
Research Article
Austin Chem Eng. 2020; 7(2): 1076.
Effect of Pressure on In-situ Catalytic Hydropyrolysis of Rice Straw
Naidu VS*, Kumar P*, Karri N, Singh PK, Gandham S and Rao BR
Fluid Catalytic Cracking Division, Hindustan Petroleum Green Research & Development Centre, India
*Corresponding author: V Satyam Naidu, Fluid Catalytic Cracking Division, Hindustan Petroleum Green Research & Development Centre, India; Pramod Kumar, India
Received: June 01, 2020; Accepted: July 02, 2020; Published: July 09, 2020
Abstract
Pyrolysis of rice straw was investigated to study deoxygenation of biooil vapours with various parameters such as pressure, gas environment, and catalyst. Pyrolysis and hydropyrolysis experiments were performed at a temperature of 500oC and pressures of 1, 5 and 10 bar in a fluidized bed reactor under N2 and mixture of N2 and H2 environments. It was observed that the biooil yield increased from 28 to 42 wt.% and the bio char yield decreased from 43 to 33 wt.% with increase in pressure from 1 to 10 bar during hydropyrolysis in the presence of ZSM-5 catalyst. Gas analysis showed that carbonylation and decarboxylation were the major pathways for deoxygenation of pyrolysis vapours using ZSM-5and lighter hydrocarbons up to 13 wt.% were obtained under catalytic hydropyrolysis. The detailed analysis of carbon balance and oxygen balance was carried out to evaluate carbon efficiency and degree of deoxygenation of bio-oil vapours.
Keywords: In-situcatalytic pyrolysis; Hydropyrolysis; Deoxygenation; ZSM- 5; Pressure effect; Rice straw
Introduction
The increase in demand for energy and huge dependence on fossil fuels create environment pollution such as greenhouse gas emissions, particulates formation etc. One of the main sources of alternate energy to replace fossil fuels is by thermo chemical conversion of biomass via gasification, pyrolysis, hydrothermal liquefaction routes to produce gaseous, liquid and solid char as fuels [1,2]. Pyrolysis of biomass is widely used technique to produce bio-oil which can replace petroleum products [3]. Lignocelluloses biomasses are widely used for the production of bio fuels, chemicals, etc.
As per the statistics of International Rice Research Institute (IRRI), rice is major crop in the Asian countries in which India being the second largest consumer with 97.35 million metric tons of rice consumption annually. Each kg of milled rice produces 0.7 to 1.4 kg of rice straw depending on the variety of rice crop, stubble cutting and moisture content during harvest. Rice straw is basically a by-product of rice when harvesting paddy and is abundantly available from the agro fields that can also be utilized to produce bio oils pyrolysis [4- 10]. In the Indian context, stubble burning by farmers of Haryana, Punjab, Uttar Pradesh and Delhi is a major contributor to the smogsoaked winters. Therefore, one of the objectives of the present study is to convert rice straw biomass to liquid fuels in order to reduce the particulates, pollutants and gas emissions into the atmosphere.
Various stages of pyrolysis kinetics involving drying, devolatilization and hydropyrolysis etc are not extensively studied in the literature [11,12]. It is widely known that higher bio-oil yield can be achieved from fluidized bed reactors [13-17]. Maximum biooil yield of 54 to 60 wt.% is obtained under fluidizing and spouted bed conditions [13,14]. Iisa et al. demonstrated that organic bio-oils with wide range of oxygen contents can be obtained by Catalytic Fast Pyrolysis (CFP), however, leaving more oxygen leads to better carbon efficiency and economics [18].
The pyrolysis techniques are classified as in-situ and ex-situ mode depending on the catalyst utilization in the pyrolysis reactor. These are defined as follows: Biomass pyrolysis and up gradation occur in the single reactor during fluidization is called in-situ catalytic pyrolysis whereas pyrolysis occurs in the first reactor and upgradation happens in the second reactor is called as ex-situ catalytic pyrolysis [18]. Recent studies on in-situ and ex-situ catalytic pyrolysis in the presence of zeolite catalysts demonstrated enhancement in the aromatic hydrocarbons [19-21]. Although ex-situ catalytic pyrolysis had inherent advantages in terms of low-oxygen content, high catalyst stability, in-situ catalytic pyrolysis is superior for high biooil yield, carbon retaining capacity with similar minimum fuel selling price in the range of $1.1 per litre [22,23]. Gamliel et al. compared in-situ and ex-situ Catalytic Fast Pyrolysis (CFP) bio-oil produced in PyGC analyser composition and reported that ex situ CFP more accurately predicts the molecular composition with spouted bed reactor [24]. Nolte et al. reported MoO3 was effective catalyst to produce hydrocarbons at higher yields in in-situ mode consisting of linear alkanes and aromatics in comparison to ex-situ HDO of bio-oil [25-26].
