
Research Article
Austin Chem Eng. 2016; 3(5): 1043.
Effect of Koh/Al2O3 Heterogenous Catalyst on Various Properties of Biodiesel by Transesterification of Palm Oil
Ughade YR and Ajabe NK*
Department of Chemical Engineering, Anuradha Engineering College, Chikhli (MH), India
*Corresponding author: Nikhil K Ajabe, M Tech, Department of Chemical Engineering, Anuradha Engineering College, Chikhli (MH), India
Received: October 12, 2016; Accepted: November 04, 2016; Published: November 07, 2016
Abstract
Transesterification of palm oil for biodiesel production are catalyzed by homogenous or heterogeneous catalyst. The present work studied the transesterification reaction used in the production of palm oil methyl ester from palm oil and methanol over synthesized KOH/Al2 O3 catalysts.
And effect on the different biodiesel properties such as density, viscosity, acid value, flash point, freezing point, pour point and yield. The reaction is proceed under following reaction condition: reaction time 6hr, 1:9 mole ratio of oil to methanol, reaction temperature 60oC. The results show that the produced palm oil methyl ester can safely be used as an alternative diesel fuel.
Keywords: Transesterification; Heterogeneous; Catalyst; Palm oil; Biodiesel properties
Introduction
Increasing uncertainty about global energy production and supply, environmental concerns due to the use of fossil fuels, and the high price of petroleum products are the major reasons to search for alternatives to petro-diesel. Their utilization has been continuously increased, which accelerates the depletion of limited petroleum reserves and unavoidably increases petroleum prices. Biodiesel can be used in compression-ignition (diesel) engines with little or no modification. transesterification of palm oil to biodiesel (fatty acid methyl ester, FAME) can be catalyzed by bases, acids, and enzymes. Commercially used homogeneous alkali catalysts are NaOH, KOH, and their alkoxides. Nowadays, most of the commercial biodiesel comes from the transesterification of vegetable oil using a basic catalyst, such as NaOH or KOH, because a basic catalyst can catalyze faster than an acid catalyst. But homogeneous catalysts have many problems and lead to a reduced yield of biodiesel. For example, hydrolysis and saponification are side reactions of transesterification resulting in the formation of soap which is hard and high cost to separate the catalyst from the product. And a large amount of wastewater is produced in separating and cleaning the catalyst and the products. In this reaction, these parameter reaction time, reaction temperature and mole ratio are constant for all different value of catalyst concentration. Common processes of biodiesel production are mixing triglyceride and alcoholic phases in the presence of catalyst in a closed reactor. Application of mechanical stirrers is the most common way for reactants mixing. Stirring speed (RPM) is also constant for every reaction.
Palm Oil
Palm oil is the most efficient oilseed crop in the world. One hectare of oil palm plantation is able to produce up to ten times more oil than other leading oilseed crops. Palm oil is used in a wide variety of food products such as cooking oil, shortenings and margarine. Palm oil is increasingly being used as feedstock for bio-fuel although its primary use remains for food. Palm oil is balanced oil with a unique chemical composition that offers greater advantages compared to other vegetable oils: It has a longer shelf life as it does not become easily rancid. Unlike other vegetable oils, palm oil is naturally semisolid and does not need to undergo hydrogenation to make it suitable for solid applications. The hydrogenation process is responsible for the formation of Tran’s fatty acids which are detrimental to health.
Catalyst Preparation
The procedure involves the following steps
- 12.5455gm of KOH was added to 300ml distilled water in a 500ml beaker.
- This solution was stirred for 20 minutes with a glass rod.
- To the solution 100.0220gm alumina was added and then stirred for 30 minutes with a glass rod.
- The beaker containing solution was kept at in a oven at 1100C for 24 hours. (The oven was already started and solution was kept at the time when it was switch on. Time required to reach 1100C from room temperature is about 20 to 25 minutes).
- Then the dried catalyst sample was kept for calcination at 5000C for 3 hours.
- The catalyst was cooled and stored in an air tight plastic container for further use.
The prepared catalyst is shown in Figure 1.

Figure 1: KOH supported on alumina catalyst used for transesterification
reaction.
Experimental Procedure for Biodiesel Preparation
Briefly, the experimental procedure consists of the following steps. Figure 2 shows the experimental setup
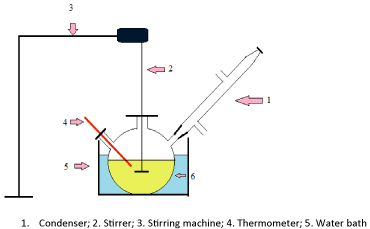
Figure 2: Experimental set-up for biodiesel production.
- A known amount of palm oil (100ml) was introduced into the round bottomed flask and was kept in the water bath and heating was started.
- Weighed amount of dried catalyst powder was added to the reactor contents.
- When the reactor contents reached the set reaction temperature, a measured quantity of methanol is quickly added to the reactor through the side opening containing the thermometer. After completion of methanol addition a thermometer was quickly fixed. This is noted as zero time, and the stirrer was started and adjusted to the required speed.
