
Research Article
Austin Chem Eng. 2017; 4(1): 1047.
Phytoremediation – Aeration System for Treatment of Urban Wastewater
Tripathi DM¹ and Tripathi S²*
¹Department of Microbiology, Bundelkhand University, Jhansi, India
²Institute of Environment and Development Studies, Bundelkhand University, Jhansi, India
*Corresponding author: Smriti Tripathi, Institute of Environment and Development Studies, Bundelkhand University, Jhansi, India
Received: December 21, 2016; Accepted: February 01, 2017; Published: February 03, 2017
Abstract
The present work was aimed to study the efficiency of Phytoremediation including aeration in treatment of secondary effluent. In the experiment, aquatic plants (Eichhornia crassipes and Lemna minor) were used individually and in combination at 0.0 – 1.6 Lmin-1 aeration for 0 – 120 hrs. In this context, the best results were obtained when the action of Eichhornia crassipes and Lemna minor, was combined with that of 0.4Lmin-1 aeration. Result exhibits promising efficiency towards the removal of BOD (85.49%), COD (85.71%), TKN (80.32%) and TP (80.92%), including heavy metals Fe (73.9%), Cr (66.8%), Cd (75.3%), Cu (73.5%), Zn (79.2%), Pb (76.9%) and Ni (74.9%). Statistical analysis showed significant reduction (p<0.001) in heavy metal content from secondary effluent after treatment. This technique is highly recommendable for tropical wastewater where sewage is mixed with industrial effluents.
Keywords: Aquatic macrophytes; Aeration; Phytoremediation; Heavy metal; Secondary effluent
Introduction
The anthropogenic activities exploit water resources to fulfill their daily need and create large amounts of wastewater, which have resulted in water pollution [1]. Heavy metals are among the most dangerous substances in the environment, because of their high level of durability and biomagnifications process [2]. Although many metals are essential but all metals are toxic at higher concentrations, cause undesirable effects and severe problems even at very low concentrations [3-5]. It also creates oxidative stress by formation of free radicals by replacing essential metals in pigments or disruption in enzymatic activities [6,7]. Due to discharge of heavy metals from wastewater from industrial, municipal and domestic origin the environment has suffered manifold detrimental effects [1,8]. During the last two decades, reuse of treated has expanded, helping to relieve water scarcity. So that, the demand for storm water treatment to prevent the anthropogenic release of heavy metals into local water bodies, is increasing rapidly [9].
Phytoremediation is a novel bioremediation technology in which plants are utilized to remove or degrade complex environmental pollutants [9]. They perform better purification due to direct contact with contaminated water. Eichhornia crassipes (Water hyacinth) and Lemna minor (duckweed) are hyper accumulator, fast growing and floating plants with a well-developed fibrous root system. It also adapts easily to various aquatic conditions and plays an important role in accumulating metals from water [10-12].
A few studies [7,13,14] have been done on the evaluation of effectiveness of aquatic floating plants, when combined with aeration. In activated sludge system aeration is a key process in wastewater treatment. Its principle role is to supply oxygen, needed for all aerobic treatment processes and to enhance the Dissolve Oxygen (DO) in treated wastewater [15]. Aeration is the dominant source of kinetic energy in an aeration tank [16]. This is one order of magnitude more than suitable mixing devices transfer into the water and several orders of magnitude more than the kinetic energy of the inflowing water or the hydraulic head loss. It also enhances the treatment capacity, facilitates operation at higher organic loading and helps to reduce the required area.
In the present study, the efficiency of the aquatic plants to produce water of higher quality from secondary effluent was investigated, under laboratory conditions. The highest possible treatment efficiency which is attainable in the presence of aquatic plants individually and in mixed culture was explored. This is done in conjunction with local standards levels, which can be reached in a system comprising aquatic plants supplemented with aeration, and the retention time required to this end.
Materials and Methods
Study area and sample collection
Present study was conducted in Varanasi city (82° 15’E to 83° 30’E and 24° 35’N to 25° 30’N). Samples were collected from Bhagwanpur Sewage Treatment Plant which utilizes conventional Activated Sludge Process including trickling filter for the treatment of wastewater.
Samples were collected in plastic containers from effluent channel and transferred to the laboratory, preserved and stored for further analytical determinations and treatment. Methods of preservation include cooling, pH control, and chemical addition. The length of time that a constituent in wastewater will remain stable is related to the character of the constituent and the preservation method used [17].