Zeolite catalysts have been extensively used for biomass catalytic pyrolysis with different silica-to-alumina ratio such as ZSM-5, H-beta, Y-Zeolite, USY, MCM-41 etc. to improve the organic bio-oil yield and quality [28-32]. However, the organic bio-oil yield has never exceeded the amount higher than organic bio-oil produced under thermal conditions and is the maximum yield produced from catalytic pyrolysis for fuel applications [33]. Two-stage zeolite catalysts such as ZSM 5 (micro pore catalyst) and MCM-41 (mesoporous catalyst) have also been used to produce bio-oil with approximately 77 % of favourable fractions, water content up to 42%, TAN of 43 mg-KOH/g and high gasoline range chemicals up to 98% were obtained [34,35].
Hydropyrolysis is evolved as an emerging technology to produce bio-oil in the presence of catalysts for the pressure range from 1 to 52 bar of H2 in an autoclave reactor [36]. Various noble metal catalysts on carbide and Al2O3 supports have been screened for hydropyrolysis and hydrodeoxygenation of bio-oil [37,38]. The rate of deoxygenation can be improved via hydrogenation with increase in H2 pressure over Ni/ZSM-5 [39]. Recent studies conducted on hydropyrolysis in the pilot-scale plants under fluidizing conditions to produce gasoline and diesel range fuels in two-stage processes yielded less than 1 wt.% oxygen at 22 to 25 bar [40-42]. Hydropyrolysis experiments are also performed in a single-stage process at 20.7 bar and produced lowoxygenate bio-oil with oxygen content up to 5 wt.% [43-45]. Although hydropyroysis experiments were performed atthe pilot scale level, the effect of pressure on bench scale and pilot scale experiments are very scarce.
The present work is focused on studying the effect of pressure on slow pyrolysis and hydropyrolysis for rice straw feedstock at 1, 5 and 10 bar at a reaction temperature of 500oC. Catalytic pyrolysis and hydropyrolysis experiments were performed using ZSM-5 catalyst to study the deoxygenation efficiency of bio-oil. The detailed analysis of bio-oils, biochar and Non Condensable Gases (NCG) is performed to determine the carbon efficiency and oxygen distribution in each of the products.
Experimental Section
Materials and characterization methods
Rice straw feedstock was procured from local agro fields in the Bangalore city in India. Rice straw was sun-dried before reducing its size to 2 to 5mm using a crusher. These particles were further reduced to 100 μm to 1000μm using a grinding mill. The crushed and ground biomass was sieved to obtain a size range of +300-700μ particles for the experiments. The reduced particles size was needed to avoid diffusion limitations and to improve the rate of heat transfer during pyrolysis under fluidizing conditions. Proximate and ultimate analysis were performed using sophisticated analytical instruments (CHNS analyzer and TGA) as per ASTM standards and the results are shown in Table 1. Commercial ZSM-5was used as catalyst in some experiments to study the deoxygenation mechanism of pyrolysis vapours under in-situ pyrolysis conditions. Catalyst was sieved to obtain a size range of +106-212μm particles in order to have proper mixing with biomass particles under fluidizing conditions.ZSM-5 catalyst was characterized for BET surface area, pore volume and acidity of the catalyst. The catalyst showed low surface area with 119m2/g and high micro pore surface area with more of weak acid sites as shown in Table 2 [46]. XRF analysis was performed to determine the metal composition of ZSM-5 catalyst as shown in Table 3 [46].
M
VM
Ash
FC
C
H
N
O*
9.27
58.47
17.93
14.33
32.8
5.2
3.4
40.67
Table 1: Schematic of fluidized bed batch reactor for in-situ pyrolysis experiments.
Catalyst
Surface area (m2/g)
Pore volume distribution (cm3/g)
Acid density (mmol/g)
SBET
Smic
Sext
Vmic
Vmeso
Vp
Weak acid sites
Strong acid sites
Total
ZSM-5
119
80.86
38.15
0.032
0.04
0.08
1.04
0.08
1.12
Table 2: Characterization of ZSM-5catalyst.
Catalyst
SiO2 (%)
Al2O3 (%)
P2O5 (%)
TiO2 (%)
ZSM-5
56.6
20.1
8.46
1.18
Table 3: XRF analysis of zeolite catalyst.
