
Research Article
Austin Chem Eng. 2019; 6(1): 1064.
Ground Water Contamination by Arsenic in Nepal: Lessons to be Learned from Geology
Mueller B*
¹Bamugeobiochem, Switzerland
*Corresponding author: Barbara Mueller, Bamugeobiochem, Horbenstrasse 4, 8356 Ettenhausen, Switzerland
Received: February 04, 2019; Accepted: March 18, 2019; Published: March 25, 2019
Abstract
In several countries of South East Asia ground water extracted from Quarternary alluvial sediments exhibits arsenic concentrations exceeding the the World Health Organization (WHO) drinking water guideline of 10 μg/L. According a widely accepted theory a reductive dissolution of Fe-bearing minerals leads to a release of As-oxyanions from these minerals. Yet, a pronounced decoupling or As and Fe concentrations in the ground water in the lowlands of Nepal (Terai) results in a loss of correlation between these two elements mainly being derived from the same minerals. Therefore, it seems obvious that other mineral sources of as have to be considered. Albeit As is mainly associated with iron (hydr) oxides, clay minerals (including micas) cannot be disregarded as s substantial source of As. This lack of correlation between aqueous As and Fe as well as the high temperature of the well water induces severe consequences for water treatment.
Keywords: Arsenic; Clay minerals; Decoupling
Introduction
The districts Nawalparasi, Bara, Parsa, Rautahat, Rupandehi, and Kapalivastu are among the regions in the lowlands (Terai) of Nepal most severely affected by the adverse effects of arsenicosis (keratosis of the palms of the hands and soles of the feet, skin lesions including pigmentation changes, mainly on the upper chest, arms and legs, and as the most severe effect, cancer of the skin and internal organs [1-3]. The arsenic concentrations in the ground water hosted in Quarternary alluvial sediments and used for consumption regularly exceed the World Health Organization (WHO) drinking water guideline (10 μg/L). The maximum permissible limit of arsenic in Nepal is as high as 50 μg/L. An estimated 0.5 million people in Terai were at risk of consuming water with an arsenic concentration above this limit [4].
Arsenic in ground water is of clear geogenic origin and its elevated concentrations in are considered to be due to natural weathering of the Himalayan belt [5-10]. The sediments are derived from the high Himalayas, carried downward by the Ganga–Brahmaputra river system and lastly build up the foreland basin and the Bengal fan [11,12]. In such regions, young sediments of low elevation and low hydraulic gradient are characteristic of many arsenic-rich aquifers.
Arsenic is mainly concentrated in the mineral pyrite, mostly being found in sulphide-bearing mineral deposits. Pyrite weathering leads to formation of hydrous iron oxides and clay minerals hosting As. In general, sediments containing 1 to 20 mg/kg (near crustal abundance) of arsenic can already give rise to high dissolved arsenic (>50 μg/L) in groundwater if one or both of two possible “triggers”-an increase in pH above 8.5 or the onset of reductive iron dissolution are initiated [13]. Solubilization of arsenic into ground waters rigorously depends on pH, redox conditions, temperature, and solution composition. In the ground water of the Terai, arsenite, the reduced trivalent form [As (III)], is principally present in ground water [14,15]. The ground water in the Terai seems mostly to be of near-neutral to alkaline state within a pH range of 6.1-8.1 [16,15]. Variations of redox potential (Eh) between -0.20 to -0.11 V suggest fairly reduced conditions in the aquifers.
There is an ongoing debate about the most probable mechanisms leading to the dissolution of As from alluvial sediments as the iron concentration in ground water is much lower than e.g. in Bangladesh or Vietnam. Beyond that, As concentration in the ground water of the Terai is weakly negatively or not correlated at al with the Fe concentration. This decoupling of As is in contrast with the positively correlated As concentration with the lithophile elements like Na, and K Mueller and Hug, [15]. Decoupling between aqueous As and Fe has also been described by [17,18,14]. Typically, Na and K are lost from alumosilicates such as clay minerals (including micas) during weathering, leading to an enrichment of immobile elements such as Fe and Al in the remnants. It is widely described that arsenic can be adsorbed efficiently by pure clay minerals [19-21] and are capable to release a substantial amount of arsenic depending on local and climatic conditions. The present article deals with geological issues leading to arsenic contaminated ground water in Nepal.
