
Research Article
Austin Chem Eng. 2016; 3(5): 1042.
Effect of Organic Binders of TiO2 Pastes in the Photoanodes of Cost-Effective Dye Sensitized Solar Cells Fabrication
Valsaraj D1,2, Subramaniam MR², Baiju G² and Kumaresan D2,3*
¹Department of Electrical & Electronics Engineering, Amrita School of Engineering, Coimbatore 641112, Amrita Vishwa Vidyapeetham, Amrita University, India
²Department of Chemical Engineering and Materials Science, Amrita School of Engineering, Coimbatore 641112, Amrita Vishwa Vidyapeetham, Amrita University, India
³CoE–AMGT, Amrita School of Engineering, Coimbatore 641112, Amrita Vishwa Vidyapeetham, Amrita University, India
*Corresponding author: Duraisamy Kumaresan, Department of Chemical Engineering and Materials Science, Amrita School of Engineering, Coimbatore 641112, Amrita Vishwa Vidyapeetham, Amrita University, India
Received: September 09, 2016; Accepted: October 06, 2016; Published: October 10, 2016
Abstract
A simple method of preparation of screen printable TiO2 paste from commercial anatase TiO2 nanopowder using two different organic binders i.e. polyvinylpyrrolidone and ethyl cellulose was tested, to study the effect of organic binders on their TiO2 films used as photoanodes in dye sensitized solar cells. In this study, TiO2 films made from the TiO2 paste with ethyl cellulose binder showed an improved power conversion efficiency of 4.47% over the TiO2 paste with polyvinylpyrrolidone binder producing power conversion efficiency of 3.59%, in their respective DSSC photoanodes. Also, the electrochemical impedance studies revealed a better charge transfer dynamics with reduced recombination processes for the DSSC photoanode made from TiO2 paste with ethyl cellulose binder. These results show a superior photovoltaic performance for the TiO2 paste with ethyl cellulose binder made by the simple method introduced in this work, which can open up options for the large-scale production of TiO2 pastes useful to the manufacturing of cost-effective DSSCs.
Keywords: Dye-sensitized solar cells; TiO2 nanopowder; TiO2 pastes; Organic binders; Photovoltaic performance
Introduction
In recent years, dye-sensitized solar cells (DSSCs) have showed enormous potential over the conventional silicon solar cells as inexpensive solar cells due to the surpassing power conversion efficiency (~13%) and their ease of fabrication. Also, the low-cost of fabrication of DSSCs has made them very attractive to the researches related to the device performance enhancement and on integrating the DSSCs in to domestic products [1-3]. Usually, DSSCs are made by the sandwich of mesoscopic TiO2 film anode, an electrolyte and a metal (platinum) coated cathode. Among these components, the techniques used for TiO2 film deposition on transparent conducting oxide (TCO) are critical to the fabrication of highly efficient DSSCs, together with the mesoscopic TiO2 film typically coated by a monolayer of high molar extinction co-efficient dye molecules to extend the visible light absorption of anode [4]. Moreover, for the fabrication of high performance DSSC, coating of nano-sized highly porous TiO2 film on TCO plays a significant role. Consequently, TiO2 nanoparticles used for the fabrication of DSSCs expected to have a particle diameter of about 20 – 25 nm, which makes the long wavelengths of visible light to penetrate the TiO2 film easily. And to improve the light scattering, an additional layer of particle diameter around 400nm is introduced on top of the smaller size nanoparticles layer. Generally, TiO2 nanomaterials exist in rutile, brookite and anatase crystalline phases. The most utilized phase of TiO2 in DSSC application is anatase phase. Brookite phase exists only in ore form and due to smaller surface area, lower fermi level and larger crystallite size rutile phase is not favorable to the DSSC applications. Therefore, the maximum efficiency achieved in DSSC fabricated with the thin film of anatase TiO2 nanoparticles which are mostly synthesized via hydrothermal method. And for the above mentioned reasons, anatase phase TiO2 film preparation adoptable to the screen printing technique for printing the uniform thickness TiO2 layer on top of fluorine doped tin oxide (FTO) coated glass has been widely preferred.
