
Research Article
Austin Chem Eng. 2016; 3(5): 1044.
Experimental Studies on Single Slope Solar Distillation Unit with Different Coatings on Basin
Krishna PV*, Sridevi V, Priya BH and Kumar KH
Department of Chemical Engineering, Andhra University, India
*Corresponding author: P Vamsi Krishna, Department of Chemical Engineering, Andhra University, India
Received: October 03, 2016; Accepted: November 09, 2016; Published: November 11, 2016
Abstract
Almost two-third of the earth’s surface is covered with water. In that 97% of the earth’s water is salty; only remaining 3% is fresh water. Less than 1% of fresh water is accessible for humans. The consumption of fresh water is increasing all over the world, mainly due to increase in the population and the rapid industrial growth. This causes a serious shortage of fresh water. So it is very important to conserve the available fresh water. In this regards solar distillation of impure water plays a very important role since its cost of construction is very less, easy to maintain and eco friendly because it uses only solar energy. A solar distillation unit operates similar to the natural hydrologic cycle of evaporation and condensation. There are many factors that affect the efficiency of a solar distillation unit like thickness of the glass plate, solar radiation, direction of the unit, volume of water, material of insulation and material of the basin. In the present research the variation in the performance of the solar distillation unit is studied by using different coatings on basin like copper electroplated Aluminium basin, Aluminium basin with black paint at different volumes of water (2, 2.5, 3 liters) in different directions. The maximum percentage of efficiency is found to be 54.66% when copper electroplated Aluminium basin is used at 3 liters of water and 65.63% when Aluminium basin with black paint is used at 3 liters of water.
Keywords: Solar distillation; Aluminium basin; Copper electroplated Al basin; Al basin with black paint
Introduction
Water is the basic necessity for human along with food and air. There is almost no water left on Earth that is safe to drink without purification. Only 1% of Earth’s water is in a fresh, liquid state, and nearly all of this is polluted by both diseases and toxic chemicals. For this reason, purification of water supplies is extremely important. Moreover, typical purification systems are easily damaged or compromised by disasters, natural or otherwise. This results in a very challenging situation for individuals trying to prepare for such situations, and keep themselves and their families safe from the myriad diseases and toxic chemicals present in untreated water. Everyone wants to find out the solution of above problem with the available sources of energy in order to achieve pure water. Fortunately there is a solution to these problems. It is a technology that is not only capable of removing a very wide variety of contaminants in just one step, but is simple, cost-effective, and environmentally friendly. That is use of solar energy [1]. In general, solar distillation process is carried out both in passive and active modes. Normally passive solar still operates in low temperature and the daily productivity is comparatively low. Whereas, to increase the evaporation rate in an active mode the extra thermal energy is fed into the basin. To increase the productivity of solar still, the various active methods are being carried out by many researchers. Most of the works were based on the flat plate collector and concentrating collector. The single basin solar still coupled with flat plat collector and found that the daily production rate was increased by 24% when compared to the simple single basin solar still by Rai [2]. The maximum yield of a simple solar still was 2.575L/m2 day while it was 5.18L/m2 day when integrated with flat plate collector was found by Tiris [3]. Its production rate was increased by 231% but efficiency has decreased by about 2.5%. The solar still productivity has increased by 36% while coupling flat plat collector was determined by Badran [4]. The productivity was proportional to the solar radiation Badran [5]. An active solar still with water flow over the glass cover has given maximum yield of 7.5L/day was found by Sanjay Kumar [6]. The annual yield was at its maximum when the condensing glass cover inclination was equal to the latitude of the place was concluded by Singh. Solar stills coupled with solar collectors and storage tank both experimentally and theoretically and found that the productivity doubled for 24 hours period was determined by Voropoulos [7-9]. Also, they have designed a hybrid solar desalination and water heating system [10]. In the present study we are mainly focus on the coatings of the basin and how they affect the efficiency of solar distillation unit the we considered are copper electroplating and black paint on a Aluminium basin.
Principle
Solar distillation uses the heat of the sun directly in a simple piece of equipment to purify water. The equipment, commonly called a solar still, consists primarily of a shallow basin with a transparent glass cover. The sun heats the water in the basin, causing evaporation. Moisture rises, condenses on the cover and runs down into a collection trough, leaving behind the salts, minerals, and most other impurities, including germs. Although it can be rather expensive to build a solar still that is both effective and long-lasting, it can produce purified water at a reasonable cost if it is built, operated, and maintained properly [11]. Figure 1 shows the schematic diagram of solar still.