Geology
Being a landlocked country in South-East Asia, Nepal’s topography is delineated by a diverging rugged and undulating topography and geology, a common cold climate and it is presented predominantly as mountainous. As many as 6,000 rivers and rivulets, with a total drainage area of about 194,471 km2, flow through the country, whereof 76% of this drainage area is contained within Nepal. The surface variations in Nepal are largely controlled by geology [22,2]. The Southern and Northern parts of Nepal are extremely distinct from each other due to the discrete geological history. So said, the marine sedimentary deposits forming the high Himalayas, the Siwalik hills built by the then east-west flowing rivers and the Archan crystalline formations deep beneath the Alluvium of the Terai can be found within comparatively short distances [23]. The Terai Plain in the southern part of Nepal close to the Indian border is built up by Quaternary sediments that include molasse units along with gravel, sand, silt, and clay and represents an active foreland basin. All major rivers originate in the high Himalayas whilst smaller rivers also arise from the nearby Siwalik Hills. Fine sediments as well as organic material are deposited in inter-fan lowlands, in wetlands and swamps [24].
Extreme monsoon precipitation (1,800-2,000 mm) and yearround snow-fed river systems replenish the Terai sediments, leading to a high potential for ground water resources. The majority of shallow aquifers (‹50 m) are unconfined or semi-confined, while the deep aquifers (>50 m) are generally confined by impermeable clay layers. The aquifer system is markedly sensitive to precipitation [6].
Nawalparasi is so far the most acute studied Terai province concerning local geology and ground water contaminated by arsenic. The district of Nawalparasi extends into the Terai plain as the continuation of Indo-Gangetic plain (Figure 1). From the Indian border, this district spreads northward across the Narayani River (one of the major rivers of Nepal) alluvium, later across the low gradient fan of locally derived alluvium and finally into the Himalayan foothill (also known as Churia hills) [25]. The Narayani River originating in the Higher Himalaya, flows along the eastern boundary of the Nawalparasi district and exerts a essential influence on the underlying unconsolidated Holocene fluvial deposits. Characteristic geomorphic features of the area include small natural ponds and meandering rivers [26]. In the areas with fine-grained sediments, elevated concentrations of As are typically recorded [27,28,14].

Figure 1: Location of the Terai Basin, Nepal in the Ganges–Brahmaputra–
Meghna watershed area (from Gurung et al., [6]).
The district of Nawalparasi is typically characterized by small natural ponds and rivers forming the morphology. Near the frontal mountain chain, the Indo-Gangetic plain is composed of boulder- to gravel-sized sediments, as well as of fine-grained sediments. Guillot et al. [8] report about the lithology of sledge core samples from five drill holes exhibiting various coarse (millimetric) to fine-grained (micrometric) sediments in the Narayani basin. They consists of distinguished light-grey to dark grey sands; grey, greenish-grey to brown-grey and yellow-brown silts; and light-grey to black-grey, yellow-brown and black clay with some gravel layers. Sands, silt and clay sediments generally consists of micas that are occasionally massive to laminated, bioturbated, and/or also containing roots and plant debris. Detrital minerals in the silt fraction are dominated by quartz, biotite, muscovite, K-feldspar, calcite and dolomite as major phases and garnet, zircon, and monazite as heavy minerals as determined by binocular observations.
Material and Methods
Several years ago concerns arose that at least some of the installed iron-assisted bio-sand filters (so-called “Kanchan” filters, [28,29] do not eliminate the arsenic from ground water as desired. Co-workers from CAWST (Centre for Affordable Water Sanitation Technology) Calgary, Canada, in cooperation with ENPHO (Environment & Public Health Organization) Kathmandu, Nepal and Eawag, Switzerland, therefore started sampling campaigns for ground water in Nawalparasi in October 2015 (post-monsoon) and in April 2017 (pre-monsoon). 35 water samples from hand pumps were collected in 2015 and again 2017. Pumps were well flushed (e.g. pumped for > 2 min) before sample collection to remove all standing water in the tube wells.