Experimental set-up
A lab-scale semi-batch reactor was set-up to perform pyrolysis experiments under fluidized bed conditions as shown in Figure 1. The reactor was made up of SS 316 with an inner diameter of 41 mm and length of 440 mm. A wire mesh of 50μm size was placed at both ends of the reactor between the two flanges to prevent the passage of catalyst or biochar particles either from the bottom or top of the reactor. The flanges were protruded in order to accommodate mesh and a gasket between the reactor and flange so that it arrests the leakage of gases or vapors under operation. The inlet gases, N2 and H2 were pre-heated to a temperature of 300oC before entering the reactor. The reactor was placed in a furnace having three heating zones. The temperature inside the reactor was measured using three thermocouples inserted into a thermo well from the top of the reactor and located at the top, middle and bottom zone of the reactor. Shell and tube type condenser was provided downstream of the reactor to condense the pyrolysis vapours and bio-oil was separated in twostage Gas-Liquid Separation (GLS) system. The pressure in the reactor was controlled by a Pressure Control Valve (PCV) provided after first GLS. Chiller was operated at -5oC to condense the vapours in the condenser and gas-liquid separators. The chilling fluid was equal mixture of propylene glycol and water to operate chiller at sub-zero temperatures. The Non Condensable Gases (NCG) such as CO, CO2, CH4, C2-C5compoundswere passed through a two-stage scrubber system. Two-stage scrubber system was provided to remove acidic compounds or any trace amounts of light hydrocarbons present in the NCG. The evolution of gases during pyrolysis were measured using Wet Gas Meter (WGM).
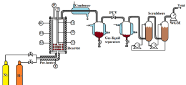
Figure 1: Schematic of fluidized bed batch reactor for in-situ pyrolysis
experiments.
Experimental procedure
Initially known quantity of biomass was placed in an oven at 105oC for three hours to remove moisture content. 50g of dried biomass was taken for both pyrolysis and hydropyrolysis experiments and the same amount of catalyst is used such that biomass-to-catalyst ratio one is maintained for deoxygenation of bio-oil vapours under in-situ mode in the case of catalytic pyrolysis. The reactor was operated at a temperature of 500oC based on optimum yields of bio-oil and bio char [5,13]. The reactor was heated from room temperature to 500oC with a heating rate of 10oC/min and was fluidized under slow pyrolysis conditions. The effect of pressure on pyrolysis reaction was studied at 1, 5, and 10 bar and the pressure was controlled by the PCV. Catalytic pyrolysis and hydropyrolysis experiments were performed using commercial zeolite catalyst for semi-batch fluidization. Noncatalytic and catalytic pyrolysis experiments were conducted under identical conditions with same amounts of biomass and gas flow rates. Alumina balls were placed at the bottom of the reactor to provide sufficient heat to the biomass during pyrolysis. Biomass and catalyst were mixed thoroughly before placing in to the reactor. Nitrogen was used for pyrolysis experiments, hydrogen and nitrogen in equal proportion were used for hydropyrolysis experiments. A fixed gas flow rate of 240 Standard Liters Per Hour (SLPH) was used for all the experiments. The vapours generated during the catalytic pyrolysis undergoes upgradation/cracking in the reactor simultaneously. The vapours generated in the reactor were passed through a condenser and in series of two gas-liquid separators to separate bio-oil from the vapours. The non-condensable gases were passed through scrubbers to further separate acidic components in the bio-oil. The evolved gases were measured using WGM for quantification before venting to the atmosphere. The amount of gas generated during pyrolysis was determined by subtracting the total amount of gas processed through the WGM and inlet carrier gas supplied using mass flow controller. A three-way valve was mounted after WGM for gas samples collection and the rest for venting to the off-gas streams. A part of the gas in the temperature range from 400 to 500oC was collected at regular intervals with Tedlar gas bags for analysis.
Product characterization
The pyrolysis products comprised of bio-oil (organic and aqueous phases), bio char and Non-Condensable Gases (NCG). The bio-oil was collected after sufficiently cooled down to room temperature from gas-liquid separators and was mostly aqueous phase in nature. The organic bio oil was stuck on to the walls of the SS tubes during the process of cooling and was flushed with acetone to quantify organic bio-oil. Acetone was recovered from the organic bio-oil by a rotary evaporator based on boiling point difference. Any trace amount of organic bio-oil in the aqueous phase was separated by vacuum suction. Bio char yield was measured after the experiment by separating alumina balls from bio char using sieving analysis for noncatalytic experiments. In the case of catalytic pyrolysis experiments, char yield was calculated by subtracting the pre-weighted catalyst amount from the mixture of bio char and alumina balls. Biochar, organic and aqueous bio-oils were characterized for oxygen content using CHNS analyzer (LECO CHNS-932 Elementary Chemical Analyzer) and oxygen analyzer. Gas analysis was performed using Rapid Gas Analyzer (RGA) as per standard UOP 539 to determine the evolved gases (CO, CO2, H2, CH4) and lighter hydrocarbons (C1-C3) during pyrolysis and hydropyrolysis experiments immediately.