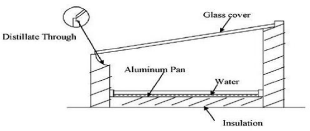
Figure 1: Schematic diagram of a single slope solar distillation unit.
Experimental Setup
A single slope solar distillation unit consists of a wooden box of length 0.715m, breadth 0.415m, height at the smaller edge 0.125m, height at the longer edge 0.36m, and thickness 0.01m. The inside of the box is insulated on all the sides with a 0.02m thick of thermocol sheets. A metal basin of length 0.64m, breadth 0.33m, and height 0.085m which will hold the waste water is placed on the insulation. The top of the box is covered by a glass sheet of thickness 0.04m at an inclination of 18o and a collector is attached at the lower end to collect the condensate. A rubber stripe is placed on the edges of the wooden box so that the glass won’t slip down and helps in reducing the losses by keeping the water vapour inside. The experimental setup with copper electroplated Aluminium basin is shown in Figure 2.

Figure 2: Solar distillation unit with copper electroplated Aluminium basin.
Details of different parts of the system
Still basin: It is the part of the system in which the water to be distilled is kept. It is therefore essential that it must absorb solar energy. Hence it is necessary that the material have high absorptivity or very less reflectivity and very less transmitivity. These are the criteria’s for selecting the basin materials. Kinds of the basin materials that can be used are as follows: 1. Leather sheet, 2. Ge silicon, 3. Mild steel plate, 4. RPF (reinforced plastic) 5. G.I. (galvanized iron), 6. Aluminium and 7. Cupper.
We have used Aluminium sheet with black paint and copper electroplated Al sheet (KAl= thermal conductivity of Aluminium = 205W/m.K. KCu= thermal conductivity of copper = 385W/m.K) (1mm thick). (SIZE: 64 X 33 X 8.5 cm BOX of Al and Cu).
Side walls: It generally provides rigidness to the still. But technically it provides thermal resistance to the heat transfer that takes place from the system to the surrounding. So it must be made from the material that is having low value of thermal conductivity and should be rigid enough to sustain its own weight and the weight of the top cover. Different kinds of materials that can be used are: 1) wood, 2) concrete, 3) Thermocol, and 4) RPF (reinforced plastic). For better insulation we have used composite wall of Thermocol (inside) and wood (outside). Size: wood (k= thermal conductivity=0.6W/ m0C) 10mm thick, Thermocol (k= thermal conductivity=0.02W/ m0C) 20mm thick.
Top cover: The passage from where irradiation occurs on the surface of the basin is top cover. Also it is the surface where condensate collects. So the features of the top cover are: 1) Transparent to solar radiation, 2) Non absorbent and Non-adsorbent of water, 3) Clean and smooth surface. The Materials Can Be Used Are: 1) Glass, 2) Polythene. We have used glass (4mm) thick as top cover having rubber tube as frame border. (size : 76 x 41.5cm).
Channel: The condensate that is formed slides over the inclined top cover and falls in the passage, this passage which fetches out the pure water is called channel. The materials that can be used are: P.V.C., 2) G.I., and 3) RPF. We have used P.V.C channel (size: 1 inch).
Experimental Procedure
A single sloped solar distillation unit with a copper electroplated aluminium basin is constructed. To determine the optimum direction 2 litres of waste water is taken in basin and distillation unit is placed in the sun light such that the sloped side is directed towards east from 9:30am to 5:30pm. At the end of every hour solar radiation is measured using pyranometer, temperature of surroundings, outer surface of the glass, inner surface of the glass, moist air inside the box, water inside the basin and condensate are measured using thermometer and thermocouple. The amount of distillate collected is also measured at the end of each and every hour. Similarly the experiments are conducted in west, south, north and east from 9:30am to 12:30pm then west from 12:30pm to 5:30pm. The direction in which the yield is high is taken as the optimum direction and the remaining experiments are carried in that direction only which is east from 9:30am to 12:30pm then west from 12:30pm to 5:30pm. After fixing the direction the volume of the water is changed (2, 2.5, and 3 lits) and the experiments are conducted again to determine the optimum volume of water. Similarly the experiments conducted by changing the material of the basin with Aluminium basin with black paint shown in Figure 3. Cylindrical ceramic packing is also used in the basins.

