
Research Article
Austin Chem Eng. 2016; 3(5): 1045.
Demulsification of a Mixture of Di-Chloro-Floro- Acetophenone, Di-Chloro-Floro-Benzene and Water
Korde M, Jadhav AJ, Pinjari DV and Pandit AB*
Department of Chemical Engineering, Institute of Chemical Technology, Matunga, India
*Corresponding author: Aniruddha B Pandit, Department of Chemical Engineering, Institute of Chemical Technology, Matunga, India
Received: October 25, 2016; Accepted: November 22, 2016; Published: November 24, 2016
Abstract
The present study investigated the various physical and chemical factors that result into the destabilization of water in organic phase emulsions and confirmed with experiments that an addition of appropriate chemical destabilizing reagents was the most effective in this case. Experimental data are presented to depict the influence of varying concentrations of surfactant and salt on the stability and the properties of the water in oil emulsion. The results obtained from this study revealed the choice of the most effective demulsifier as Sodium Dodecyl Sulphate, coupled with Sodium Chloride salt and their optimum concentrations were found. The relative rates of separation of water from the emulsion were characterized by density measurements and were confirmed by droplet size analysis. 250ppm of Sodium Dodecyl Sulphate along with 0.5g of Sodium Chloride per 100g of emulsion proved to be most effective in demulsifying the given water in oil emulsion.
Keywords: Demulsification; Water-in-organic fluid emulsions; Surfactant; Chemical demulsifiers; Salt concentration; Stirring, Interfacial science; Coalescence
Introduction
With an exponential growth of industries making a wide range of products such as fuels, surfactants, oils and oleo-chemicals, perfumery, pharmaceuticals and cosmetics, painting and in many other industries generate various types of emulsions as a by-product [1,2]. Water-in-oil emulsions are commonly formed during the production of crude oil, where droplets of water are finely dispersed throughout the continuous crude oil phase [3]. They can also be formed during the production or processing of bulk organic compounds. The emulsions formed may be intentional in case of a few products based on their formulations or they may be accidently formed with one or more side products or with leftover reactants and impurities getting associated with the desired product. Hence, the necessity arises in developing new means of demulsification, for the recovery of the product or the separation of the side product.
The stability of all emulsions in general is affected by various factors like droplet size distribution, viscosity of the continuous phase, density difference between both the phases, nature of interfacial film, amount of surfactant added or present, etc [1]. The commonly used methods of demulsification include: physical and chemical means. The physical means include factors like - temperature change, agitation or shear or stirring, bubbling of air and the residence time or retention, whereas the chemical means include - salting, addition of demulsifying chemical agents, electrical means and centrifugation [4]. All the major as well as minor aspects which can physically and chemically affect the emulsion stability need to be considered for demulsification. Over the years many of these properties have been exploited for the demulsification of case specific products. However, it has also been observed that the demulsification approach useful in one particular case is totally ineffective in another. Hence, every time a new emulsion is formed, a new method for demulsification has to be developed and optimized.
The three predominant steps of chemical demulsification are flocculation followed by coalescence and then finally the breakdown of the emulsion into two immiscible liquids. As suggested by Bancroft’s theory, the nature of the adsorbed layer of demulsifiers affects the emulsion stability and the interfacial and surface elastic properties after the adsorption of the surfactant and the interfacial viscosity of the film affects the stability of the interfacial film [4,5]. Here, we aim to destabilize the emulsion by targeting both these factors by choosing an appropriate demulsifier/demulsifiers. An effective demulsifier is a surface-active compound that can adsorb onto the interface of the water droplets dispersed in the organic liquid, and change its properties such that the water droplets aggregate and coalesce [6].
In this research, an emulsion of Di-Chloro Floro Acetophenone (DCFA), Di-Chloro Floro Benzene (DCFB) and Water has been taken and a simple, direct method of demulsification has been developed involving the addition of Sodium Dodecyl Sulphate (SDS) and common salt to the emulsion under optimum conditions. The degree of separation of the components has been analyzed by density measurements of both the separated layers. Thus, the optimum surfactant and salt concentrations for demulsification of this particular emulsion have been determined. Phase separation enhancement occurs in the presence of small amount of demulsifiers (usually 1-1000 ppm) [7]. The amount of SDS and NaCl added to the solution for demulsification also lies in this range.