Experimental design
Phytoremediation with aeration of secondary treated wastewater: The aquatic macrophyte E. crassipes L. and L. minor L. were collected from the Agro farm pond of the Banaras Hindu University, Varanasi, India. The selected macrophytes were cultured individually and in combination with 100% coverage of the total surface area of the 150 L capacity of glass aquariums (0.39 m×0.59 m floor area per container) filled with 95 L of secondary effluent. An aerator was placed on the top of aquarium to maintain the air flow (0, 0.2, 0.4, 0.8 and 1.6 Lmin-1) through porous diffusers positioned into bottom of the aquarium (Figure 1). Control experimental sets contained secondary effluent with plants but no aeration. Five replicates of each experimental set containing macrophytes and control were prepared, i.e., total 30 sets.
Figure 1: Experimental design for the treatment of wastewater.
Analytical procedure
Water analysis: The samples of secondary effluent were collected from Bhagwanpur Sewage Treatment Plant and examined for pH, Acidity, Alkalinity, DO, BOD, COD, Color, Total Kjeldhal Nitrogen (TKN), Total Phosphorus (TP), toxic heavy metals (Fe, Cr, Cd, Cu, Zn, Ni, Pb) and microbial biomass (E. coli, Total coliform and Fecal coliform).
Physicochemical analysis as for BOD, COD, turbidity, temperature, dissolved Oxygen (DO), pH and color of wastewater was conducted according to Standard Methods for the Examination of Water and Wastewater [17], total nitrogen-N by the micro Kjeldahl method and total phosphorus by the wet oxidation method. For heavy metal analysis, Buck Scientific Flame Atomic Absorption Spectrophotometer (AAS) Model 205 was used. Sample blanks were also analyzed and results that were between 1% and 5% of each metal determined in samples were used to correct for any contamination in the course of the analysis. Water samples were also examined for microbiological content including total coliforms, fecal coliforms, and E. coli, after being kept at 480C for 14hours, using the method of the Most Probable Number [17].
Plant analysis: Aquatic plants were obtained from natural specimens, grown (for 1–2 months) in a fresh water pond. The shoot and root samples from each treatment were separately digested in a tri-acid mixture (5:1:1 HNO3:H2SO4:HClO4) and HM concentrations were determined by ICP. The Translocation Factors (TF) of metals was calculated as the ratio of metal concentrations in the shoots to that in the roots. The digested samples were analyzed for metals (Fe, Pb, Cd, Cu, Cr, Ni and Zn) using AAS Buck Scientific Model 205A.
Bioconcentration factor
Bioconcentration of heavy metal by aquatic organisms is described as the Bioconcentration Factor (BCF), which is the ratio of heavy metal accumulated by plants to that dissolved in the surrounding medium. For this, two bioconcentration factors were computed from the plant compartment concentrations as;
BCFa = Croots/Cwater (1)
BCFb = Caerial(peduncle+leaf) / Cwater (2)
Results and Discussion
Characteristics of secondary effluent
Mean values of physicochemical parameters, microbial biomass and heavy metals in raw sewage and in secondary effluent incorporating efficiency (%) of Bhagwanpur STP was given in Table 1. Results revealed that secondary effluent was slightly alkaline (pH- 7.9) had high amount of BOD (44.64mgL-1), COD (277.46mgL-1), TKN (153.21mgL-1), TP (10.49mgL-1), FC (36.05x103 MPN), TC (72.95x103 MPN), E. Coli (310.05 x103 MPN) and heavy metals (Fe-5.79mgL-1, Cd-1.082mgL-1, Cu-1.13mgL-1,Cr-0.21mgL-1, Zn- 4.001mgL-1, Ni-1.05mgL-1 & Pb-6.40mgL-1). The analysis was run in triplicates and the results obtained were averaged which showed that the parameters were always above to prescribed limit (Table 1). At Dinapur Sewage Treatment Plant including treatment efficiency of combination of aquatic plants with 0.4 Lmin-1 aeration rate.
