
Research Article
Austin Chem Eng. 2016; 3(4): 1041.
Using Response Surface Methodology for Modeling and Optimization of Propene/Propane Separation Column
Kazemi A*
Chemical Engineering Department, Isfahan University of Technology, Isfahan, Iran
*Corresponding author: Abolghasem Kazemi, Chemical Engineering Department, Isfahan University of Technology, Isfahan, Iran
Received: September 09, 2016; Accepted: September 27, 2016; Published: September 29, 2016
Abstract
Propylene/Propane separation system is widely encountered in chemical and petrochemical processes. One of the conventional methods used for this separation is distillation. In this study a propylene/propane separation column with the capacity of purification of 70,000 tons/year of propylene has been considered. The primary aim of this study was the modeling and optimization of this column with the use of response surface methodology (RSM). Four input variables have been taken into account, which are columns reflux ratio, number of theoretical stages, condenser pressure and the recovery of propylene in the top product. A central composite design with 31 data points has been used, the data has been derived from simulation, and three different system responses have been studied and modeled which are propylene purity in the top product and condenser and reboiler duties. Based on the models derived and the surface plots of the models, several suggestions have been presented to enhance the system’s performance.
Keywords: Response Surface Methodology; Propane; Propylene; Optimization; Modeling
Introduction
Propylene is a building block for the petrochemical industry and it is the base material for production of a wide range of other materials such as propylene oxide, polypropylene and acrylonitrile and the world propylene demand has been increasing in recent years [1-3]. Propylene can be produced via several different methods, while production of propylene in the FCC and Steam cracking of naphta process are the main sources for production of propylene [4, 5]. However nowadays a lot of other processes such as dehydrogenation of propane and methanol to propylene (MTP) are being developed for production of propylene [1,5]. The separation of propylene/ propane is required in each of the introduced propylene production processes. Since propylene and propane have close boiling points, the separation of these two gases is very energy consuming. Different methods like hybrid membrane distillation, cryogenic distillation, extractive distillation and adsorption can been used to separate these components [6-9]. In this study an ordinary distillation column is considered, modeled and optimized for separation of the two components. The response surface methodology has been used for modeling and optimization of this column.
The response surface methodology (RSM) is a method which can be used to find the relation between several input variables and system response (responses) [10]. RSM is extensively used in situations where several factors influence the response of the system and it is desired to optimize the performance of the system [11]. This method provides a way to choose some of the points of a design space, and having the system response at those points, it represents an estimation of the response at any other point in the design space. It uses a statistical approach for modeling and optimization of the system. The real relation between the input variables and the system response remains unknown, however the behavior of the system response is fitted by a polynomial in which variables are the input variables which have been selected for study [12]. The response surface methodology has been used in recent studies for a wide range of different systems like water and wastewater treatment, membrane systems, electronics and chemical systems like distillation [13-18]. The main of this study was the modeling and optimization of a propylene/propane distillation column with the use of response surface methodology.
Basis of Simulation
For the purpose of optimization of the propylene/ propane separation column, a feed of propylene/propane mixture based on production of 70,000 tons/year propylene has been taken into account. It has been assumed that the feed is available at a temperature of 173oC and a pressure of 500 psia. A distillation column model in Aspen HYSYS is selected for the modeling of the column [19].
Since the components in the feed mixture are hydrocarbons, and the pressure of the column doesn’t exceed 5,000 psia, the SRK property package is used for simulation of this process [20]. This property package exploits enhanced binary interactions for all library hydrocarbon-hydrocarbon systems [20]. As suggested by earlier studies and because of the close boiling point of the two components, this separation is a rather difficult separation process [21] and thus, in order to reach appropriate purities in the products, relatively high values of column reflux ratio and number of stages have been selected for the simulation. Figure 1 shows the simulated PFD of the separation process.
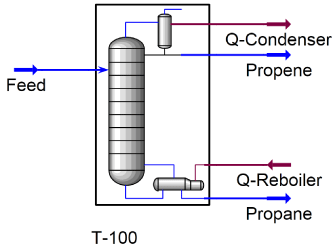
Figure 1: Process flow sheet [19].
Two specifications have been applied to the distillation column model. Column reflux ratio and the propene recovery in the top product have been specified to run the distillation column model and obtain results for the top and the bottom products. Also 10 psi pressure drop has been assumed to occur in the column.