Materials and methods
Materials
The emulsion was provided as a gift sample by Val Organics Pvt. Ltd. as a product of a Friedel Crafts reaction whose exact manufacturing procedure was not disclosed. The data provided by the industry (Val organics Pvt. Ltd.) is given in Tables 1 and 2.
Sodium Dodecyl Sulphate (Ultrapure), Sodium Chloride (Ultrapure), polyethylene glycol (PEG), Tween 80 and polymethylacrylic acid were procured from S.D. Fine Chemicals, Mumbai. The distilled water used in the experiments was prepared by using Millipore apparatus. All the chemicals used during the experiments were of Analytical grade.
Component
Percent Component (%)
Density (g/ml)
2,4-Chloro 1-Floro Benzene (DCFB)
42
1.409
Isomers of (DCFB)
2
1.409
2,4-Chloro 5-Floro Acetophenone (DCFA)
52
1.425
Other heavy components
4
1.45
Table 1: Bottom layer: Average density=1.419g/ml.
Component
Density (g/ml)
Water and organic mixture in the layer collected at the top
1.203(calculated in laboratory after 2 weeks)-1.245(specified data measured at the industry)
Table 2: Density of the top layer.
Instruments
The Zetasizer (Malvern) was used to find the charge on the emulsion. Magnetic stirrer and magnetic needle, hot water bath, Mini Centrifuge is other instruments used during the experiments.
Representative sample extraction from the bulk
The emulsion provided was unstable and due to long storage a noticeable quantity of the aqueous components had already been separated on the top of the emulsion. To obtain accurate measurements the entire liquid was shaken vigorously and a sample was quickly poured out before the layer separation occurred.
Selection of suitable demulsifying agent
PEG, Tween 80, SDS, NaCl and polymethylacrylic acid in different proportions were added to the emulsion individually and the separation into two layers was observed for each one of them over a period of time, in the initial exploratory experiments. Amongst these, the most effective ones were found to be SDS and NaCl, by visual observation and hence they have been chosen in the further experiments.
Preparation of the solutions and the process of demulsification
To analyse the effect of surfactant concentration on the emulsion stability, 8.3mM (2.3936g/L), 12mM (3.4606g/L), 15mM (4.3258g/L) solutions of SDS were prepared in distilled water. 1.1968g of SDS was added to 500ml of distilled water in a volumetric flask, was shaken till the SDS was completely dissolved and then kept standing till a clear solution was obtained. This stock solution will be further referred to as Stock 8.3. Similarly, for making the 12mM solution, 1.7303g of SDS was added to 500ml of distilled water to form a stock solution which will be further referred to as Stock 12 and for the 15mM solution, 2.1629g of SDS was added to 500ml of distilled water to form the stock solution which will be further referred to as Stock 15.
Furthermore, investigating the impact of electrolyte concentration on the stability of the emulsion, an aqueous solution of varying quantity of NaCl (0.1-0.5 g of salt per 100ml of distilled water) was prepared. NaCl in the solid crystalline form was also directly used and the results of the aqueous solution form as well as the direct crystalline form were compared. 50 to 350ppm of the SDS solution (Stock8.3 or Stock12 or Stock15) along with NaCl (0.1 to 0.5g of salt per 100ml emulsion) was added to 175ml of emulsion in a 250ml beaker and stirred to form a solution, further referred to as solution A.
Studying the effect of physical factors
Determination of the nature of the emulsion: A small amount of the emulsion was spread on a clean transparent glass plate to observe the nature of the emulsion by identifying the dispersed phase and the dispersion medium (continuous phase).
Effect of centrifugation: Solution A was taken in various cuvettes and was centrifuged at high speeds of about 2000 to 2500rpm for 15 minutes and the changes in the emulsion were observed. For the centrifuge used in the laboratory, the force exerted can be considered equivalent to 675 - 1050 of g force
Checking the thermal stability: Solution A was heated in a water bath at 65°C for one to two hours and its effects on the degree of separation of the two layers was analyzed.