For the industrial scale manufacturing of inexpensive DSSC, screen printing of TiO2 layer on top of FTO or polymer surface is one of the widely used technologies. The applications of TiO2 paste also covers areas including gas sensors, Gamma – radiation sensors, corrosion resistance, and microwave absorption and so on [5-7]. Since titanium dioxide is nontoxic and eco-friendly, it has also found a wide variety of applications in health care products, cosmetics and paints. The main factor which influences the screen printing is the characteristics and quality of TiO2 paste. The preparation of TiO2 paste with controllable nanoparticles size via hydrothermal method takes minimum of 28 hrs to complete and follows a lengthy procedure. A lengthy procedure of TiO2 paste making may not fit industrially due to the economic constraints and the slow processes. So, to achieve an easy TiO2 paste preparation with comparable efficiency as that of nanoparticles, several methods are tried using commercially available nanopowder (P25, Degussa) [8].
In this study, a commonly available TiO2 nanopowder (Sigma Aldrich, USA) is converted into paste in lesser time when compared to the hydrothermal method. A faster and easier procedure makes the industrial manufacturing of DSSC more easy and rapid, without compromising the performance and quality. So, we have prepared the TiO2 pastes by following a simple method with different binders and tested their photovoltaic performances, and also studied the influence of dispersion agent with the best performing paste made from commercially available TiO2 powder. The paste preparation doesn’t involve any sophisticated equipments and the overall paste preparation has been completed in less than 6 hours. In contrast, the paste preparation using laboratory made nanoparticles requires trained hands and well equipped machineries. Moreover, the commercially available TiO2 nanopowder based paste provides almost similar performance throughout and high reliability regardless of number of batches of the TiO2 paste prepared.
Experimental
Materials
Commercial TiO2 nanopowder (Anatase) was procured from Sigma-Aldrich, USA. Ethyl cellulose powder (18-22 cps) was purchased from Loba Chemie, India. Polyvinylpyrrolidone was purchased from Himedia, India. Fluorine doped tin oxide (FTO) glass slides (12-14 ohm/sq) were purchased from Dyesol Corporation, Australia. Commercial N719 dye (4- tertbutylpyridine, cis di(thiocyanato)-N, N’-bis (2,2’ bipyridyl- 4-carboxylic acid-4’- tetrabutylammonium carboxylate) ruthenium (II) were procured from Dyesol Corporation, Australia. Adhesive film (Surlyn, Meltronix 1170-25PF) was purchased from Solaronix, Switzerland.
Preparation of screen-printable TiO2 pastes
Different type of TiO2 pastes were prepared using commercially available TiO2 nanopowder by varying different organic binder concentrations. Then their TiO2 films adhesion and performance were tested. Based on the literature, the widely known two organic binders ethyl cellulose, and polyvinyl pyrrolidone were selected for this study and compared with the TiO2 paste made without binder.
Preparation of TiO2 paste without binder: In the beginning, anatase TiO2 powder was mixed with few drops of DI water and ethanol thoroughly. Then, the mixture was crushed in an agate mortar until it converts as viscous state [9] by following the detailed procedure given below:
TiO2 powder was taken in a crucible and heated at 400°C for 30 minutes to remove the absorbed moisture and impurities. 2mL ethanol was then added to 1 gram TiO2 powder drop wise in an agate mortar followed by constant grinding, until the mixture turns in to a smooth white viscous paste. This mixture was then ultrasonicated for an hour and kept for magnetic stirring up to 12 hours at a speed of 300 rpm to obtain a homogenous, viscous paste. This paste was then used for TiO2 doctor blading/screen printing on substrates.