Properties
Dinapur STP
After treatment with aquatic plants (Ec + Lm)
Untreated
Treated
With aeration (0.4 Lmin-1)
% reduction
Temperature (0C)
27.09 ± 3.68
25.48 ± 1.87
25.11± 1.19
1.45
pH
7.74 ± 1.08
7.48 ± 0.99
7.39 ± 0.72
1.2
Acidity (mgL-1)
37.00 ± 3.01
21.13 ± 1.18
18.02 ± 1.61
14.7
Alkalinity (mgL-1)
495.67 ± 13.75
267.97 ± 6.34
210.11 ± 9.65
21.6
DO (mgL-1)
1.51 ± 0.09
2.67 ± 0.21
4.23 ± 1.01
58.43 ?
BOD (mgL-1)
84.32 ± 3.65
44.64 ± 2.39
5.6 ± 1.39
85.49
COD (mgL-1)
496.98 ± 15.41
277.46 ± 9.18
39.9± 5.98
85.71
Color (Hazen Unit)
25.3 ± 3.18
19.9 ± 2.18
12.51 ± 1.26
37.2
Phosphorus (mgL-1)
13.52 ± 2.33
10.49 ± 1.95
2.0 ± 0.56
80.92
TKN (mgL-1)
330.79 ± 15.01
153.21 ± 9.99
30.1 ± 3.59
80.32
Fe (mgL-1)
6.71 ±0.99
5.79 ±0.95
1.52 ± 0.74
73.9
Cr (mgL-1)
0.32 ±0.05
0.21 ±0.06
0.14 ± 0.05
66.8
Cd (mgL-1)
1.11 ±0.09
1.082 ±0.007
0.262 ± 0.08
75.3
Cu (mgL-1)
1.19 ±0.17
1.13 ±0.02
0.35 ± 0.10
73.5
Zn (mgL-1)
4.58 ±0.97
4.001 ±0.99
0.831 ± 0.75
79.2
Ni (mgL-1)
1.59 ±0.09
1.05 ±0.05
0.264 ± 0.05
74.9
Pb (mgL-1)
6.92 ± 2.39
6.40 ± 1.29
1.48 ± 0.77
76.9
Table 1: Average concentration of untreated sewage and treated secondary effluent water quality.
Performance of phytoremediation
Analysis of the water quality parameters demonstrated that the removal efficiency (%) was always higher for mixed culture (Ec + Lm) with aeration when compared with individual plant study and control experiment. The highest removal rate of physico-chemical parameters (BOD, COD, TKN, TP and color) were achieved in mixed culture (Ec + Lm) on 120 hrs (21.4%, 29.9%, 45.3.1%, 47.3%). Figure 2 revealed that removal efficiency of individual and mixed culture of aquatic plant with no aerations ranged between 10.0 – 50%. Continuous decrease was also recorded with increasing days during the experiment [7]. Reported the similar results by using mixed culture (Ec + Lm) of aquatic plant [18]. Showed the efficiency of L minor individually in reduction of physico-chemical parameters which ranged between 64 – 75% in 5 – 10 days and [14] reported 30 – 60% reduction in BOD and COD by E. crassipes within 1 – 4 days.
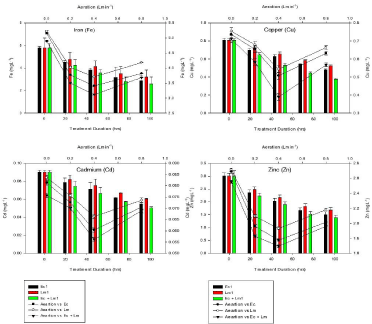
Figure 2: Effect of aeration and exposure duration on efficiency of aquatic macrophytes for heavy metal accumulation.
Table 2 designates the accumulation of some toxic heavy metals (Fe, Pb, Cr, Cu, Cd, Ni and Zn) in macrophytes under present study. The results of the regression analysis confirm that the metal removal by the macrophytes individually and in mixed culture were proportional to metal concentrations (p < 0.01). The results obtained from the present study indicated that metal removal percentages were highest during 2nd – 4th day for all the three sets for most of the times. The heavy metals removal by mixed culture (E. crassipes + L minor) were very high and ranged between 30 - 45% in 120hrs of incubation period, a small decrease was observed further. The metal removal efficiencies were increased initially with increasing time. Previous workers [11,12,19] also reported the reduction in heavy metal by using aquatic plants (E. crassipes, Pistia,, T. lattifolia and L. minor).