Results and Discussion
Response surface methodology
The response surface methodology is used to find the relationship between several input variables and one or more system responses [22]. This methodology is also applicable to find the interactions between the effects of the parameters under investigation. The main application of the response surface methodology is to find the optimum (maximum or minimum) value of a specific response in the ranges of change of the control parameters [12]. In this work the decisive variables in a distillation column have been chosen and their effects on three different system responses have been investigated. For investigation of the effects of the four parameters under investigation, a central composite design of experiment (CCD) has been used. 31 set of variables have been introduced and the data of the three responses have been obtained from the simulator after applying each set of variables and running the simulation. Since the results have been derived from the simulator, the results for the replicated experiments have been the same and thus for the replicated data points, only one data has been derived from the simulator. For instance one of the data points has to be repeated 7 times in the experiments. However, since the results have been derived from simulation, the responses for this point have been derived once and used for the other 6 data points.
Parameters under investigation
In this work four different variables in the distillation column have been taken into account and their effects on the three system responses have been investigated. The variables are column reflux ratio, number of stages, propene recovery in the top product and the condenser pressure. Effects of these variables on three different system responses (propylene purity in the top product, condenser duty and reboiler duty) have been investigated.
Since the propylene/ propane separation is an energy consuming and rather difficult separation process, a relatively large number of stages has been used for the purpose of separation. This number varied between 45 and 145 in different data points. Because of difficulty of separation, relatively large values of column reflux ratio (maximum of 19.5) have been used for this separation. In Table 1 the range of change of each of the variables under investigation are shown.
#
Parameter
Lower limit
Upper limit
1
Reflux ratio
1.5
19.5
2
Number of stages
45
145
3
Propylene recovery (fraction)
0.87
0.99
4
Condenser pressure (psia)
100
500
Table 1: Range of change of parameters under investigation.
Response surface analysis
The data has been derived from the simulator for the 31 data points and then analyzed to reveal the relationship between the four factors and the responses. The surface plot relating the column reflux ratio, condenser pressure and the purity of methanol in the top product is shown in Figure 2. As it is clear in Figure 2, decreasing the column pressure in the range of change of pressure under investigation (100 – 500 psia) would cause higher purities of propylene at the top product at a certain column reflux ratio. Also at a certain column pressure, increasing the column reflux ratio would increase the purity of the top product which is a legitimate result. Because increasing column reflux ratio almost in any distillation column helps better separation of the components [23,24]. However, two other system responses have been obtained which are the condenser and reboiler duties needed for the separation. As the reboiler duty – reflux ratio – pressure and condenser duty – reflux ratio – pressure surface plots (Figure 3 and Figure 4) depict, increasing reflux ratio and decreasing pressure causes the condenser and reboiler duties to rise dramatically which is a negative change because the hot and cold utilities needed for the purpose of separation would rise which means higher energy consumption of the column. It is observed from reboiler duty – reflux ratio – pressure and condenser duty – reflux ratio – pressure surface plot (Figure 3 and Figure 4), that although reduction of pressure causes the column energy consumption to increase, reboiler and condenser duty changes by pressure are negligible when compared to their changes by reflux ratio. Since the purity of propylene at the top product tends to flatten at high reflux ratios, decreasing the column pressure at a moderate reflux ratio would be a better change in the column operating parameters than increasing reflux ratio to very high values.
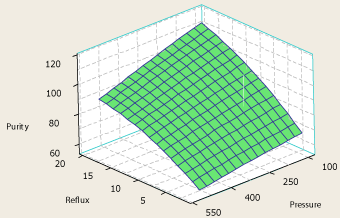
Figure 2: The surface plot of propylene purity – reflux ratio – pressure.
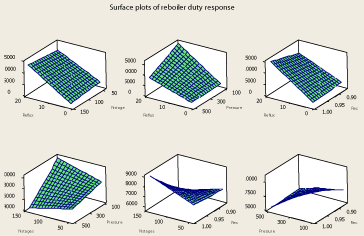
Figure 3: Surface plots of reboiler duty response.
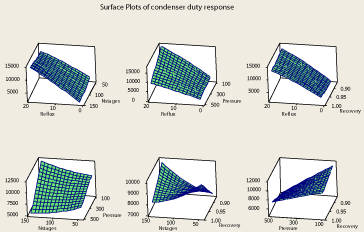
Figure 4: Surface plots of condenser duty response.