Stability of emulsion by measuring its zeta potential: Samples of the emulsion were analyzed in the Zetasizer to assess the changes in the droplet size distribution as a function of the treatment type and duration.
Effect of agitation: Effect of agitation alone (without the destabilising agents) on Solution A was studied. Gentle stirring with a magnetic needle at about 250-500 rpm for 15 to 20 minutes was carried out and the changes in the emulsion quality were observed. The rpm was chosen such that the flow in the beaker remains laminar and gradual mixing of the components occurs, which facilitates soft collision of the dispersed phase droplets.
Optimizing the concentrations of both the reagents along with the effect of heat and stirring
The effect of salt addition was investigated first, both in the crystalline form and in the aqueous solution form to the emulsion. Stock 8.3 was used here. Table 3 shows the results obtained. From Table 3, it can be inferred that adding crystalline salt is more advantageous than the addition of the salt solution. Also, heating of the emulsion facilitated the separation but was not the most preferred solution for emulsion destabilization as the organic compounds were heat sensitive.
Reagent added to 175ml emulsion
Bottom Layer
Top Layer
Density (g/ml)
Organic %
Aqueous %
Density (g/ml)
Organic %
Aqueous %
50+salt solution(10ml of 2g/100ml solution added to 100 ml emulsion)+heat
1.399
95.26
4.74
1.132
31.8
68.2
100+salt solution(10 ml of 2g/100ml solution added to 100 ml emulsion) +heat
1.384
91.69
8.31
1.102
24.67
75.33
150+salt solution(10 ml of 2g/100ml solution added to 100 ml emulsion) +heat
1.381
90.98
9.02
1.096
23.24
76.76
50+ crystalline salt (0.2g salt per 100ml emulsion) +heat
1.4
95.49
4.51
1.215
51.52
48.48
50+ crystalline salt (0.1g salt per 100ml emulsion) +heat
1.402
96.02
3.98
1.225
53.9
46.1
100+ crystalline salt (0.2g salt per 100ml emulsion) +heat
1.39
93.12
6.82
1.221
52.95
47.05
150+ crystalline salt (0.2g salt per 100ml emulsion) +heat
1.387
92.4
7.6
1.145
34.89
65.11
200+ crystalline salt (0.2g salt per 100ml emulsion) +heat
1.398
95.02
4.98
1.116
28
72
250+ crystalline salt (0.2g salt per 100ml emulsion) +heat
1.4
95.49
4.51
1.121
29.18
70.82
Table 3: Density and composition of the respective layers on salt addition.
Further, Stock 12 was used and the same experiments were carried out, the results of which are tabulated in Table 4. Also, Stock 15 was used and the same experiments were carried out, the results of which are tabulated in Table 5.
Reagent
Bottom Layer
Top Layer
Density (g/ml)
Organic %
Aqueous %
Density (g/ml)
Organic %
Aqueous %
50+ crystalline salt (0.5g salt per 100ml emulsion)
1.3723
88.91
11.09
1.2292
54.9
45.1
100+ crystalline salt (0.5g salt per 100ml emulsion)
1.3734
89.17
10.83
1.2234
53.52
46.48
150+ crystalline salt (0.5g salt per 100ml emulsion)
1.372
88.88
11.12
1.1577
37.91
62.09
200+ crystalline salt (0.5g salt per 100ml emulsion)
1.3755
89.67
10.33
1.1827
43.85
56.15
250+ crystalline salt (0.5g salt per 100ml emulsion)
1.3801
90.76
9.24
1.0937
22.7
77.3
300+ crystalline salt (0.5g salt per 100ml emulsion)
1.3941
94.09
5.91
1.1411
33.96
66.04
350+ crystalline salt (0.5g salt per 100ml emulsion)
1.39
93.12
6.82
1.2156
51.67
48.33
Table 4: Density and composition of the layers on addition of salt and varying concentrations of 12mM SDS.