Competence of aeration with Phytoremediation
The batch study conducted with combination of aquatic macrophytes E. crassipes and L. minor, clearly indicate that the removal efficiency of system improves with decrease in required duration with aeration. The effect of aeration on physicochemical parameters with applied aeration rate (0.4 Lmin-1) was given in Table 1. With aeration, BOD and COD level of 5.6mgL-1 and 39.9mgL-1 respectively was reached after 24 hrs of treatment, while 4 days were required to this end without aeration. In the continuous treatment process, retention time and aeration rates were the controlling factors [13] reported the similar results. Higher flow rates work better in shorter periods of operation.
Mixed culture (Ec+Lm) and the imposed aeration produced better kinetic features for purification of secondary effluent as compared to the control. Notwithstanding the considerable fluctuation of the feed and treated effluent quality, the laboratory-scale tests confirm the capacity of the plants to reach and hold reasonably low levels of BOD (5.6mgL-1) and COD (39.9mgL-1), very low levels of Total Phosphorus (2.0mgL-1) and Total Nitrogen (30.1mgL-1). These results are in agreement with those already reported [7,13,14] where a combination of naturally growing aquatic macrophytes removed nitrates and phosphates [20]. Observed that the interaction between macrophytes and bacteria might amplify the assimilation of nitrogen compounds as opposed to situations in which macrophytes and bacteria act separately. Over the last 20 years, many experiments were conducted, in order to reduce organic carbon concentration of domestic sewage, using aquatic plants but this is a unique work in which plants (Eichornia crassipes and Lemna minor), supplemented with 0.4Lmin- 1 aeration, are capable of lowering BOD, COD, color, TP and TKN to 85.49%, 85.71%, 37.2%, 80.92% and 80.3% respectively which required by national and local guidelines for irrigation water within 24 – 48 hrs, while 5–8 days were required to this end without aeration. As a plant-based technology, the success of Phytoremediation depends upon several plant characteristics. Accumulation of heavy metals such as Cr, Cd, Zn, Ni, Cu, Pb and Fe by the plants in our study showed their greater efficiency because of being rooted plants. Various other studies indicate the same [21,22]. The plant should have the ability to produce large amounts of biomass rapidly using standard crop production and management practices [23] together with high efficiency of metal accumulation in shoot biomass [24,26].
At 0.4 Lmin-1 aeration rate Eichhornia individually removed 65.3±4.4, 63.9±1.4, 61.4 ± 3.45, 62.0±2.4, 62.4 ± 2.87, 60.8 ± 1.76, 65.9 ± 4.65% Fe, Cr, Cu, Cd, Zn, Ni and Pb respectively. Among small leaved aquatic macrophytes L. minor showed the highest removal efficiency (Table 2) but significant (p < 0.05) reduction in heavy metal concentration was observed in combination of broad leaved macrophyte Eichhornia crassipes and small leaved macrophyte Lemna minor i.e. 70 – 80% (Figure 2). The experimental plants showed excellent performance in removing the metals as they were able to remove up to 95% of heavy metals in 120hrs incubation period. ANOVA revealed that concentration of heavy metals in the samples decreased at 0.2 - 0.4 Lmin-1 within 24 – 48 hrs while further slightly decrease was observed at 0.8 – 1.6 Lmin-1 (p < 0.01). Modifications in metal concentration in water with time frame showed >60% removal within 120hrs of the experiment. Aeration is seen to enhance the kinetics of processes that lower the levels of organic, inorganic and toxic metal contents from the municipal sewage [13,26]. Positive and significant correlations have been observed between percent removal of heavy metals from the Secondary treated wastewater and the incubation period with aeration (p < 0.001). The aquatic plant species utilized in this study showed a large range of heavy metal tolerance [7,27,28].
Heavy metals
Plant sp.