Results and Discussion
Mass balance was performed for bio-oil including organic and aqueous phases, char and NCGs for all the pyrolysis experiments. The recovery of products (bio-oil, char and NCGs) in the range from 91% to 98.5% was observed. The bio-oil recovery was slightly low for noncatalytic experiments and high for catalytic experiments. The low recovery in the non-catalytic experiments may be due to high viscous nature of the bio-oil. In the section, the effect of gas environment (N2 and N2+H2), zeolite catalyst and pressure were discussed on biomass pyrolysis products. The detailed analysis for the effect of these three parameters on the pyrolysis products were illustrated below:
Effect of gas environment
The fluidizing gas for pyrolysis experiments was nitrogen and equal proportion of nitrogen and hydrogen was used as fluidizing gas for hydropyrolysis experiments. The total bio-oil content up to 31.5 wt.% was observed at 1 bar for pyrolysis and is increased to 42 wt.% at 5 bar as shown in Figure 2. Similar observations were made for hydropyorlysis as shown in Figure 3. The total bio-oil yield was higher for hydropyrolysis than pyrolysis due to water formation. The organic bio-oil yield was slightly lower for hydropyrolysis than pyrolysis due to the hydrodeoxygenation of bio-oil resulting in more of aqueous bio-oil. The amount of bio char was varied in the range from 40 to 43 wt.% in the case of pyrolysis and was decreased up to 34 wt.% for hydropyrolysis. The decrease in char may also be due to conversion of carbon in the char into methane formation. The detailed gaseous species evolved in each experiment were reported in the Table 5. It was also clear from the gas analysis that formation of CO2 was predominant up to 73% in the case of pyrolysis and CO up to 33 % and lighter components up to 10%were observed in hydropyrolysis.
Pressure (bar)
51
5
10
Gas/Catalyst
Component
None
ZSM-5
None
ZSM-5
None
ZSM-5
N2
CH4
5.61
5.37
4.25
0
6.76
3.27
C2H6
2.43
0
0
0
4.22
2.16
C2H4
0
0
0
0
0
1.44
C3H8
0
0
0
0
1.85
0
C3H6
0
2.51
0
0
1.38
1.8
CO
24.28
27.76
24.62
27.41
17.89
22.29
CO2
67.68
64.36
70.95
72.47
67.75
69.05
N2+H2
CH4
1.22
3.46
4.65
6.17
2.8
6.5
C2H6
0.813
3.7
3.33
2.78
1.8
3.96
C2H4
0
2.53
0
0
0.633
2.66
C3H8
0
1.25
0
0
0.804
0
C3H6
0
2.24
0
2.24
0.864
3.76
CO
45.34
42.82
34.25
26.79
21.76
0
CO2
52.63
45.65
54.87
62.02
70.55
83.12
Table 5: Non-Condensable Gas (NCG) analysis (wt.%).

Figure 2: Effect of pressure on biomass products yield for a) non-catalytic and b) catalytic pyrolysis.