Reagent
Bottom Layer
Top Layer
Density (g/ml)
Organic %
Aqueous %
Density (g/ml)
Organic %
Aqueous %
62.5+ crystalline salt (0.5g salt per 100ml emulsion)
1.385
91.93
8.07
1.2254
54
46
125+ crystalline salt (0.5g salt per 100ml emulsion)
1.3912
93.4
6.6
1.211
50.58
49.42
200+ crystalline salt (0.5g salt per 100ml emulsion)
1.3925
93.71
6.29
1.2179
52.22
47.78
250+ crystalline salt (0.5g salt per 100ml emulsion)
1.3971
94.8
5.2
1.1622
38.98
61.02
300+ crystalline salt (0.5g salt per 100ml emulsion)
1.4003
95.56
4.44
1.1756
42.16
57.84
350+ crystalline salt (0.5g salt per 100ml emulsion)
1.3876
92.55
7.45
1.2066
49.53
50.47
Table 5: Density and composition of the layers on addition of salt and varying concentrations of 15mM SDS.
The effect of physical factors on the prepared solution (Solution A) as well the optimization of these two demulsifying agents (SDS and NaCl) has been carried out. After cooling down the emulsion (If heating was involved) it was added to a separating funnel and kept standing for 10-15 minutes till the layers separated clearly and a distinct rag layer was formed in between the two layers as shown in Figure 1. Finally, the two layers were separated.

Figure 1: Visual appearance of (A) emulsions before treatment and (B)
emulsified after treatment.
Results and Discussion
As studied earlier, a variety of operational principles can lead to demulsification. Out of this wide range, we have exploited some of those properties in this work. Initially, we analyzed the effect of each factor and the extent to which it can cause demulsification of the given emulsion. Further, we studied the combined effect of two or more factors on the breaking down of the target emulsion, to give a single synergistic optimum solution for demulsification.
Nature of the emulsion
It was evident from observation that it was a water-in-oil type of emulsion with big and small drops of aqueous phase dispersed in the continuous organic phase.
The effect of physical factors
Effect of centrifugation: The aqueous layer separated clearly from the organic layer and an extremely small amount of irregularities were observed at the interface of the aqueous and the organic layers indicating no rag layer formation. This method alone is the most environment-friendly method with no chemical additions and less energy intensive but can only be used if the load of emulsion to be treated is not high, or if operated with an expensive continuous centrifuge.
Effect of supply of heat: On heating for 1-2 hours, it was observed that demulsification was facilitated and the fraction of DCFA and DCFB in the bottom layer became more concentrated. However, heating for a long duration is not cost effective; hence the time of heating should be kept as low as possible. At elevated temperatures, the higher amount of energy provided as well as the reduced viscosity lead to a higher degree of demulsification [8]. Taking measurements after 2 days revealed that the changes caused by heating were irreversible. This technique can only be used if none of the components in the emulsion are heat sensitive. Table 3 reveals the effect of heat on the given emulsion.
Finding out the charge on the surface of the droplets dispersed in the emulsion: Samples of the emulsion were analyzed in the Zetasizer and the instrument returned the values of the zeta potential and the standard deviation. As the emulsion was unstable, the zeta potential varied at each reading, but the charge on the surface of the droplets dispersed in the emulsion was found to be positive. This positively charged oil film forming the interphase between the dispersed phase and the dispersion medium needed to be destabilized. Hence, a surface active, water-soluble anionic surfactant should be used to destabilize this interfacial film [9]. Analyzing all the available options, SDS which is an anionic long chain surfactant was identified as a potential demulsifying agent.
Changes caused by agitation: Shaking vigorously did not affect the emulsion much. However, gentle stirring with a magnetic stirrer at about 250-500 rpm without any additives caused flocculation of the drops which were collected at the interface of the two layers. An optimum magnetic needle speed of 300-400 rpm was selected.
Selection and optimization of demulsifying agents
SDS solution: The use of SDS as an emulsifier in oil-inwater emulsions has been widely exploited [10-12] but its use as a demulsifier in water-in-oil emulsions is still at a preliminary stage. For destabilizing this particular emulsion, the major factor was considered to be the positive charge on the surface of its droplets. Exploiting this very property, we experimented with SDS which is an anionic water-soluble surfactant and the results obtained were encouraging. The emulsion was demulsified to a large extent due to the surface active behaviour and charge destabilization caused by SDS. This particular anionic surfactant with its Amphiphilic nature, having a polar head group which interacted with and stabilized the aqueous part of the solution and its long hydrocarbon chain interacted with and stabilized the organic part of the solution thus reducing the surface tension between them and bringing molecules of similar nature closer to one another. Increasing the concentration of SDS beyond its CMC leads to the formation of aggregates or micelles, this in turn increases the rate of creaming and flocculation in the emulsion [13].