All ground water samples were directly taken from privately owned hand pumps within the urban area of Ramgram (capital of the district Nawalparasi, Ramgram labeled “Nawalparasi” in Figure 1) or in the villages Manari, Panchanagar, Sukauli and Tilakpur. The depth of the tube wells is commonly 25 m and the soil consists broadly of clayey sediments. Households for sample collections were chosen in relation to a register provided by ENPHO for wells from which filtered water exceeded the Nepal drinking water quality standard value (50 μg/L).
Kanchan filters are the most important water treatment method used in Nepal. These iron-assisted bio-sand filters are constructed on the basis of arsenic removal from water using Zero-Valent Iron (ZVI) media. In order to achieve acceptable levels of removal, a second filtration step to remove formed arsenic containing colloidal Fe(III) (hydr)oxide has to be implied [30].
In the field, ground water samples were collected into 4.5 mL preacidified polypropylene vials (containing 150 μl 1M HNO3) and sent to Eawag for prompt examination. The acidified samples were diluted 1:5 and 1:20 into 0.1M HNO3 for ICP-MS analysis (Agilent 7500cx) in the laboratory. Standards for all reported elements were prepared by dilution of J.T. Baker single element standards with 0.1M HNO3, covering the relevant concentration ranges concerning the samples. Results were intermittently verified by comparison with spiked ground water samples and with 1:10 diluted X CertiPur multi element standard solution (Merck). To measure As(III), non-acidified water samples were filtered through metal soft arsenic speciation cartridges which adsorb only As(V), after which As was quantified by ICPMS as described above. Triplicate analysis agreed to within 2-5%. Detection limits were at least 10 times lower than the lowest reported concentrations for all elements, except for P, B, and Fe, for which they were 0.03 mg/L, 5 μg/L, and 0.03 mg/L, respectively, with dilutions of 1:5.
Results and Discussion
Range, average and median concentrations of As, Fe and P for 35 ground water samples in collected in post-monsoon (2015) and pre-monsoon (2017) seasons from five different communities in the district of Nawalparasi are listed in Table 1. Arsenic concentrations exceed the WHO guideline of 10 μg/l in all samples. Concerning the average molar Fe/As ratio for the 35 samples analyzed, it has to be stated clearly that the geological situation in Nepal is explicitly dissimilar compared to e.g. Vietnam or Bangladesh. A fact so far completely neglected. In Nepal the mentioned ratio sums up to 9.4 (post-monsoon) and to 6.0 (pre-monsoon). In contrast, Berg et al. [31] write about an average molar Fe/As ratio between 60 and 68 for arsenic contaminated ground water in the Hanoi area of Vietnam. The molar Fe/As ratio in Bangladesh sums up to 14.88 or 90.49, respectively [32,33]. The correlation between As and Fe concentrations in the 35 ground water samples from Nawalparasi is depicted in Figure 2. There is obviously no correlation between As in Fe in both climatic seasons. Figure 3 exhibits the the pH-dependence of Fe and As in pre-monsoon season 2017. Only pH measurements from this time are available as the pH-meter was not fully functional in post-monsoon season 2015. A apparent negative correlation is reported for Fe, and a slightly positive correlation for As. The pH in ground water from Nepal (range: 7.0-7.8, neutral to alkaline) is slightly higher than in ground water samples for Bangladesh [range: 6.6-7.4 Ahmed et al. [32] and for Vietnam, Berg et al., [31]. According analysis from post-monsoon 2015, As(III) was the dominating species in Nepali ground water pointing to a fairly reduced environment (81 % As(III) on average). The significance of this dominance of As(III) in the well water of the Terai is two fold: (i) As(III) is more mobile than As(V) and (ii) reductive processes are immanent concerning As mobilization [34-36].
Element
Range 2015
Average 2015
Median 2015
Range 2017
Average 2017
Median 2017
As
95.5 - 798.7
285.6
265.4
54.8 - 774.0
257.9
211.2
Fe
0.01 - 5.91
2.03
1.76
0.02 - 5.66
1.44
1.44
P
<0.01 - 0.89
0.17
0.15
0.00 - 0.86
0.09
0.09
Table 1: Range, average and median concentrations of As (μg/l), Fe (mg/l), Mn (mg/l) and P (mg/l) in groundwater from tube wells in several municipalities in Nawalparasi, Nepal. Samples collected in October 2015 (post-monsoon) and April 2017 (pre-monsoon). Average values of two determinations of concentrations by ICP-MS. Standard deviation 3-5 % (Wenk et al., [43]).