Figure 3: Solar distillation unit with Aluminium basin with black paint.
Energy Balance
The energy balance over the distillation unit is given by
Is Ag/Ab = Isrg Ag/Ab + qg,s Ag/Ab +qh,g Ag/Ab+ qk,airAk,air/Ab + qk,lAk,l /Ab+qk,bAb/Ab+(mcwh sat,g) /Ab.
Where
Is=Solar radiation intensity W/m2, Ag Area of glass surface = 0.3154m2; Ab Area of black surface= 0.2112m2; rg Reflectivity of the glass cover for visible light = 0.04. Ak,air Circumferential area of solar still covered by inside moist air = 0.3154m.2 Ak,l Circumferential area of solar still covered by sea water = 0.3154m2. mcw = Mass flow rate of condensed water, Kg/m2.sec hsat,g = Enthalpy of water at saturation temperature KJ/kg. Considering the heat transfer from the cover to the atmosphere by convection:
qh,g = hg (Tg - Ta) W/m2
Where Tg is the glass temperature, Ta is ambient temperature hg is the convective heat transfer coefficient and is given by the following formula:
hg = 5.7+ 3.8w W/m2
Where the forced convection coefficient dependent on the wind velocity, w (m/s) = 3.75 m/sec.
Radiative heat transfer from the glass cover to the atmospheric air is given by the following formula:
qg,s=eg Cs [(Tg/100)4- (Tsky/100)4] W/m2.
Where emissivity of the glass, eg, is 0.88 for infrared radiation, the constant, Cs, is 5.667W/m2K4 and Tsky is the sky temperature. Generally for practical purposes the average sky temperature during operations hours can be assumed as about 20°C below the ambient temperature i.e. Tsky = Ta- 20°C; Tg=Temperature of glass K ; Ta=Ambient temperature K .
The conductive heat transfer from the bottom to the atmosphere may be formulated as:
qk,b = kb (Tb - Ta) W/m2
where (1/kb)=(1/hin)+(Σ(δi/λi))+(1/ha) m2 K/W.
hin =Convective heat transfer coefficient at sea water interface, W/ m2.
Σ(δi/λi)= (δg/λg)+(δb/λb)+(δw/λw)+(δth/λth) m2 K/W.
δg Thickness of glass=4mm; λg Thermal conductivity of glass=0.96 W/m.K
δb Thickness of basin=1mm; λb Thermal conductivity of basin =205 (Al), 385 (Cu) W/m.K
δw Thickness of wood=10mm ; λw Thermal conductivity of wood=0.17 W/m.K
δth Thickness of Thermocol=20mm; λth Thermal conductivity of Thermocol =0.036W/m.K
ha = Convective heat transfer coefficient at ambient temperature,W/m2. K.
Tb= Temperature of the basin K; Ta=Ambient temperature K.
Considering the heat transfer from the circumferential area of the still by conduction;
From inside moist air to the atmosphere,
qk,air=km (Tr - Ta ) W/m2.
where (1/kr) = (1/hr) + (Σ (δi/λi)) + (1/ha) W/m2. K
hr = convective heat transfer coefficient at moist air, W/m2.
From liquid to the atmosphere,
qk,l= kl (Tb - Ta ) W/m2.
Where (1/kl)=(1/∞)+(Σ(δi/λi))+(1/ha)m2.K/W.
The efficiency of a still can be calculated by the following equation:
η = (Water output × Latent heat of evaporation of water/Daily solar radiation) × 100%
η = (m×2500.7/I×Ag) X 100%
Results and Discussion
For two liters of waste water among the direction like east, west, north, south and east from 9:30am to 12:30pm then west from 12:30pm to 5:30pm the maximum efficiency of 53.85% is found in the direction of east from 9:30am to 12:30pm then west from 12:30pm to 5:30pm.
For copper electroplated Aluminium basin among different volumes like 2, 2.5 and 3 litres of waste water the maximum efficiency of 54.66% is found at three liters and for Aluminium basin with black paint the maximum efficiency of 65.63% is found at three litres
Hourly variation of temperature at different places in the unit
The variation of temperatures at different places like basin, moist air, inside surface of the glass, outside surface of glass, condensate and ambient temperatures are measure at the end of each and every hour and plotted against time for cupper electroplated aluminium basin is shown in Figure 4 and for aluminium basin with black paint is shown in Figure 5.