Effect of pressure
The effect of pressure on bio-oil yield was studied in the pressure range from 1 to 10 bar. As shown in Figure 2, it was observed that bio-oil yield increased from 31.5 wt.% to 39 wt.% with increase in pressure from 1 to 10 bar under non-catalytic pyrolysis conditions. The bio-oil was further separated into organic and aqueous phases where organic bio-oil decreased from 13.6 wt.% to 10 wt.% and aqueous bio-oil increased from 17.9 wt.% to 28.7 wt.% with increase in pressure. There was a linear decrease in oxygen content in organic bio-oil which showed that deoxygenation effect was prevalent in the presence of catalyst with increase in pressure as shown in Figure 2. However, increase in oxygen content of aqueous bio-oil was due to the formation of more of water via dehydrogenation. Bio char formation in the range from 40.4 to 43.4 wt.% was observed with change in pressure and Non Condensable Gas (NCG) formation was decreased with increase in pressure from 22.3 wt.% to 18.1 wt.%. The major gas components and lighter hydrocarbons include CH4, C2H6, C3H6, C3H4 and CO, CO2 formation via decarbonylation, decarboxylation as shown in Table 5. Similar experiments were performed for hydropyrolysis with equal proportion of N2 and H2 gas mixture under identical conditions.In the hydropyrolysis, bio-oil yield was increased to 40 wt.% at 5 bar and there was insignificant variation in the biooil generation with further increase in pressure to10 bar as shown in Figure 3 for non-catalytic and catalytic conditions, respectively. There was an increase in organic bio-oil and proportional to increase in pressure for hydropyrolysis as shown in Figure 4. Organic bio-oil yield increased by 2 to 3 wt.% in the presence of catalytic hydropyrolysis at 10 bar compared to normal or catalytic pyrolysis under atmospheric conditions. The experimental observations were compared for the difference in bio-oil yield of catalytic slow pyrolysis and catalytic slow hydropyrolysis and showed good agreement for the increased organic bio-oil yield by 2.5 wt.% at a pressure of 35 bar in the presence of catalytic fast hydropyrolysis [47]. The increase in the pressure has aggravated the formation of aqueous bio-oil up to 5 bar and remains constant with further increase in pressure as shown in Figure 4. Similar observations are made for bio-oil where organic bio-oil decreased and aqueous bio-oil increased with increase in pressure by Chen et al. [48]. There was a slight change in the biochar formation with variation of 1 to 2 wt.% for hydropyrolysis conditions. The formation of NCGs were higher at 1 bar compared to 5 and 10 bar as shown in Figure 2 and Figure 3. The formation of methane was favoured with increase in pressure as shown in Table 5.

Figure 3: Effect of pressure on biomass products yield for a) non-catalytic and b) catalytic hydropyrolysis.

Figure 4: Effect of pressure on a) organic and b) aqueous bio-oil yield for pyrolysis and hydropyrolysis.
Effect of catalyst
The effect of ZSM-5 catalyst was studied for pyrolysis and hydropyrolysis conditions at 1, 5 and 10 bar as shown in Figure 2 and Figure 3. Under catalytic pyrolysis, organic bio-oil decreased as like pyrolysis from 14.3 wt.% to 9 wt.% and the bio char content was decreased from 40% to 33 wt.% with increase in pressure from 1 bar to 10 bar as shown in Figure 2. There was a significant increase in gas formation up to 27 wt.% under catalytic pyrolysis which was 5 to 9% rise in comparison to pyrolysis conditions. Under hydropyrolysis catalytic conditions, the formation of aqueous bio-oil increased in comparison to normal pyrolysis, however, organic bio-oil formation decreased with increase in pressure as shown in Figure 3. Catalyst also played a significant role in the deoxygenation of bio-oil up to 18.6 wt.% and conversion of bio char to CH4, CO, and CO2 where char formation of gases decreases by 3 to 5 wt.% in comparison to normal pyrolysis conditions as shown in Figure 3. Gas analysis shows that catalyst also had more influence on decarbonylation over decarboxylation of bio-oil to form CO and CO2 as given in Table 5.
CHN Analysis
The elemental analysis for organic bio-oil is given in Table 4. The effect of pressure depicts that carbon content increased from 64.5 wt.% to 72.1 wt.% and hydrogen content from 8.2 to 9.8 wt.% in organic bio-oil with increase in pressure from 1 bar to 10 bar for pyrolysis conditions as shown in Table 4. The carbon and hydrogen contents were further increased by 1 to 4 wt.% in the presence of ZSM-5 catalyst. Under hydropyrolysis conditions, the increase in carbon content was predominant up to 5 bar and decreased for 10 bar which is due to the formation of more of CO2 decarboxylation as is evident in Table 5. The decrease in hydrogen content is due to water formation via dehydration.
Pressure (bar)
1
5
10
Gas
Element/Catalyst
None
ZSM-5
None
ZSM-5
None
ZSM-5
N2
C
64.5
65.2
64.1
68.3
72.1
73.7
H
8.2
8.4
9.2
9.5
9.8
6.4
N
1.6
1.8
0.6
1.1
1.4
-
N2+H2
C
65.9
61.7
67.1
70.6
66.2
59.7
H
8.5
9.8
9.5
8.7
8.5
7.2
N
1.7
0.8
1.5
1.7
1.6
-
Table 4: CHN analysis for organic bio-oil.