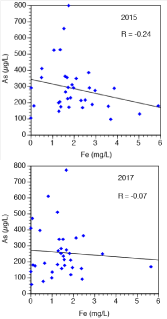
Figure 2: Apparent decoupling between As (μg/l) and Fe (mg/l) from 35
ground water samples (raw water only). Correlation coefficients (r) are
included in the diagrams for illustration though they are not significant.
Moreover, Figure 2 illustrates the obviously absent correlation between As and Fe demonstrating a decoupling between mobilization of As and Fe in general. The correlation between As and Fe in groundwater was so far discussed controversially: McArthur et al. [37] report about a positive correlation between As and Fe in West Bengal whereas Bhattacharya et al. [16] describe a positive correlation between As and Fe (r=0.77) at some areas in the aquifer of the Nawalparasi district. Contrary to these reports, a clear decoupling between these elements has been attributed by [17,18,14]. Whilst Horneman et al. [38] propose processes mobilizing As and Fe are decoupled, because Fe reprecipitates could be formed again after reduction of Fe(III) oxyhydroxides, while the mobilized As would remaining in solution. Diwakar et al. in turn specifies that decoupling between Fe and As may result from sorption of iron to various mineral surfaces (i.e. clays) or precipitation of Fe(II) minerals, such as siderite [14]. Ground water in Nawalparasi is nearly saturated with respect to siderite in most samples according to the mentioned study by [14].
Some preliminary X-ray inspections of nails from some Kanchan filters (University of Bern, oral communication) revealed that siderite was formed on the surface of the nails being prearranged to adsorb As. The diagram in Figure 4 illustrates undoubtedly how the concentration of Fe and As in groundwater depend on temperature. As the solubility of siderite decrease with increasing temperature [39] its formation is enhanced with raising temperature. Siderite will seal the surface of the nails in the Kanchan filters and will therefore prevent the adsorption of As. The implication of the findings concerning siderite formation is double: As seen in Figure 4 in the climatic hot post-monsoon season 2015, As is positively whilst Fe is not correlated with temperature, illustrating Fe is precipitated as siderite and As remains in solution. During the colder and more pleasant pre-monsoon season 2017 both Fe and As are negatively correlated with temperatures preventing the formation of the undesirable siderite. In Figure 5 calcite and siderite are precipitated at the discharge of a naturally occuring hot spring near Manaslu (8156 m) proving formation of siderite at higher temperature of the source water.
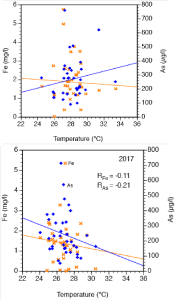
Figure 4: Temperature-dependence of Fe and As concentrations in 35
groundwater samples from Nawalparasi district.

Figure 5: The author close to a calcite and siderite precipitating natural hot
spring near Manaslu (8156 m).
Moreover, the decoupling between Fe and As in the ground water of Nawalparasi reported above clearly points out the importance of reflecting the original mineralic hosts of arsenic. This decoupling clearly enhances our understanding of As being incorporated and released from clay minerals and to a minor extent from iron(hydro) xides in Nawalparasi. The degree of As mobilization obviously depends on the affinity of the original and reworked minerals for arsenic species [40]. As is known, Fe fits well in octahedral layer of clay minerals such as biotite and much less in its interlayer; therefore preventing Fe to be exchanged against K and Na. In addition, both biotite and muscovite only contain Fe as a minor constituent. Beyond that, as stated eg. by [19-21] fine grained minerals like clay minerals are habile of adsorbing arsenic efficiently and consequently can also release a substantial portion arsenic depending on local and chemical conditions. XRF analysis published by Yadav et al. revealed that high As concentration were mostly associated with fine-grained clay minerals [23]. According to Guillot et al. As is heavily concentrated in clayey sediments in the entire Terai and is usually associated with specific elements (Fe, Al, K and C) [6]. The fine grain-size fractions of the sediments exhibit larger surface areas and are therefore capable to adsorb the major part of As. Taking into account that Fe, Al and Mn oxides and hydroxides form the major part of the fine grained minerals, they are likely to incorporate significant amounts of As under specific pH condition. As reducing conditions are prevailing in the ground water from Nawalparasi, denominating clay minerals as fundamental carriers of As does not mean to deviate from the reduction hypothesis as the main source of As(III). The water samples from a study of Mueller and Hug confirm reducing conditions where As remains relatively mobile [15]. In a arising consensus view then described by Stanger, the dominant process was delineated as follows [19]: The fixation of aqueous arsenic by sorption onto Fe-, Mnoxide or clay surfaces was initiated during high-redox medium-pH conditions (i.e. about 5.5-6.5).