The different strengths of SDS, namely 8.3mM, 12mM and 15mM helped in optimizing the demulsification of the given mixture. However, the addition of SDS (at all feasible concentrations) caused flocculation but not coalescence of the droplets. Tables 3, 4 and 5 reveal the extent to which the added SDS affects the emulsion.
NaCl solution: Addition of a salt reduced the surface tension as well as suppressed the electrostatic force of repulsion between the droplets [9] facilitating their coalescence. The added electrolyte causes a drop in the zeta potential of the droplets which promotes creaming and further coalescence, resulting into two separate phases as desired [14]. Hence varying quantity of NaCl (0.1-0.5 g of salt per 100ml of emulsion) was added to the SDS solution and the density measurements were taken. Measurements were also taken after adding the same quantity of salt, but separately in the solution form (0.02g salt per ml of water). Both the results, as tabulated in Tables 3, 4, and 5 were compared. As the salt was soluble in water, the organic phase was not contaminated by salt addition. This behavior can be explained by using interfacial science. The polar head group area on addition of electrolytes (NaCl here) decreases hence reducing the net Critical Micellar Concentration of the solution and hence causing better surface activity. However the concentration of salt to be added must be minimum to avoid settling down of the excess salt at the bottom of the container. Addition of a lesser quantity of salt will prevent the collection of salt at the bottom of the container.
Interpretation of the results and reasoning
The Tables 4 and 5 tabulate the results obtained from the experiments conducted by integrating all the factors together. Varying each factor one at a time, enabled us to optimize the effect of each parameter. The final percentage of separation achieved has been quantified by the density of both the top and the bottom layers obtained after separation. Hence, the mean density of each layer is plotted against the ppm of SDS added and the trends obtained are represented in Figure 2 for the 12mM SDS solution and Figure 3 for the 15mM SDS solution.
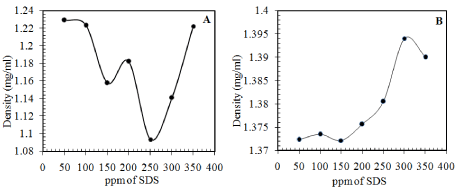
Figure 2: Density of the layers obtained on addition of salt and varying
concentrations of 12mM SDS solution (A) Top Layer (B) Bottom Layer.
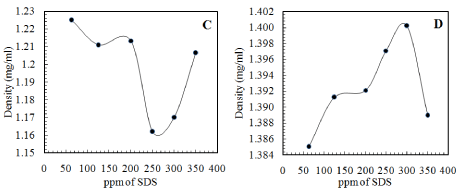
Figure 3: Density of the layers obtained on addition of salt and varying
concentrations of 15mM SDS solution (C) Top Layer (D) Bottom Layer.
The trend observed for both the 12 and the 15mM SDS solutions addition is that there is an optimum total concentration of the added Sodium Dodecyl Sulphate and salt solution at which the separation of the organic and the aqueous components is the maximum. This behaviour is not as expected, as the emulsion destabilization was expected to increase with increase in added destabilizer concentration. This can be attributed to various reasons:
- The chemical demulsifier (SDS here) first displaces the stabilizing layers around the drops, and with an increase in the demulsifier concentration, the adsorbed layer of demulsifier becomes close-packed. Hence, the emulsions become progressively more stable by preventing coalescence. Hence, at high concentrations of the added surface active agent, coalescence becomes the rate determining step [15].
- Addition of excess surface active agent beyond the concentration at which it neutralizes the surface charge on the emulsion droplets, increase the repulsion amongst them again due to adsorption of excess SDS on the interface and actually stabilizes the emulsion by preventing coalescence.
- Multimolecular adsorption may occur at the interface, delaying the process of coalescence [13].
- As the droplets approach a limiting size and relatively uniform size, the rate of coalescence reduces to zero due to the phenomenon of limited coalescence [16]. Limited coalescence is the phenomenon where the stability of suspensions depends inversely on the droplet size and the exact relationship has been explained [16].