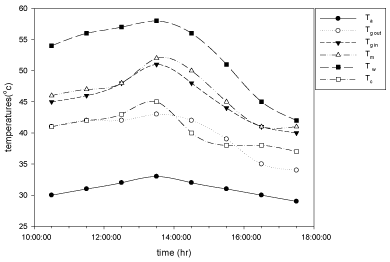
Figure 4: Hourly variation of temperature for copper electroplated Aluminium
basin with 3 liters of waste water in the direction of east from 9:30am to
12:30pm then west from 12:30pm to 5:30pm.
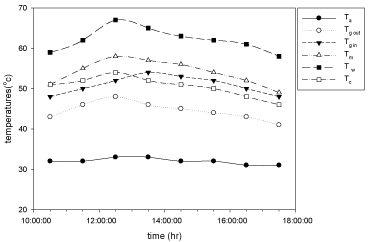
Figure 5: Hourly variation of temperature for Aluminium basin with black paint
and 3 liters of waste water in the direction of east from 9:30am to 12:30pm
then west from 12:30pm to 5:30pm.
Hourly variation of distillate collected
The variation of distillate collected with respect to time for copper electroplated Aluminium basin is shown in Figure 6 and for Aluminium basin with black paint is shown in Figure 7. Maximum distillate is collected around 1:30pm in both the cases but the total amount of distillate collected using Aluminium basin with black paint is higher than the amount collected using cupper electroplated Aluminium basin.
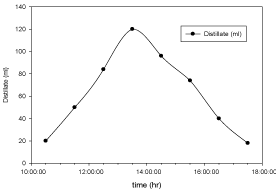
Figure 6: Hourly variation of distillate collected for cupper electroplated
Aluminium basin with 3 liters of waste water in the direction of east from
9:30am to 12:30pm then west from 12:30pm to 5:30pm.
Hourly variation of solar radiation
The variation of solar radiation with respect to time during the experiment for copper electroplated Aluminium basin is shown in the Figure 8 and for Aluminium basin with black paint is shown in the Figure 9.
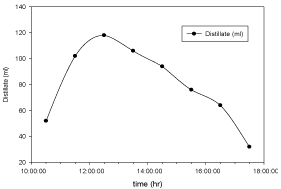
Figure 7: Hourly variation of distillate collected for Aluminium basin with
black paint and 3 liters of waste water in the direction of east from 9:30am to
12:30pm then west from 12:30pm to 5:30pm.
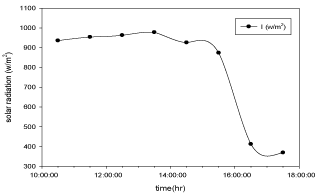
Figure 8: Hourly variation of solar radiation for cupper electroplated
Aluminium basin with 3 liters of waste water in the direction of east from
9:30am to 12:30pm then west from 12:30pm to 5:30pm.
Hourly variation of energy input, utilized and lost
The variation of energy input through solar radiation, energy utilized by the solar distillation unit and energy lost with respect to time for copper electroplated Aluminium basin is shown in the Figure 10 and for Aluminium basin with black paint is show in Figure 11.
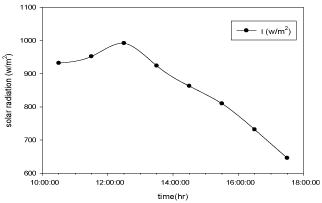
Figure 9: Hourly variation of solar radiation for Aluminium basin with black
paint and 3 liters of waste water in the direction of east from 9:30am to
12:30pm then west from 12:30pm to 5:30pm.
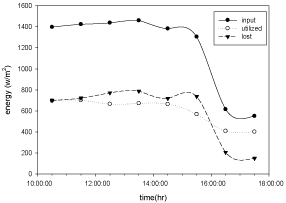
Figure 10: Hourly variation of energy input, utilized and lost for copper
electroplated Aluminium basin with 3 liters of waste water in the direction of
east from 9:30am to 12:30pm then west from 12:30pm to 5:30pm.