Increasing pH leads to a desorptive release of adsorbed As(V)-a process explaining in turn the positive correlation between As concentration and pH as shown in Figure 3 as the ground water in Nawalparasi becomes more alkaline (pH >6.5). Arsenic mobilization depends sensitively on the pH. An alkaline pH measured in ground water from Nawalparasi points to a well known trigger of As dissolution as shown in Figure 3. Unfortunately, Fe dissolution is inhibited at higher pH values (Figure 3). Beyond this said, the immense variation of the As concentration in the ground water mirrors the extremely heterogeneous sediments in this a region on a municipality-scale [41,8].
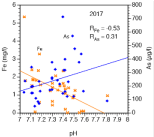
Figure 3: PH-dependence of Fe and As in 35 groundwater samples from
Nawalparasi district. A significant negative correlation is given for Fe whereas
the positive correlation for As is weaker.
Regarding the removal efficiency concerning As by the widely in Nawalparasi installed Kanchan filters, it has to be stated clearly that geological factors like high As concentration, combined with low Fe concentration, high temperature of the source water as well as an unfortunate pH influence the mode of operation of the filters considerably. From previous work [42,28,29] it is known that Fe and P are crucial elements in terms of the As elimination process in the in the iron-assisted bio-sand filters based on Zero-Valent Iron (ZVI) used throughout the Terai. The total iron in the ground water over time is strongly correlated with the arsenic elimination efficiency of these filters [43]. As reported by Mueller and Hug, P is only a minor constituent of the ground water of Nawalparasi [15]. Phosphate is well known as a powerful competitor of arsenic for sorption and incorporation on mineral surfaces. The apparent decoupling between Fe and As restricts the performance of the As eliminating ironassisted bio-sand filters installed in the district Nawalparasi. The unsatisfying elimination of arsenic is controlled by the low quantity of iron released to ground water from sediments as well as with high pH and high temperature.
Acknowledgement
My sincere thanks for support and valuable discussions are expressed to Stephan Hug (Eawag), Tommy Ngai and Candice Young-Rojanschi from CAWST, Calgary, Canada; Bipin Dangol and Hari Boudhatoki from ENPHO, Kathmandu, Nepal; Gyan Prakash Yadav, Parasi and to Som Rai, my loyal expedition and trekking guide in Nepal and responsible for all logistics over many years. I thank Thomas Ruettimann, Eawag, for analysis of the samples by ICP-MS. This research was supported by various private foundations from Switzerland.
References
- Adhikari HJ, Ghimire TR. Prevalence of arsenicosis in Ramgram municipality, Nawalparasi, Nepal. Int J Health Res. 2009; 2: 183-188.
- Thakur JK, Thakur KR, Ramanathan A, Kumar M, Singh SK. Arsenic contamination of Groundwater in Nepal - An overview. Water. 2011; 3: 1-20.
- Shrestha RK, Regmi D, Kafle BP. Seasonal variation of arsenic concentration in groundwater of Nawalparasi district of Nepal. Int J Appl Sci Biotechnol. 2014; 2: 59-63.
- Shrestha RR, Shrestha MP, Upadhyay NP, Pradhan R, Khadka R, Maskey A, et al. Groundwater arsenic contamination in Nepal: A new challenge for water supply sector. Chappell WR, Abernathy CO, Calderon RL, Thomas DJ, editors. In: Arsenic Exposure and Health Effects. Elsevier BV. 2003; 25-37.