GC-MS Analysis
The variation in the chemical composition of bio-oil for pressure at 1 and 10 bar, change in gas environment, and catalytic effects were monitored by GC-MS analysis as shown in Figure 5. The detailed analysis of bio-oil shows that formation of acetic acid, hexanioc acid, 2-pentanone and drastic increase in phenol derivatives from 12 % to 47.5% with increase in pressure from 1 to 10 bar. Under noncatalytic hydropyrolysis conditions, acids and ketones are converted to phenols and are increased from 54.5 % to 70% with increase in pressure from 1 to 10 bars. In the case of catalytic pyrolysis, acids, ketones and alcohols were converted to more of phenols and aromatics (12.5%). Catalytic hydropyrolysis data showed that ketones were decreased from 26% to 13% and phenols were increased from 48% to 60% along with 15% of aromatics formation. Phenols and aromatics were favoured under catalytic pyrolysis and hydropyrolysis conditions by converting ketones and furans which are precursors for the preparation of chemicals.

Figure 5: Effect of pressure, gas environment and catalyst on composition of
bio-oil from GC-MS analysis.
Carbon balance
The effect of pressure on carbon yield and its comparison with literature have shown in Figure 6. The carbon yields in the range from 19 to 28 wt.% were obtained with increase in pressure in both pyrolysis and hydropyrolysis. The carbon yield decreased with increase in pressure under pyrolysis conditions, however, it increased slightly with pressure under hydropyrolysis conditions. The carbon yields were very well comparable with literature which were in the range from 20 to 28 wt.% under in-situ pyrolysis conditions [18,33,49].
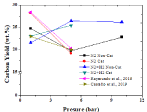
Figure 6: Effect of pressure on carbon yield of organic bio-oil under noncatalytic
and catalytic Hydropyrolysis and comparison with literature.
Oxygen balance
Mass balance calculations were done for oxygen distribution in organic, aqueous bio-oils, bio char and NCGs based on CHNS analysis. The distribution of oxygen in biomass pyrolysis products for pyrolysis and hydropyrolysis experiments under catalytic and non-catalytic conditions is shown in Figure 7 sequentially. In the case of non-catalytic pyrolysis, the oxygen content decreased in organic bio-oil from 9.4 wt.% to 4.7 wt.% and increased in aqueous bio-oil from 32.5 wt.% to 52.2 wt.% with increase in pressure up to 10 bar. The oxygen content decreased for bio char from 22.2 wt.% to 15.3 wt.% and for gases, it decreased from 34.6 wt.% to 26.4 wt.%. In the case of catalytic pyrolysis, oxygen content in the bio-oil decreased from 8.6 wt.% to 4.6 wt.% and for bio char decreased from 19.6 wt.% to 13.5 wt.%. The oxygen content in aqueous bio-oil decreased drastically and increased in gases with increase in pressure. Oxygen distribution in hydropyrolys is having different behaviour compared to pyrolysis conditions as depicted in Figure 7. In the case of non catalytic hydropyrolysis experiments, oxygen content was not varied widely in the organic bio-oil with increase in pressure, however, it increased in aqueous bio-oil with increase in pressure. The bio char content showed to decrease with increase in pressure resulting in more of methane formation. The effect of catalyst on hydropyrolys is showed better results where oxygen content decreased from 8.6 wt.% to 5.3 wt.% in organic bio-oil and increased in aqueous bio-oil from 38.5 wt.% to 51.2 wt.% with increase in pressure as shown in Figure 7. It can be concluded that the increase in pressure under catalytic conditions showed better deoxygenation efficiency compared to non-catalytic and pyrolysis conditions. The oxygen distribution was in good agreement with the yield of oxygen under in-situ catalytic conditions as reported in Iisa et al. [18] which were in the range from 7.7 to 8.2 wt.% in organic bio-oil and 45.5 wt.% to 49.4 wt.% in aqueous bio-oil.
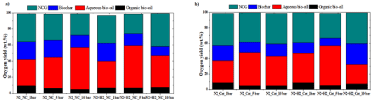
Figure 7: Effect of pressure on oxygen yield under a) non-catalytic pyrolysis and hydropyorlysis and b) catalytic pyrolysis and hydropyrolysis conditions.
Conclusions
In the present study, pyrolysis and hydropyrolysis of rice straw feedstock arestudied under catalytic and non-catalytic conditions. The study is focused on the influence of three parameters namely, effect of pressure, gas environment and ZSM-5 catalyst. The influence of these parameters on the pyrolysis products is summarized below:
1. The maximum bio-oil yield up to 42 wt.% is obtained with increase in pressure from 1 to 10 bar at 500oC under hydropyrolysis conditions and in the presence of ZSM-5 catalyst, 50% deoxygenation efficiency is noticed in the organic bio-oil with increase in pressure up to 10 bar under in-situ mode.
2. There was a 10-point wt.% decrease in bio char yield in the presence of catalytic hydropyrolysis at a pressure of 10 bar resulting in higher amounts of lighter hydrocarbons such as CH4, C2H4, C2H6 and C3H6 formation with increase in pressure up to 10 bar.