Polyvinyl pyrrolidone as binder: TiO2 powder was taken in a crucible and heated up to 400°C for 30 minutes to remove the absorbed moisture and organic impurities. Then the powder was mixed with polyvinylpyrrolidone (PVP) in different concentrations. Polyvinylpyrrolidone (PVP), also commonly called polyvidone or povidone, is a water-soluble binder extracted from the monomer N-vinylpyrrolidone. Therefore, at the outset TiO2 films were made using TiO2 paste with different concentrations of poly(vinylpyrrolidone) as a binder to understand the effect of PVP. Then, Triton X-100, acetic acid and ethanol were used in different stages of the TiO2 paste preparation. The amount of each chemical used for making the TiO2 paste with different PVP concentrations was followed as per the literature procedure [10]. TiO2 powder mixed with PVP in different proportions was crushed using 2mL of ethanol in an agate mortar for 20min to avoid aggregation. After that, 0.2mL of acetic acid was added. Triton X-100 was used as a dispersing agent; subsequently ethanol was used to dilute the mixture. After thorough mixing, the mixture was concentrated by heating on the hotplate to obtain a viscous paste [10,11].
As shown in the Figure 1 the photovoltaic properties of TiO2 films in DSSC photoanode are usually influenced by the methods of TiO2 paste made. PVP was used as a binder to regulate the viscosity of the paste, inhibit the aggregation of TiO2 nanopowder and improve the mechanical stability and continuity of the sintered film. An optimal paste composition, suitable for superior dye adsorption to improve the DSSC performance was obtained. Even though PVP enables good adhesion of paste on the FTO, the dye absorption was found to be poor, because the film was forming non-porous surfaces in several places after the sintering.
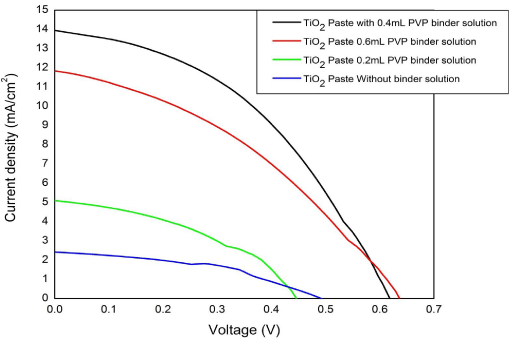
Figure 1: J-V characteristics of fabricated DSSC on varying the concentration
of PVP binder solution.
Ethyl cellulose as binder: The commercial TiO2 powder was taken in a crucible and heated up to 400°C for 30 minutes to remove the absorbed moisture and organic impurities. Figure 2 shows the scheme for the preparation of TiO2 paste by following a modified procedure obtained from the literature [8]. Then the preheated nanopowder was added with 0.2mL glacial acetic acid and 0.2mL DI water. This mixture was grinded well in an agate mortar. The dispersion of TiO2 nanopowder into the dilute acetic acid was controlled by mortar grinding. This assisted in reducing the mean particle size by increasing the dispersion time. Transparency of the coated film also improved on increasing the grinding time. Powder which stuck inside the mortar was removed using a plastic spatula, to obtain a smooth homogenous paste. A well dispersed TiO2 particles in homogenous paste were then transferred using ethanol (or any appropriate solvent) to a round bottom flask. This solution was then subjected to ultrasonication about 15 minutes for homogenous mixing of particles. Followed by sonication, the obtained homogenous white liquid was subjected to magnetic stirring for 2 hours at a speed of 300rpm. Then rotary evaporation of homogenous white liquid for 15 minutes at 40°C produced a viscous paste. The viscous paste was again ultrasonicated for another 15 minutes to get a fine homogenous paste. This prepared paste was protected from moisture before coating on the substrates.

Figure 2: Steps for fabrication of TiO2 paste using nanocrystalline powder.
Fabrication of porous-TiO2 electrodes
To avoid surface contamination by ions or iron oxides, which will be formed during the sintering of TiO2 film causing enhanced charge recombination in photocells, plastic spatulas and glass rods was used. This charge recombination will reduce the photo current (JSC) and the efficiency of DSSCs. Prior washing with acidic solution can remove the iron contamination and hence a 0.1M HCl was used to remove the iron contamination.