Initial concentration (mgL-1)
Concentration after treatment (mgL-1)
Net accumulation in plant
(mgL-1)
R
(mgL-1)
% Reduction
Fe
Ec + Lm
5.79 ±0.95
1.52 ± 0.74
3.62 ± 0.42
0.65 ± 0.09
73.9
E. crassipes
5.79 ±0.95
2.09 ± 0.32
3.32 ± 0.23
0.65 ± 0.09
65.3
L. minor
5.79 ±0.95
2.78 ± 0.29
2.72 ± 03.45
0.65 ± 0.09
51.9
Cr
Ec + Lm
0.65 ±0.14
0.14 ± 0.05
0.46 ± 0.16
0.05 ± 0.01
76.8
E. crassipes
0.65 ±0.14
0.23 ± 0.05
0.41 ± 0.09
0.05 ± 0.01
63.9
L. minor
0.65 ±0.14
0.31 ± 1.56
0.32 ± 0.03
0.05 ± 0.01
54.2
Cu
Ec + Lm
1.19 ± 0.84
0.35 ± 0.10
0.76 ± 0.19
0.08 ± 0.01
73.5
E. crassipes
1.19 ± 0.84
0.47 ± 0.15
0.62 ± 0.13
0.08 ± 0.01
61.4
L. minor
1.19 ± 0.84
0.56 ± 0.125
0.55 ± 0.11
0.08 ± 0.01
46.8
Cd
Ec + Lm
1.082 ±0.007
0.262 ± 0.08
0.79 ± 0.01
0.04 ± 0.003
75.3
E. crassipes
1.082 ±0.007
0.41 ± 0.03
0.61 ± 0.28
0.04 ± 0.003
62.7
L. minor
1.082 ±0.007
0.557 ± 0.05
0.42 ± 0.28
0.04 ± 0.003
48.5
Zn
Ec + Lm
4.001 ±0.99
0.831 ± 0.75
3.072 ± 0.76
0.098 ± 0.01
79.2
E. crassipes
4.001 ±0.99
1.51 ± 0.65
2.95 ± 0.39
0.098 ± 0.01
62.4
L. minor
4.001 ±0.99
2.08 ± 0.32
2.40 ± 2.07
0.098 ± 0.01
48.1
Ni
Ec + Lm
1.05 ±0.05
0.264 ± 0.05
0.73 ± 0.07
0.056 ± 0.005
74.9
E. crassipes
1.05 ±0.05
0.416 ± 0.07
0.58 ± 0.04
0.056 ± 0.005
60.8
L. minor
1.05 ±0.05
0.612 ± 0.104
0.30 ± 0.03
0.056 ± 0.005
41.6
Pb
Ec + Lm
6.40 ± 1.29
1.48 ± 0.77
4.03 ± 1.25
0.89 ± 0.12
76.9
E. crassipes
6.40 ± 1.29
2.18 ± 0.22
3.29 ± 0.96
0.89 ± 0.12
65.9
L. minor
6.40 ± 1.29
2.99 ± 0.27
2.87 ± 0.17
0.89 ± 0.12
53.2
Table 2: Concentration of heavy metals in aquatic plants individually and their mixed culture with its % reduction at aeration of 0.4Lmin-1.
Conclusion
Results confirm the capacity and effectiveness of aquatic plants, Eichhornia crassipes and Lemna minor, in mixed culture when supplemented with 0.4Lmin-1 aeration. The mixed culture of two aquatic macrophytes (Ec + Lm) at 0.4 Lmin-1 aeration rates indicate reduction of organic and inorganic content 80 - 85.7% with of heavy metals 70 – 80% within 24 – 96 hrs from treated secondary effluent.
This is one of the most significant results of this work, as no related data are available elsewhere. Thus this technique is highly recommended in the developing countries for the wastewater treatment.
References
- Mani D, Kumar C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: an overview with special reference to Phytoremediation. Int. J. Environ. Sci. Technol. 2014; 11: 843–872.
- Bennicelli R, Stezpniewska Z, Banach A, Szajnocha K, Ostrowski J. The ability of Azolla caroliniana to remove heavy metals (Hg(II), Cr(III), Cr(VI)) from municipal waste water. Chemosphere. 2014; 55: 141–146.
- Kara Y. Bioaccumulation of Cu, Zn and Ni from the wastewater by treated Nasturtium officinale. Int. J. Environ. Sci. Technol. 2005; 2: 63–67.
- Arora M, Kiran B, Rani S, Rani A, Kaur B, Mittal N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008; 111: 811–815.
- Memon A.R, Schroder P. Implications of metal accumulation mechanisms to phytoremediation. Environ. Sci. Pollut. Res. 2009; 16: 162–175.
- Ali H, Khan E, Shajad M. A. Phytoremdiation of heavy metal: concept and application. Chemosphere. 2013; 91: 869 – 881.
- Tripathi S, Tripathi B.D. Efficiency of combined process of ozone and bio-filtration in the treatment of secondary effluent. Bioresource Technology. 2011; 102: 6850–6856.