- The emulsion could be stabilized by solid particles by the Pickering effect and the addition of surfactant could displace a certain percent of these solid particles but not all [4,14,17]. However, this may not be the case here, as after filtration of the emulsion, no solid residue was obtained.
- Insufficient or excess demulsifier addition changes the droplet size at the microscopic level and hence affects the degree of demulsification [18].
The process of demulsification is complex and is thoroughly explained by [19,20] where this behaviour of demulsifiers has been justified.
Comparison of the solutions to get an optimum result
The degree of separation was highest for 250 to 300ppm 12mM SDS solution with 0.5g salt per 100ml emulsion than the 50 or 250ppm 8.3mM SDS solution with heating with 0.2g salt per 100ml emulsion. The 250 to 300 ppm 15mM SDS solution with 0.5g salt per 100ml emulsion gave more concentrated organic phase, but it’s percentage loss of the organic phase into the aqueous phase in the top layer (38.98% - 42.16%) was more than that of the 12mM solution (22.7% -33.96%). The organic phase which was lost into the aqueous phase could not be retrieved as it was completely solubilized in the aqueous phase. Hence the 12mM SDS solution was finalized as the optimum solution. The highest degree of demulsification observed in terms of salt concentration is at a lower concentration of 0.5g NaCl per 100ml emulsion or even lower. This is in accordance with the stated fact that the lower electrolyte concentrations lead to the highest degree of demulsification [14]. Theoretically, the combined effect of both the demulsifiers has to be such that they have the lowest interfacial tension and highest surface activity [5]. This further explains why only this particular combination the two demulsifiers leads to the highest degree of demulsification. The optimized method is shown in Figure 4.
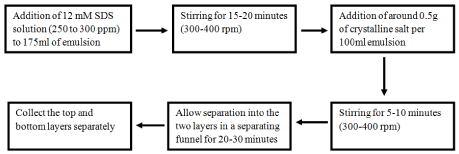
Figure 4: The optimized process.
Effect on the surface tension of the top aqueous layer
After separating the top aqueous phase from the emulsion (after following the aforesaid procedure) by a separating funnel, the surface tension was measured by the capillary rise method. The results were compared with the top aqueous phase provided by the company before the addition of any surfactants (Table 6). The solutions used were those where different quantities of 250ppm of SDS were added to the emulsion, as that was the optimum concentration of surfactant as obtained from the experiments. It was found that all quantities of the 250ppm SDS added to the emulsion reduced the surface tension. It was also found that all quantities of the 500ppm SDS added to the emulsion had similar effects of reduction in surface tension. The exact surface tension was not found as it was challenging to find cos(θ) due to the limitation of instruments available for measuring the contact angle. Instead ratios of capillary rise height were taken, considering that cos(θ) does not change much by addition of minute quantities of surfactant to water.
Phase
Rise in Height of Capillary Tube (cm)
Percent Decrease in Surface Tension as Compared to Original
Original aq. phase
0.525
-
After addition of 250ppm SDS + salt
0.4 to 0.4125
About 21% to 24%
After addition of 500ppm SDS + salt
0.375
About 29%
Table 6: Reduction in surface tension due to this process.
Conclusion
The separation of emulsion components into two layers was characterized by the flocculation and coalescence of the droplets thus destabilizing the emulsion. The demulsification of emulsion was successfully carried out, by effectively separating the DCFA and DCFB from the aqueous solution. Since it was a cationic emulsion, the negatively charged surfactant SDS was chosen along with NaCl as the most effective combination. Heating the emulsion for a limited period of time (15 - 20 minutes) at 65°C followed by natural cooling was certainly beneficial. 92.14% pure organic phase was obtained by heating alone. The percentage loss of the organic phase into the aqueous phase was reduced from 58.66% to 53.90% - 51.52%. However, this is not recommended as it was found to be detrimental (being heat sensitive) to the organic molecules in the emulsion to be demulsified. An exceptional separation efficiency with a maximum concentration of organic phase of 95.56% was obtained. The optimum combination of the aforesaid factors was found to be: (i) Addition of 12.5-15 ml of the 12mM SDS solution to175ml of emulsion in a 250ml beaker followed by 15-20 minutes of stirring at 300-400 rpm. (ii) Addition of around 0.5g salt per 100ml emulsion followed by stirring for 5-10 minutes at 300-400 rpm. The separating time required to separate both the layers in the separating funnel was about 20 to 30 minutes, after which the layers were collected separately. A thin brown coloured rag layer was formed at the interface of the two layers, which was about 2 to 3ml for the 190ml solution. The disposed rag layer contained a small amount of the valuable organic compounds (about1 to 1.2% of the total organic content in the emulsion) along with the excess surfactant and salt. If the rag layer formation can be avoided, an additional organic phase recovery of 1 to 1.2% can be obtained.