Hourly variation of efficiency
The variation of efficiency with respect to time for copper electroplated Aluminium basin is shown in the Figure 12 and Aluminium basin with black paint is shown in the Figure 13.
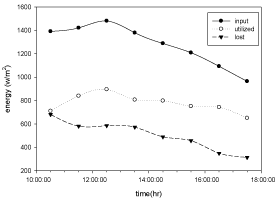
Figure 11: Hourly variation of energy input, utilized and lost for Aluminium
basin with black paint and 3 liters of waste water in the direction of east from
9:30am to 12:30pm then west from 12:30pm to 5:30pm.
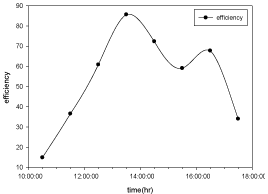
Figure 12: Hourly variation of efficiency for copper electroplated Aluminium
basin with 3 liters of waste water in the direction of east from 9:30am to
12:30pm then west from 12:30pm to 5:30pm.
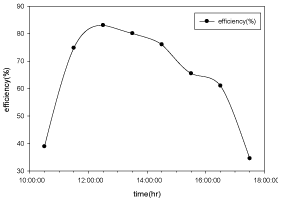
Figure 13: Hourly variation of efficiency for Aluminium basin with black paint
and 3 liters of waste water in the direction of east from 9:30am to 12:30pm
then west from 12:30pm to 5:30pm.
Water characteristics
The characteristics of waste water before treatment and after treatment are shown in the Table 1.
Property
untreated water
Treated
pH
6.7
7.2
TSS(ppm)
22620
20
TDS(ppm)
28432
15
Hardness(ppm)
3620
10
Chlorides(ppm)
11360
12
COD(ppm)
600
22
BOD(ppm)
350
16
Table 1: The characteristics of waste water before treatment and after treatment.
Conclusion
Solar distillation is one of the best methods for waste water treatment. For a single slope solar distillation unit east from 9:30am to 12:30pm then west from 12:30pm to 5:30pm is the direction in which maximum efficiency was obtained. For both copper electroplated Aluminium basin and Aluminium basin with black paint 3 litres volume of waste water showed the maximum efficiency. An Aluminium basin with black paint showed better efficiency when compared to that of a copper electroplated Aluminium basin. The efficiency has increased from 54.66 to 65.63 by replacing a copper electroplated Aluminium basin with an Aluminium basin with black paint.
References
- Prof Alpesh Mehta, Arjun Vyas, Nitin Bodar, Dharmesh Lathiya. Design of Solar Distillation System. International Journal of Advanced Science and Technology. 2011; 29: 12-17.
- Rai SN, Tiwari GN. Single Basin Solar Still Coupled with Flat Plate Collector. Energy Conversion and Management. 1983; 23: 145-149.
- Tiris C, Tiris M, Erdalli Y, Sohmen M. Experimental Studies on a Solar Still Coupled with a Flat Plate Collector and a Single Basin Still. Energy Conversion and Management. 1998; 39: 853-856.
- Badran AA, et al. A Solar Still Augmented with a Flat-Plate Collector. Desalination. 2005; 172: 227-234.
- Badran OO, Al-Tahainesh HA. The Effect of Coupling Flat-Plate Collector on the Solar Still Productivity. Desalination. 2005; 183: 137-142.
- Sanjay Kumar, Tiwari GN. Performance Evaluation of an Active Solar Distillation System. Energy. 1996; 21: 805-808.
- Singh HN, Tiwari GN. Monthly Performance of Passive and Active Solar Stills for Different Indian Climatic Condition. Desalination. 2004; 168: 145-150.
- Voropoulos K, Mathioulakis E, Belessiotis V. Experimental Investigation of a Solar Still Coupled with Solar Collectors. Desalination. 2001; 138: 103-110.
- Voropoulos K, Mathioulakis E, Belessioti, V. Solar Stills Coupled with Solar Collectors and Storage Tank – Analytical Simulation and Experimental Validation of Energy Behaviour. Solar Energy. 2003; 75:199-205.
- Voropoulos K, Mathioulakis E, Belessiotis V. A Hybrid Solar Desalination and Water Heating System. Desalination. 2004; 164: 189-195.
- S K Shukla. Application of Solar Distillation Systems with Phase Change Material Storage. Modern Mechanical Engineering. 2014; 15-42.