As shown in Figure 3, FTO glass used as a current collector, therefore clean FTO was doctor-bladed with TiO2 paste to prepare the anode. The TiO2 nanopowder based paste was coated on top of the FTO to produce the TiO2 thin film. This film was air dried to reduce the surface irregularity. This process is known as leveling, and the leveling time must be observed visually, which depends on the viscosity of the paste applied. The air dried and leveled film was then gradually heated in a muffle furnace at 325°C for 5 min, 375°C for 5 min, 450°C for 15 min and 500°C for 15 min. Then the film was allowed to cool down naturally to a temperature of 70°C. This electrode was then immersed into a 0.5 mM N-719 dye solution and kept for 24 hours for the dye loading.
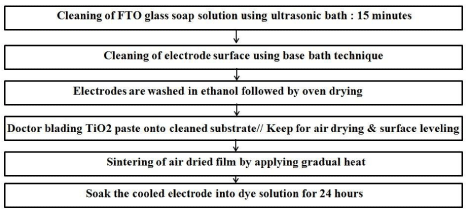
Figure 3: Schematic representation for fabrication of dye-sensitized-TiO2
electrodes.
Preparation of counter electrodes
To prepare the platinum counter electrode, the conducting glass was subjected to all the pre-cleaning procedures. The residual contaminations including the iron contamination were cleaned from the FTO surface by heating it in the oven, H2PtCl6 solution was drop casted on its surface. After air-drying, the drop casted film was heat treated for 400°C for 15 minutes [4].
Electrolyte
The iodine/tri-iodide electrolyte solution was prepared by adding 0.5M I2, 0.1M LiI, and 0.5M of 4-tert-butylpyridine in acetonitrile. This solution mixture was then kept for magnetic stirring for 24 hours for the uniform mixing.
DSSC fabrication
Figure 4 shows the schematic diagram of DSSC. The dye coated TiO2 electrode and platinum counter electrode were assembled into a sandwich type cell. In between the working electrode and the cathode, a Surlyn® spacer film was kept. Then a drop of iodine/tri-iodide electrolyte solution was added in between the electrodes.
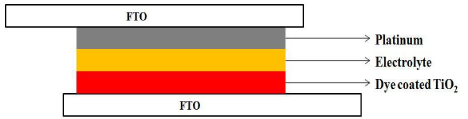
Figure 4: Schematic diagram of DSSC fabricated.
J-V Measurements
Photocurrent-photovoltage (J-V) measurements of DSSCs were obtained by using an AM 1.5 solar simulator (Newport corporation, USA) equipped with xenon light irradiation of 1000 W/m2 at ambient conditions. With a Keithley model 2400 digital source meter, I-V curves were obtained by applying an external bias to the cell, and the generated photocurrent was also measured. According to equations (1) and (2), fill factor and efficiency were calculated.
JSC and VOC are the short circuit current density (mA cm-2) and open circuit voltage (V) respectively. Pin is the power of incident light. Where, Jmax and Vmax are the current density, voltage at the point of maximum power output in the J-V curves, respectively.
Results and Discussions
The films prepared without water and acetic acid found to have large cracks and peeling-up from the FTO glass. This indicates for the TiO2 paste preparations acetic acid and water are necessary to obtain more homogeneity in the paste. Also, the film structures after sintering, due to the presence of water and/or acetic acid is improved many times as confirmed by the optical images [not shown]. To make strong bonding between the TiO2 nanoparticles and the FTO surface, the presence of hydroxides (-OH) groups can play a vital role. The hydroxide groups can ensure the increased chemical bonding of each other by the dehydration while sintering at high temperature. Moreover, by adding DI water, paste provides more hydroxide groups on the surfaces and helps in reducing the inter-particle aggregations. If the particles are aggregated, it can cause more shrinkage in the film upon high temperature sintering, which reduces bonding between the TiOTiO2 particles and the FTO surface. By adding acetic acid, the rate of aggregation of particles is inhibited, due to its adsorption on the surface of TiOTiO2 [1TiO2].