- Acharyya SK, Lahiri S, Raymahashay BC, Bhowmik A. Arsenic toxicity of groundwater in parts of the Bengal basin in India and Bangladesh: The role of Quaternary stratigraphy and Holocene sea-level fluctuation. Environ Geo. 2000; 39: 1127-1137.
- Gurung JK, Ishiga H, Khadka M. Geological and geochemical examination of arsenic contamination in groundwater in the Holocene Terai Basin, Nepal. Environ Geol. 2005; 49: 98-113.
- Guillot S, Charlet L. Bengal arsenic, an archive of Himalaya orogeny and paleohydrology. J Environ Sci Health A. 2007; 42: 1785-1794.
- Guillot S, Garçon M, Weinman B, Gajurel A, Tisserand D, France-Lanord C, et al. Origin of arsenic in Late Pleistocene to Holocene sediments in the Nawalparasi district (Terai, Nepal). Environ Earth Sci. 2015.
- Mueller B. Arsenic in groundwater in the southern lowlands of Nepal and its mitigation options: A review. Environ Rev. 2017; 25: 296-305.
- Mueller B. Preliminary trace element analysis of arsenic in Nepalese groundwater may pinpoint its origin. Environ Earth Sci. 2018; 77: 35-40.
- France-Lanord C, Derry L, Michard A. Evolution of the Himalaya since Miocene time: isotopic and sedimentologic evidence from the Bengal fan. Himalayan tectonics. Treloar, PJ, Searle M, editors. In: Geol. London Soc Spec Publ. 1993; 74: 445-465.
- Garzanti E, Vezzoli G, Ando S, France-Lanord C, Singh SK, Foster G. Sand Petrology and focused erosion in collision orogens: the Brahmaputra case. Earth Plan Sci Lett. 2004; 220: 157-174.
- Amini M, Abbaspour KC, Berg M, Winkel L, Hug SJ, Hoehn E, et al. Statisctical Modeling of Global Geogenic Arsenic Contamination in Groundwater. Environ Sci Technol. 2008; 42: 3669-3675.
- Diwakar J, Johnston SG, Burton ED, Shrestha SD. Arsenic mobilization in an alluvial aquifer of the Terai region, Nepal. J Hydrol Regional Studies. 2015; 4: 59-79.
- Mueller B, Hug SJ. Climatic variations and de-coupling between arsenic and iron in arsenic contaminated ground water in the lowlands of Nepal. Chemosphere. 2018; 210: 347-358.
- Bhattacharya P, Tandukar N, Neku A, Valero AA, Mukherjee AB, Jacks G. Geogenic arsenic in groundwaters from Terai Alluvial Plain of Nepal. J Phys IV France. 2003; 107: 173-176
- Dowling CB, Poreda RJ, Basu AR, Peters SL, Aggrawal PK. Geochemical study of arsenic release mechanisms in the Bengal Basin groundwater. Water Air Soil Pollut. 2002; 38: 1173.
- van Geen A, Zheng Y, Cheng Z, Aziz Z, Horneman A, Dhar RK, et al. A transect of groundwater and sediment properties in Araihazar, Bangladesh: further evidence of decoupling between As and Fe mobilization. Chem Geol. 2006; 228: 85-96.
- Stanger G. A palaeo-hydrogeological model for arsenic contamination in southern and south-east Asia. Environ Geochem Healt. 2005; 27: 359-367.
- Chakraborty S, Wolthers M, Chatterjee D, Charlet L. Adsorption of arsenite and arsenate onto muscovite and biotite mica. J Colloid Interface Sci. 2007; 309: 392-401.
- Uddin MK. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J. 2017; 308: 438-462.
- British Geological Survey (BGS) Report. Groundwater Quality: Nepal. 2001; 4.
- Yadav IC, Devi NL, Sing S. Reductive dissolution of iron-oxyhydroxides directs groundwater arsenic mobilization in the upstream of Ganges River basin, Nepal. J Geochem Explor. 2015; 148: 150-160.