- The reaction was allowed to proceed to the required pre determined reaction time, afterwards the heating and stirring was stopped, and the reactor was placed in cold water bath to arrest the reaction. It was kept for 10-15 min in cold water bath.
- The entire contents of the reactor was transferred into a conical flask and equal amount of water was added then this mixture is shaken for 5 minutes, transferred into a separating funnel and left overnight for settling. Next day two layers were observed. The upper layer and lower layer having colours white and light grey respectively.
- The lower layer was discarded and the upper layer was treated with hot water which is double in the quantity than that of upper layer and this mixture was shaken for 5 minute and transferred into separating funnel from conical flasks.
- The lower layer was discarded and the upper layer was collected.
- The washed upper layer was centrifuged for 15 to 20 minutes to separate the suspended particles.
- In subsequent next three washings equal quantity of water was used for washing.
- The volume of biodiesel obtained was recorded as a yield, and is further analyzed for properties like Density, Viscosity, and Acid Value.
Analysis of the Reaction Products
The product obtained from the above steps was analyzed for density, viscosity, and acid value, and a brief discussion of these methods is given below.
Density
10ml product sample was pipette out into a weighing bottle and weight the sample at room temperature. From this weight density was determined.
Viscosity
- The viscosity of the fuel samples was determined using the Ostwald viscometer conforming to the British Standards. The viscometer used was a size D BS/U, borosilicate glass. The viscometer was cleaned with chromic acid solution and thoroughly washed with distilled water several times to remove the traces of acid.
- About 50ml of oil sample was filled into clean dried viscometer tube.
- The viscometer was placed in an electrically heated water bath, fitted with automatic temperature controller.
- The viscometer was filled with oil sample. This was done by inverting the thinner tube arm in to the oil sample and then using suction force to draw up the oil to the upper mark of the viscometer.
- The viscometer was inserted into a holder and placed in a constant temperature bath set at temperature of 400C and allowed to stand for 15-20 minutes for the sample to reach 400C.
- The suction force was then applied to the thinner arm to draw the sample slightly above the upper mark.
- The afflux time was recorded by timing the flow of the sample as it flow freely from the upper mark to the lower mark.
The kinematic viscosity (v) was calculated by means of the following equation:
v = C.Tavg
Where
v = Viscosity in (mm2/sec. or cst)
Tavg = Average time in seconds, three reading were recorded for each sample and their average value was taken.
C = Viscometer constant
1
Feed palm oil
100 ml
2
Methanol
39 ml
3
Mole ratio (Oil: Methanol)
1:09
4
Initial oil temperature
26°C
5
Initial water bath temperature
26°C
6
Reaction time
6 hrs.
7
Reaction temperature
60°C
Table 1: Parameter in reaction.
Sr. No.
Catalyst Amount (wt%)
Density (gm/ml)
Viscosity (cst.)
Acid value
Yield%
(mg of KOH/gm of sample)
1
1
0.8645
17.15
2.12
63
2
2.5
0.8766
19.09
1.63
65
3
3
0.8615
5.21
1.23
72
4
4.25
0.8563
4.09
0.86
72
5
7.75
0.8608
3.2
0.49
72
Table 2: Parameter in reaction.
Sr. No.
Catalyst Amount (wt%)
Flash point (o c)
Freezing point
Pour point
(o c)
(o c)
1
1
126.7
-12.4
-9.4
2
2.5
125.2
-13.9
-10.5
3
3
128.5
-13.2
-9.7
4
4.25
122.8
-14.6
.9.9
5
7.75
124.2
-13.2
-10.7
Table 3: Parameter in reaction.
Acid value
I. Preparation of solutions required for standardization and titration
- About 0.28 gm of potassium hydroxide was dissolved in 50ml ethanol to prepare 0.1N alcoholic KOH solutions.
- 0.315gm of oxalic acid was dissolved in 50ml distilled water to prepare 0.1N oxalic acid solution.
II. Standardization of alcoholic KOH solution
- 10ml of 0.N oxalic acid was pipette in to a conical flask.
- 2-3 drops of phenolphthalein indicator were added to the solution.
- 0.1N alcoholic KOH solution was titrated till the colour of the solution turns to faint pink.
- Volume of alcoholic KOH solution added was recorded.
N1V1 = N2V2
N2 = N1V1 / V2
Where
N1= Normality of oxalic acid solution
V1 =Volume of oxalic acid solution
N2= Normality of alcoholic KOH solution
V2 =Volume of alcoholic KOH solution
III. Acid value determination
- 5gm of oil sample was taken in a conical flask.
- 50ml of iso-propanol or neutralized ethanol was added to it.
- The contents of the flask were heated in a water bath at 400C for about 10 minutes.
- 2-3 drops of phenolphthalein indicator was added to the solution and then titrated with 0.1N alcoholic KOH solutions.
- The solution was titrated till the solution turns into faint pink colour.
- The volume of alcoholic KOH solution required was recorded.