References
- James-Smith MA, Alford K, Shah DO. A novel method to quantify the amount of surfactant at the oil/water interface and to determine total interfacial area of emulsions. Journal of colloid and interface science. 2007; 310: 590-598.
- Abdurahman HN, Yunus RM, Jemaat Z. Chemical demulsification of water-in-crude oil emulsions. Journal of Applied Sciences. 2007; 7: 196-201.
- Hajivand P, Vaziri A. Optimization of Demulsifier Formulation for Separation of Water from Crude Oil Emulsions. Brazilian Journal of Chemical Engineering. 2015; 32: 107-118.
- Kokal SL. Crude oil emulsions: A state-of-the-art review. SPE Production & facilities. 2005; 20: 5-13.
- Kim YH, Wasan DT. Effect of demulsifier partitioning on the destabilization of water-in-oil emulsions. Industrial & engineering chemistry research. 1996; 35: 1141-1149.
- Wu J, Xu Y, Dabros T, Hamza H. Effect of demulsifier properties on destabilization of water-in-oil emulsion. Energy & fuels. 2003; 17: 1554-1559.
- Fan Y, Simon S, Sjöblom J. Chemical destabilization of crude oil emulsions: effect of nonionic surfactants as emulsion inhibitors. Energy & Fuels. 2009; 23: 4575-4583.
- Fortuny M, Oliveira CB, Melo RL, Nele M, Coutinho RC, Santos AF. Effect of salinity, temperature, water content, and pH on the microwave demulsification of crude oil emulsions. Energy & fuels. 2007; 21: 1358-1364.
- Shetty CS, Nikolov AD, Wasan DT, Bhattacharyya BR. Demulsification of water in oil emulsions using water soluble demulsifiers. Journal of dispersion science and technology. 2007; 13: 121-133.
- Staples E, Penfold J, Tucker I. Adsorption of mixed surfactants at the oil-water interface. The Journal of Physical Chemistry B. 2000; 104: 606-614.
- Yamamura H, Harayama S. Method for selective isolation of mycobacteria using olive oil emulsified with SDS. Bioscience biotechnology and biochemistry. 2007; 71: 1553-1556.
- Bratskaya S, Avramenko V, Schwarz S, Philippova I. Enhanced flocculation of oil-in-water emulsions by hydrophobically modified chitosan derivatives. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2006; 275: 168-176.
- Cockbain EG. The aggregation of oil particles in emulsions. Transactions of the Faraday Society. 1952; 48: 185-196.
- Rios G, Pazos C, Coca J. Destabilization of cutting oil emulsions using inorganic salts as coagulants. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1998; 138: 383-389.
- Sherman P. Research (London). 1955; 8: 396.
- Wiley RM. Limited coalescence of oil droplets in coarse oil-in-water emulsions. Journal of Colloid Science. 1954; 9: 427-437.
- Pickering SU. Cxcvi. — emulsions. Journal of the Chemical Society, Transactions. 1907; 91: 2001-2021.
- McClements DJ. Protein-stabilized emulsions. Current opinion in colloid & interface science. 2004; 9: 305-313.
- Van den Tempel M. Stability of oil-in-water emulsions III: Measurement of the rate of coagulation of an oil-in-water emulsion. Recueil des Travaux Chimiques des Pays-Bas. 1953; 72: 442-461.
- Masse P, Verdier A. Chemical Method of Destabilizing Emulsions Using Polyelectrolytes. Studies in Environmental Science. 1982; 19: 129-149.