- Robinson B.H, Banuelos G, Conesa H.M, Evangelou M.W.H, Schulin R. The phytomanagement of trace elements in soil. Crit Rev Plant Sci. 2009; 28: 240–266.
- Huang L, Zhuo J, Guo W, Spencer R.G.M, Zhang Z, Xu J. Tracing organic matter removal in polluted coastal waters via floating bed Phytoremediation. Marin. Pollut. Bull. 2013; 71: 74 – 82.
- Liao S.W, Chang W.L. Heavy metal phytoremediation by water hyacinth at constructed wetlands in Taiwan. J. Aquat. Plant Manage. 2004; 42: 60–68.
- Mishra VK, Tripathi BD. Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes). Journal of Hazardous Materials. 2009; 164: 1059–1063.
- Rai PK, Mishra A, Tripathi BD. Heavy metal and microbial pollution of the river Ganga: A case study at Varanasi. Aquatic Ecos. Health & Manegm. 2010; 13: 352 – 361.
- Zimmels Y, Kirzhner F, Kadmon A. Effect of circulation and aeration on wastewater treatment by floating aquatic plant. Separation and Purification Technology. 2009; 66: 570–577.
- Zimmels Y, Kirzhner F, Malkovskaja A. Application of Eichhornia crassipes and Pistia stratiotes for treatment of urban sewage in Israel. J. Environmental Management. 2006; 81: 420 – 428.
- Gresch M, Armbruster M, Braun D, Gujer W. Effects of aeration patterns on the flow field in wastewater aeration tanks. Water Research. 2011; 45: 810 – 818.
- Tchobanoglous G, Burton FL, Stensel HD. Wastewater Engineering (Treatment Disposal Reuse), fourth ed. Metcalf & Eddy, Inc. Boston. McGraw-Hill Book Company. 2003.
- APHA, AWWA, WEF. Standard Methods for the Examination of Water and Wastewater. (American Public Health Association, American Water Works Association, and Water Environment Federation. Washington DC. 2005.
- Shafai SA, EFatma A, Gohary E, Fayza AN, Peter NS, Gijzen HJ. Nutrient recovery from domestic wastewater using a UASB-duckweed ponds system. Bioresource Technology. 2007; 98: 798 – 807.
- Upadhyay A, Mishra VK, Pandey SK, Tripathi BD. Biofiltration of secondary treated municipal wastewater in a tropical city. Ecological Engineering. 2007; 30: 9 –15.
- Chang H, Yang X, Fang Y, Pu P, Li Z, Rengel Z. In situ nitrogen removal from the eutrofic water by microbial - plant integrated system. 2006; 7: 521-531.
- Deng H, Ye ZH, Wong MH. Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal contaminated sites in China. Environ. Pollut. 2004; 132: 29–40.
- Gupta M, Chandra P. Bioaccumulation and toxicity of mercury in rooted submerged macrophyte Vallisneria spiralis. Environ. Pollut. 1998; 103: 327–332.
- Das M, Maiti SK. Metal accumulation in A. baccifera growing naturally on abandoned copper tailings pond. Environmental Monitoring and Assessment. 2007; 127:119–125.
- MCGRATH SP. Phytoextraction for soil remediation. In: BROOKS, R.R. (Ed.), Plants Those Hyperaccumulate Heavy Metals: Their Role in Phytoremediation, Microbiology, Archaeology, Mineral Exploration and Phytomining. CAB International, New York, 1998; 261–288.
- Shah K, Nongkynrih JM. Metal hyperaccumulation and bioremediation. Biologia Plantarum. 2007; 51: 618–634.
- Yedla S, Miltra A, Bandyopadhyay M. Purification of pulp and paper mill effluent using Eichhornia crassipes. Environ. Technol. 2002; 23: 453–465.
- Mishra VK, Upadhyaya AR, Pandey SK, Tripathi BD. Heavy metal pollution induced due to coal mining effluent on surrounding aquatic ecosystem and its management through naturally occurring aquatic macrophytes. Bioresource Technology. 2008; 99: 930–936.
- Sauja FA, Dziedzic M, Aparecida SC, Maranho LT. Restoration of polluted waters by phytoremediation using Myriophyllum aquaticum (Vell.) Verdc., Haloragaceae. Environmental Management. 2013; 120: 5 – 9.