3. Decarbonylation, decarboxylation and dehydration are favoured in the presence of zeolite catalyst with CO, CO2 and more of aqueous bio-oil formation. The optimal composition of phenols and aromatics up to 60% and 15% were obtained under catalytic hydropyrolysis conditions, respectively.
Acknowledgment
The authors are thankful to the HP Green R&D Centre (HPGRDC) management for providing support and facilities to perform experiments.
References
- Asomaning J, Omidghane M, Chae,M, BresslerDC. Thermal processing of algal biomass for biofuel production. Current opinion in Green in Sustain Chem. 2016; 2: 1-5.
- Kan T, StrezovV, EvansTJ. Lignocellulosic biomass pyrolysis. A review of product properties and effects of pyrolysis parameters. Renew Sustain Energy Rev. 2016; 57: 126-1140.
- Perkin G, Bhaskar T, Konarova M. Process development status of fast pyrolysis technologies for the manufacture of renewable transport fuels from biomass Renew Sustain. Energy Rev. 2018; 90: 292-315.
- Zhang H, Xiao R, Jin B, Shen D, Chen R, Xiao G. Catalytic fast pyrolysis of straw biomass in an internally interconnected fluidized bed to produce aromatics and olefins Effect of differentcatalysts Bioresour Tehnol. 2013; 137: 82-7.
- LeeY, Eum PRB, Ryu C, Park YK, Jung JH, Hyun S. Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1 Bioresour Technol. 2013; 130: 345-50.
- Biswas B, Singh R, Kumar J, Singh R, Gupta P, Krishna BB, et al. Pyrolysis behavior of rice straw under carbon dioxide for production of bio-oil. Renewable Energy. 2018; 129: 686-94.
- Tsai WT, Lee MK, Chang YM. Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal Appl. Pyrol. 2006; 76: 230-37.
- Worasuwannarak N, Sonobe T, Tanthapanichakoon W. Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique. J. Anal Appl Pyrol. 2007; 78: 265-71.
- Putun AE, Apaydin E, Putun E. Rice straw as a bio-oil source via pyrolysis and steam pyrolysis. Energy 2004; 29: 2171-80.
- Kai X , Li R, Yang T, Shen S, Ji Q, Zhang T. Study on the co-pyrolysis of rice straw and high-density polyethylene blends using TG-FTIR-MS. Energy Conv. Manage. 2017; 146: 20-33.
- Chen D, Zhang Y, Zhu X. Drying kinetics of rice straw under isothermal and nonisothermal conditions. A comparative study by thermogravimetric analysis. Energy fuels. 2012; 26: 4189-94.
- Waheed QMK, Williams PT. Hydrogen production from high temperature pyrolysis/steam reforming of waste biomass. Rice husk, sugar cane bagasse and wheat straw. Energy fuels. 2013; 27: 6695-704.
- Jung SH, Kang BS, KimJS. Production of bio-oil from rice straw and bamboo sawdust under various reaction conditions in a fast pyrolysis plant equipped with a fluidized bed and a char separation system. J. Anal Appl Pyrol. 2008; 82: 240-7.
- Pattiya A, Suttibak S. Influence of a glass wool hot vapour filter on yields and properties of bio-oil derived from rapid pyrolysis of paddy residues. Bioresour Technol. 2012; 116: 107-13.
- Guedes RE, Luna AS, Torres AR. Operating parameters for bio-oil production in biomass pyrolysis. A review. J Anal Appl Pyrol. 2018; 129: 134-49.
- Eom IY, Kim JY, Lee SM, Cho TS, Yeo H, Choi J W. Comparison of pyrolytic products produced from inorganic-rich and demineralized rice straw by fluidized bed pyrolyzer for future biorefinery approach. Bioresour. Technol. 2013; 128: 664-72.
- Nam H, Capareda SC, Ashwath N, Kongkasawan J. Experimental investigation of pyrolysis of rice straw using bench-scale auger, batch and fluidized bed reactors. Energy. 2015; 93: 2384-94.
- Iisa K, French RJ, Orton KA, Yung MM, Johnson DK, Ten Dam J, et al. In situ and ex situ catalytic pyrolysis of pine in a bench-scale fluidized bed reactor. J Anal Appl Pyrol. 2016; 30: 2144-57.
- Shafaghat H, Rezaei PS, Ro D, Jae J, Kim BS, Jung SC , et al. In-situ catalytic pyrolysis of lignin in a bench-scale fixed bed pyrolyzer. J Ind Eng. Chem. 2017; 54: 447-53.