- Sharma CK. Shallow (phreatic) aquifers of Nepal. 1st edn. Kathmandu: Sangeeta Publishing. 1995.
- Hagen T. Report on geological survey of Nepal Preliminary Reconnaissance. Memoires de la Soc. Helvetique des Sci. Naturekkes, Zurich, Switzerland. 1969.
- Shrestha SD, Brikowski T, Smith L, Shei TC. Grain size constraints on arsenic concentration in shallow wells of Nawalparasi, Nepal. J Nepal Geol Soc. 2004; 30: 93-98.
- Brikowski TH, Smith LS, Shei TC, Shrestha SD. Correlation of electrical resistivity and groundwater arsenic concentration, Nawalparasi, Nepal. J Nepal Geol Soc. 2004; 30: 99-106.
- Ngai TKK, Murcott SE, Shrestha RR, Dangol B, Maharjan M. Development and dissemination of KanchanTM arsenic filter in rural Nepal. Water Sci Technol. 2006; 6: 137-146.
- Ngai TKK, Shrestha RR, Dangol B, Maharjan M, Murcott SE. Design for sustainable development-Household drinking water filter for arsenic and pathogen treatment in Nepal. J Environ Sci Health-A Tox Hazard Subst Environ Eng. 2007; 42: 1879-1888.
- Farrell J, Wang J, O’Day P, Conklin M. Electrochemical and spectroscopic study of arsenate removal from water using zero-valent iron media. Environ Sci Technol. 2001; 35: 2026-2032.
- Berg M, Trang PTK, Stengel C, Buschmann J, Viet PH, Dan NV, Giger W, Stüben D. Hydrological and sedimentary controls leading to arsenic contamination of groundwater in the Hanoi area, Vietnam: The impact of iron-arsenic ratios, peat, river bank deposits, and excessive groundwater abstraction. Chem Geol. 2008; 249: 91-112.
- Ahmed F, Hawa Bibi M, Ishiga H, Fukushima T, Maruoka T. Geochemical study of arsenic and other trace elements in groundwater and sediments of the Old Brahmaputra River Plain, Bangladesh. Environ Earth Sci. 2010; 60: 1303-1316.
- Rahman MM, Dong Z, Naidu R. Concentrations of arsenic and other elements in groundwater of Bangladesh and West Bengal, India: Potential cancer risk. Chemosphere. 2015; 139: 54-64.
- Nickson RT, McArthur JM, Ravenscroft P, Burgess WG, Ahmed KM. Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem. 2000; 15: 403-413.
- Bhattacharya P, Jacks G, Ahmed KM, Khan AA, Routh J. Arsenic in groundwater of the Bengal delta plain aquifers in Bangladesh. Bull. Environ Contam Toxicol. 2002; 69: 538-545.
- Smedley PK, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem. 2002; 17: 517-568.
- McArthur JM, Ravenscroft P. Safiullah S, Thirlwal MF. Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour Res. 2001; 37: 109-117.
- Horneman A, van Geen A, Kent D, Mathe PE, Zheng,Y. Dhar RK, et al. Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions Part I: evidence from sediment profiles. Geochim Cosmochim Acta. 2004; 68: 3459-3473.
- Bénézeth P, Dandurand JL, Harrichoury CJ. Solubility product of siderite (FeCO3) as a function of temperature (25-250°C). Chem Geol. 2009; 265: 3-12.
- Dixit S, Hering JG. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implication for arsenic mobility. Environ Sci Technol. 2003; 37: 4182-4189.
- Brikowski TH, Neku A, Shrestha SD, Smith LS. Hydrologic control of temporal variability in groundwater arsenic on the Ganges floodplain of Nepal. J Hydrol. 2014; 518: 342-353.
- Ngai TKK, Walewijk S. The Arsenic Bio Sand Filter (ABF) Project: Design of an Appropriate Household Drinking Water Filter for Rural Nepal. Report Prepared for RWSSSP and ENPHO, Kathmandu, Nepal. 2003.
- Wenk C, Kaegi R, Hug SJ. Factors affecting arsenic and uranium removal with zero-valent iron: laboratory tests with Kanchan-type iron nail filter columns with different groundwaters. Environ Chem. 2014; 11: 547-557.