The acid value is calculated by means of the following equation:
Acid value = (N2 * 56.1 * V) / W [mg KOH / gm of sample]
Where,
N2= Normality of alcoholic KOH solution
V =Volume of alcoholic KOH solution
W = Weight of oil sample
Flash point
This flash point was determined in this work according to the ASTM D93 standard test method. The flash point is the minimum temperature at which the fuel will flash upon application of an ignition source. It is important advantages of biodiesel that its flash point is greater than that of diesel fuel.
Freezing point and pour point
Liquids have a characteristic temperature at which they turn into solids, known as their freezing point. Pour points is the lowest temperature at which the fuel can still be moved, before it has gelled. Here pour point and freezing point are used to characterize the cold flow operability of biodiesel, because the pour point and the freezing point of biodiesel affect its utility. The pour point and the freezing point were determined in the present study according to the ASTM D97 and ASTM D2386 standard test method.
Result and Discussion
For the different amount of catalyst, following are the fix parameter in reaction.
The effects of KOH/Al2O3 were studied for the transesterification of palm oil with methanol. It was observed that KOH/Al2O3 was the best catalyst and gave a methyl ester content of rich properties. Under the following conditions: 600C, oil /methanol ratio of 1:9, 6hr of reaction (Figure 3-6). The palm oil methyl ester obtained was characterized to determine its suitability for use as a fuel in diesel engines. The viscosity of palm oil reduces substantially after transesterification and becomes comparable to that of diesel fuel. The flash point and calculated index of our palm oil methyl ester were higher than those of diesel fuel. The higher flash point of the palm oil methyl ester makes it a safer fuel. The produced biodiesel’s fuel characteristics indicate that it can be used as a substitute diesel fuel.
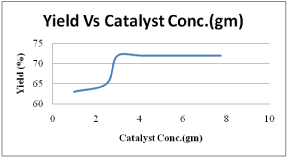
Figure 3: Effect of catalyst concentration on biodiesel yield.
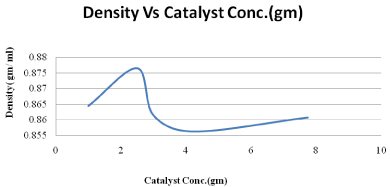
Figure 4: Effect of catalyst concentration on density.
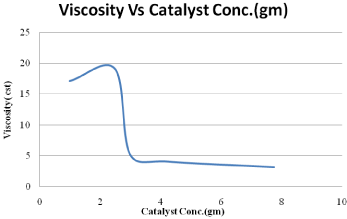
Figure 5: Effect of catalyst concentration on viscosity.
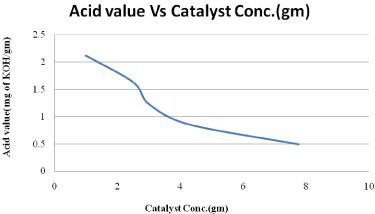
Figure 6: Effect of catalyst concentration on acid value.
References
- Kambiz Tahvildari, Hamid Reza Chitsaz, Paria Mozaffarinia. Heterogeneous Catalytic Modified Process in the Production of Biodiesel from Sunflower Oil, Waste Cooking Oil and Olive Oil by Transesterification Method. Academic Research International. 2014l; 5: 60-68.
- Sanjay Basumatary. Transesterification with heterogeneous catalyst in production of biodiesel: A Review. J Chem Pharm Res. 2013; 5: 1-7.
- Krisada Noiroj, Pisitpong Intarapong, Apanee Luengnaruemitchai, Samai Jai- In. A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renewable Energy. 2009; 34: 1145–1150.
- Nezahat Boz, Miray Kara, Oylum Sunal, Ertan Alptekin, Nebahat Degirmenbasi. Investigation of the fuel properties of biodiesel produced over an alumina-based solid catalyst. Turk J Chem. 2009; 33: 433- 442.
- Onkar S Tyagi, Neeraj Atray, Basant Kumar and Arunabha Datta. Production, Characterization and Development of Standards for Biodiesel - A Review. Journal of Metrology Society Of India. 2010; 25: 197-218.
- Achanai Buasri, Nattawut Chaiyut, Vorrada Loryuenyong, Chao Rodklum, Techit Chaikwan and Nanthakrit Kumphan. Continuous Process for Biodiesel Production in Packed Bed Reactor from Waste Frying Oil Using Potassium Hydroxide Supported on Jatropha curcas Fruit Shell as Solid Catalyst. Appl Sci. 2012; 2: 641-653.
- Wenlei Xie, Haitao Li. Alumina-supported potassium iodide as a heterogeneous catalyst for biodiesel production from soybean oil. Journal of Molecular Catalysis A Chemical. 2006; 255: 1–9.
- Ivana Lukic, Jugoslav Krstic, Sandra Glisic, Dsan Jovanovic and Dejan Skala, Biodiesel synthesis using K2CO3/Al–O–Si aerogel catalysts. J Serb Chem Soc. 2010; 75: 789–801.