The surface plot of propylene purity – reflux ratio – propylene recovery in the top product is show in Figure 5. As it is clear from Figure 5, high propylene recoveries are obtainable without reduction in top products purity. It is also found from reboiler duty – reflux ratio – propylene recovery and condenser duty– reflux ratio – propylene recovery surface plot (Figure 3 and Figure 4) that at a certain reflux ratio, recovery doesn’t have a significant effect on the condenser duty while affects the reboiler duty. It is found that when the propylene recovery increases, the reboiler duty increases slightly. This means higher energy consumption of the column would occur if higher propylene recoveries are needed. However operating the column at a condition resulting in high recovery is necessary because at lower recoveries, an extent of the propylene in the columns feed is wasted in the propane product. Thus, this change in the column is desirable despite causing the column to require more energy for the purpose of separation of the two components.
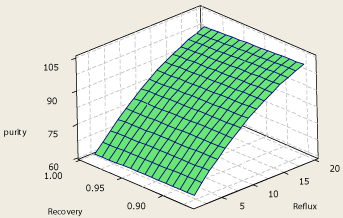
Figure 5: Surface plot of propylene purity – reflux – propylene recovery.
The surface plot of propylene purity – reflux ratio – number of stages is shown in Figure 6. As it is clear from Figure 6, increasing column reflux ratio causes the product purity to increase at a certain number of theoretical stages. However at a certain reflux ratio, increasing the number of theoretical stages causes affects the product purity slightly when number of stages is lower than 60 and when the number of theoretical stages increases further, it doesn’t have any effect on the product purity. Increasing number of stages causes the columns capital cost to increase, also it causes the column pressure drop to increase while it doesn’t have a significant effect on the product purity in the top product. From these statements it is concluded that the best possible number of theoretical stages in this separation process is 60 stages.
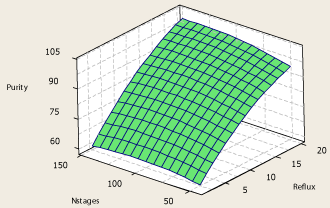
Figure 6: Surface plot of propylene purity – reflux ratio – number of theoretical
stages.
The same behavior of the system primary response with respect to pressure, number of theoretical stages and propylene recovery in the top product is observed in Figure 7 and Figure 8.
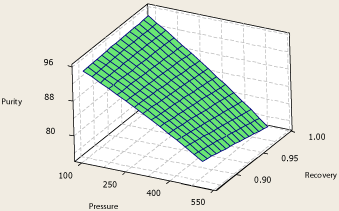
Figure 7: Surface plot of propylene purity – pressure – propylene recovery.
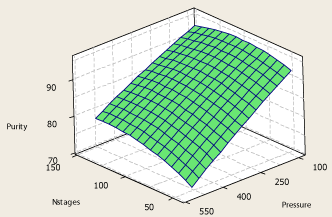
Figure 8: Surface plot of propylene purity – number of theoretical stages –
pressure.
The surface plot of the top product purity – propylene recovery – number of theoritical stages is shown in Figure 9. It is observed that at a certain obtainable recovery of propylene, when the number of stages is increased, a maximum in top product purity would appear at 115-130 stages which depend in the value of recovery which is desired. However it is clear from the Figure 9 that the difference in the product purity at the maximum and higher or lower number of stages is little. For instance when 96% propylene recovery is desired, if number of stages reduces from the extermum point (115) to the value introduced earlier as the best number of theoritical stages (60), about one percent reduction in product purity is observed.
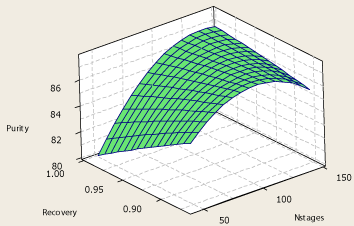
Figure 9: Surface plot of propylene purity – number of theoretical stages –
propylene recovery.
Conclusion
Response surface methodology has been used for modeling and optimization of a propylene/propane separation column. Three models have been obtained for the three decision-making factors under investigation. The surface plots of the three responses have been analyzed to find the best conditions for operating the column. It is found that at theoretical stages higher than 60, the number of stages doesn’t have a significant effect on the product purity and thus 60 theoretical stages has been found as the best number of stages of the column. Also, there is an optimal place for entering the feed to the column located in the intermediate stages. This location varies upon variation of other operational parameters. At constant operational parameters, increasing reflux ratio results in higher purity of the top product at the expense of higher energy requirements.
