self-assembling organogelators based on photochromicdihydroindolizines and N-acyl-I, amino acid units. J Photochem Photobiol. 2008; 200: 57-67.
- AL Jahdaly BA, Althagafi II, Abdallah M, Khairou KS, Ahmed SA. Fluorenone hydrazone derivatives as efficient inhibitors of acidic and pitting corrosion of carbon steel. J Mater Env Sci. 2016; 7: 1798-1809.
- Fawzy A, Ahmed SA, Althagafi II, Morad MH, Khairou KS. Kinetics and mechanistic study of permanganate oxidation of fluorenone hydrazone in alkaline medium. Adv Phys Chem. 2016.
- Ahmed SA. Photochromism of dihydroindolizines.III [1]: synthesis and photochromic behavior of novel photochromic dihydroindolizines incorporating a cholesterol moiety, Monatsh. Chem. 2004; 135: 1173-1181.
- Ahmed SA, Khairou KS, Asghar BH, Muathen HA, Nahas NMA, Al Shreef HF. Photochromism of tetrahydroindolizines. Part XIV: Synthesis of cis-fixed conjugated photochromic pyridazinopyrrolo[1,2-b]isoquinolines incorporating carbon-rich linkers. Tetrahed. Lett. 2014; 55: 2190-2197.
- Vogel IA. A Text book of quantitative inorganic analysis. 4th edn ELBS and Longman, New York. 1978.
- Day MC, Selbin J. Theoretical Inorganic Chemistry, Reinhold Publishing Corporation, New York. 1985; 344.
- Zahedi M, Bahrami H. Kinetic and mechanism of autocatalytic oxidation of L-asperagine in moderately concentrated sulphuric acid medium. Kinet Catal. 2004; 45: 351–358.
- Hosahalli RV, Savanur AP, Nandibewoor ST, Chimatadar SA. Kinetics and mechanism of the autocatalyzed oxidation of theophylline by permanganate in aqueous perchloric acid medium. J Solution Chem. 2012; 41: 567–580.
- Wiberg KB. Oxidation in Organic Chemistry, Part A, pp. 48, Academic Press, New York. 1965.
- Chimatadar SA, Hiremath SC, Raju JR. Oxidation of thallium(I) by permanganate in aqueous perchloric acid medium. Indian J. Chem. 1990; 30A: 190–192.
- Bailar JC, Emeleus HJ, Nyholm R, Dickenson AFT. Comprehensive Inorganic Chemistry, pp. 771, Pergamon Press Ltd., New York. 1975.
- Michaelis L, Menten ML. The kinetics of invertase action. Biochem. Z. 1913; 49: 333–369.
- Frost AA, Person RG. Kinetics and mechanism, Wiley Eastern, New Delhi. 1970; 147.
- Laidler K. Chemical kinetics. McGraw-Hill, New York. 1965.
- Weissberger A. In Investigation of rates and mechanism of reactions in techniques of chemistry, John Wiley & Sons (New York: Interscience Publication). 1974; 421