Furthermore, Figure 1 shows the J-V performance variations of different DSSCs fabricated using TiOTiO2 pastes made with various PVP binder concentrations. When compared to the paste without binder, TiOTiO2 photoanodes fabricated from the TiOTiO2 paste with PVP binder showed an improved FF and current density. However by increasing the quantity of PVP in binder solution, it was found that the efficiency was decreasing. Even though, the adhesion of TiOTiO2 particles to the FTO was improved, on sintering, it was observed that the porous structures of the film was reduced considerably, which resulted to the poor dye adsorption and hence the poor efficiency. Due to this problem, ethyl cellulose was introduced as the binder and dye adsorption issue was resolved. This resulted in an improved power conversion efficiency of 4.47% when compared to the efficiency 3.59% that of TiOTiO2 paste with PVP binder as shown in the Figure 5 and in Table 1. And also, better dye adsorption resulted in good solar cell stability and an increased fill factor.
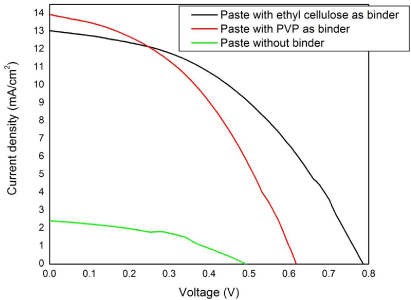
Figure 5: Comparison of J-V characteristics of the DSSCs fabricated with
two different pastes.
Type of paste
JSC (mA cm-2)
VOC (V)
Fill factor
Efficiency (%)
Without binder
1.25
0.33
0.41
0.50
Paste with PVP as binder
14.04
0.61
0.42
3.59
Paste with ethyl cellulose as binder
13.00
0.78
0.44
4.47
Table 1: Table on J-V characteristics performance of DSSC made with various paste.
From Table 1, it is evident that, on improving the dye adsorption i.e., for the paste with ethyl cellulose binder the open circuit voltage improved significantly. The main reason behind the improved efficiency was due to the increase in the open circuit voltage and fill factor. Furthermore, electrochemical impedance spectroscopy (EIS) was performed to analyze the charge transfer dynamics in DSSC fabricated using TiOTiO2 photoanodes made from paste with different binders. Figure 6 shows the Nyquist plot obtained under dark condition for the DSSC fabricated using TiO2 pastes made with PVP and ethyl cellulose as a binder. The internal impedance components of DSSC usually contain three semi-circles that are attributed to the low-frequency, mid- frequency and high-frequency regions.
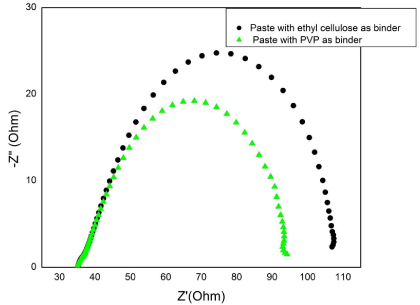
Figure 6: Nyquist plot of the DSSCs fabricated with two different pastes.
These semicircles are related to charge transfer at cathodeelectrolyte interface which is denoted as R1, charge transfer at TiO2/ dye/redox electrolyte interface in the middle frequency range denoted as R2 and charge transport within the electrolyte including Nernstian diffusion process usually observed in the low-frequency range, which is R3. RS is the component which is the magnitude of origin to the start point of the first semi circle, which is the sheet resistance of the cell including the resistance of the conducting glass. The high resistance at the middle frequency range accounts the reduced charge recombination processes and an increased electron transport within the TiO2 layer. The high charge transfer resistance (R2) obtained for the DSSC fabricated using TiO2 paste with ethyl cellulose binder indicates the superior charge transfer at the TiO2–electrolyte interface which can produce a high photovoltaic performance in the DSSC when compared to the TiO2 paste with PVP binder [4].