Also at a certain moderate column reflux ratio, it was found that reduction of column’s pressure to 100psia would result in more efficient separation of the two components, while it doesn’t cause the columns temperature to drop dramatically which could have resulted in more capital investment for purchasing materials with high resistance at cryogenic conditions and the column in this case, would require less energy for separation of the two components than the case where the reflux ratio increases further. Propylene purities (as high as 99%) have been obtained at high reflux ratio and low pressures while the propylene recovery in the top product was very high.
References
- Brown K, Kang SC, Choi S, Park Y K. The Advanced Catalytic Olefins (ACO) Process. 2007.
- Plotkin JS. The changing dynamics of olefin supply/demand. Catalysis today. 2005; 106: 10-14.
- Hong-zhong Z. Market Situation and Trend of Propylene Market Worldwide. Chemical Techno-Economics. 2004; 9.
- Ren T, Patel M, Blok K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy. 2006; 31: 425-451.
- Aitani AM. Propylene production. Encyclopedia of Chemical Processing. 2006; 5: 2461-2466.
- Benali M, Aydin B. Ethane/ethylene and propane/propylene separation in hybrid membrane distillation systems: Optimization and economic analysis. Separation and Purification Technology. 2010; 73: 377-390.
- Alcantara-Avila JR, Gomez-Castro FI, Segovia-Hernandez JG, Sotowa KI, Horikawa T. Optimal design of cryogenic distillation columns with side heat pumps for the propylene/propane separation. Chemical Engineering and Processing: Process Intensification. 2014; 82: 112-122.
- Liao B, Lei Z, Xu Z, Zhou R, Duan Z. New process for separating propylene and propane by extractive distillation with aqueous acetonitrile. Chemical Engineering Journal. 2001; 84: 581-586.
- Plaza MG, Ferreira AFP, Santos JC, Ribeiro AM, Muller U, Trukhan N, et al. Propane/propylene separation by adsorption using shaped copper trimesate MOF. Microporous and Mesoporous Materials. 2012; 157: 101-111.
- Montgomery DC. Design and analysis of experiments. John Wiley & Sons. 2008.
- Carley KM, Kamneva NY, Reminga J. Response surface methodology (No. CMU-ISRI-04-136). Carnegie-Mellon Univ Pittsburgh Pa School of Computer Science. 2004.
- Khuri AI, Mukhopadhyay S. Response surface methodology. Wiley Interdisciplinary Reviews: Computational Statistics. 2010; 2: 128-149.
- Kirmizakis P, Tsamoutsoglou C, Kayan B, Kalderis D. Subcritical water treatment of landfill leachate: Application of response surface methodology. Journal of Environmental Management. 2014; 146: 9-15.
- Tezcan Un U, Kandemir A, Erginel N, Ocal SE. Continuous electrocoagulation of cheese whey wastewater: An application of Response Surface Methodology. Journal of Environmental Management. 2014; 146: 245-250.
- McCarthy E, Flick S, Merida W. Response surface methods for membrane humidifier performance. Journal of Power Sources. 2013; 239: 399-408.
- Somasundaram M, Saravanathamizhan R, Basha CA, Nandakumar V, Begum SN, Kannadasan T. Recovery of copper from scrap printed circuit board: modelling and optimization using response surface methodology. Powder Technology. 2014; 266: 1-6.
- Leong WC, Abdullah MZ, Khor CY. Optimization of flexible printed circuit board electronics in the flow environment using response surface methodology. Microelectronics Reliability. 2013; 53: 1996-2004.
- Noshadi I, Amin NAS, Parnas RS. Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid: Optimization using response surface methodology (RSM). Fuel. 2012; 94: 156-164.
- Aspen-one. Examples.
- Aspen, user online documentation.
- Mauhar SM, Barjaktarovic B, Sovilj M. Optimization of Propylene-Propane Distillation Process. Chemical Papers. 2004; 58: 386-390.
- Habib SS. Study of the parameters in electrical discharge machining through response surface methodology approach. Applied Mathematical Modelling. 2009; 33: 4397-4407.
- Treybal RE, Treybal RE. Mass-transfer operations. McGraw-Hill New York. 1968; 3.
- Noble R, Terry. Principles of chemical separations with environmental applications. Cambridge University Press Cambridge, UK. 2004.