Conclusion
In summary, we prepared TiO2 pastes with two different organic binders and compared their DSSC photoanodes’ J-V characteristics and impedance results. TiO2 paste made with ethyl cellulose binder showed better open circuit voltage and fill factor compared that of TiO2 paste made with PVP binder, due to the better TiO2 film formation with increased porous structures in the photoanode. Furthermore, EIS results showed a high charge transfer resistance (R2) obtained for the DSSC fabricated using TiO2 paste with ethyl cellulose binder due to the reduced recombination processes. These results suggests a superior DSSC photovoltaic performance for the TiO2 nanopowder based paste made with ethyl cellulose binder which opened up options for TiO2 pastes industrial scale production useful for the manufacture of cost-effective DSSCs. Also, the additional scattering layer and TiCl4 treatment to these TiO2 paste based photoanodes can increase the performance of their DSSCs further. By using this TiO2 nanopowder based paste, sustainably efficient TiO2 anodes can be made for the large scale DSSC production.
Acknowledgment
We would like to thank, Dr. Murali Rangarajan and Dr. Thirugnanasambandham, of CoE of AMGT for providing the electrochemical analyzer for the impedance studies. Financial support from DST-CERI and CoE of AMGT for this research is gratefully acknowledged.
References
- O’Regan B, Gratzel M. A low-cost, high-efficiency solar cell based on dyesensitized colloidal TiO2 films. Nature. 1991; 353: 737-740.
- Mathew S, Yella, Gao P, Baker R H, Curchod B F, Astani N A, Tavernelli I, et al. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nature Chemistry. 2014; 6: 242-247.
- Jacoby M. The future of low-cost solar cells. Chemical and Engineering News. 2016; 94: 30-35.
- Subramaniam M R, Devanathan S, Kumaresan D. Synthesis of micrometersized hierarchical rutile TiO2 flowers and their application in dye sensitized solar cells. RSC Advances. 2014; 4: 36791-36799.
- Arshak K, Corcoran J, Korostynska O. Gamma radiation sensing properties of TiO2, ZnO, CuO and CdO thick film pn-junctions. Sensors and Actuators A. 2005; 123: 194-198.
- Shi H, Liu F, Yang L, Han E. Characterization of protective performance of epoxy reinforced with nanometer-sized TiO2 and SiO2. Progress in Organic Coatings. 2008; 62: 359-368.
- Phule P P, Risbud S H. Low-temperature synthesis and processing of electronic materials in the BaO-TiO2 system. Journal of Materials Science. 1990; 25: 1169-1183.
- Lee J K, Jeong B H, Jang, Kim Y G, Jang Y W, Lee S B, et al. Preparations of TiO2 pastes and its application to light-scattering layer for dye-sensitized solar cells. J Ind Eng Chem. 2009; 15: 724-729.
- Ito S, Chen P, Comte P, Nazeeruddin MK, Liska P, Pechy P, et al. Fabrication of screen-printing pastes from TiO2 powders for dye-sensitised solar cells. Progress In Photovoltaics: Research And Applications. 2007; 15: 603-612.
- Karthick SN, Hemalatha KV, Justin Raj C, Subramania A, Hee-Je Kim. Preparation of TiO2 paste using poly(vinylpyrrolidone) for dye sensitized solar cells. Thin Solid Films. 2012; 520: 7018-7021.
- Yune J. H, Karatchevtseva I, Triani G, Wagner K. and Officer D. A study of TiO2 binder-free paste prepared for low temperature dye-sensitized solar cells. Journal of Materials Research. 2013; 28: 488-496.
- Hee-Gon Bang, Jun-Ki Chung, Rae-Young Jung, Sang-Yeup Park. Effect of acetic acid in TiO2 paste on the performance of dye-sensitized solar cells. Ceramics International. 2012; 38S: S511–S515.