- Lee HW, Kim YM, Jae J, Sung BH, Jung SC, Kim SC, et al. Catalytic pyrolysis of lignin using a two-stage fixed bed reactor comprised of in-situ natural zeolite and ex-situ HZSM-5. J Anal Appl Pyrol. 2016; 122: 282-8.
- Wise HG, Dichiara AB, Resende FLP. Ex-situ catalytic fast pyrolysis of Beetlekilled lodgepole pine in a novel ablative reactor. Fuel. 2019; 241: 933-40.
- Li B, Ou L, Dang Q, Meyer P, Jones S, Brown R, Wright M. Techno-economic and uncertainty analysis of in situ and ex situ fast pyrolysis for biofuel production. Bioresour. Technol. 2015; 196: 49-56.
- Dutta A, Schaidle JA, Humbird D, Baddour FG,S ahir A. Conceptual process design and techno-economic assessment of ex situ catalytic fast pyrolysis of biomass. A fixed bed reactor implementation scenario for future feasibility. Top Catal. 2016; 59: 2-18.
- Gamliel DP, Du S, Bollas GM, Valla JA. Investigation of in situ and ex situ catalytic pyrolysis of miscunthus xgiganteus using a PyGC-MS microsystem and comparison with a bench-scale spouted-bed reactor. Bioresour. Technol. 2015; 191: 187-96.
- Nolte MW, Zhang J, Shanks BH. Ex situ hydrodeoxygenation in biomass pyrolysis using molybdenum oxide and low pressure hydrogen. Green Chem. 2016; 18: 134-8.
- Murugappan K, Mukarakate C, Budhi S, Shetty M, Nimlos MR, Roman- Leshkov Y. Supported molybdenum oxides as effective catalysts for the catalytic fast pyrolysis of lignocellulosic biomass. Green Chem. 2016; 18: 5548-57.
- Iisa K, French RJ, Orto K, Dutta A, Schaidle JA. Production of low-oxygen bio-oil via ex situ catalytic fast pyrolysis and hydrotreating. Fuel. 2017; 207: 413-422.
- Wang L, Lei H, Bu Q, Ren S, Wei Y, Zhu L, et al . Aromatic hydrocarbons production form ex situ catalysis of pyrolysis vapor over zinc modified ZSM-5 in a packed-bed catalysis coupled with microwave pyrolysis reactor. Fuel . 2014; 129: 78-85.
- Li Y, Li B, Zhang X. Continuous pyrolysis and catalytic upgrading of corncob hydrolysis residue in the combined system of auger reactor and downstream fixed-bed reactor. Energy Conv Manage. 2016; 122: 1-9.
- Kim YM , Jae J, Lee WH, Han TU, Lee H, Park SH, et al. Ex-situ catalytic pyrolysis of citrus fruit peels over mesoporous MFI and Al-MCM-41. Energy Conv. Manage. 2016; 125: 277-289.
- Xie W, Liang J, MorganJr HM, Zhang X, Wang K, Mao H, et al. Ex-situ catalytic microwave pyrolysis of lignin over Co/ZSM-5 to upgrade to bio-oil. J Anal Appl. Pyrol. 2018; 132: 163-170.
- Chen H, Cheng H, Zhou F, Chen K, Qiao K, Lu X, et al. Catalytic fast pyrolysis of rice straw to aromatic compounds over hierarchical HZSM-5 produced by alkali treatment and metal-modification J Anal Appl Pyrol z. 2018; 131: 76-84.
- Castello D, He S, Ruiz MP, Westerhof JM, Heeres HJ, Seshan K, et al. Is it possible to increase the oil yield of catalytic pyrolysis of biomass. A study using commercially-available acid and basic catalysts in ex-situ and in-situ modus. J Anal Appl. Pyrol. 2019; 137: 77-85.
- Kim BS, Jeong CS, Kim JM, Park SB, Park SH, Jeon JK, et al. Ex situ catalytic upgrading of lignocellulosic biomass components over vanadium contacted H-MCM-41 catalysts. Catalysis Today. 2016; 265: 184-91.
- Ratnasari DK, Yang W, Jonsson PG. Two-stage ex-situ catalytic pyrolysis of lignocellulose for the production of gasoline-range chemicals. J Anal. Appl. Pyrol. 2018; 134: 454-64.
- Balagurumurthy B, Srivastava V, Vini T, Kumar J, Biswas B, Singh R, et al. Value addition to rice straw through pyrolysis in hydrogen and nitrogen environments. Bioresour. Technol. 2015; 188: 273-9.
- Chang Z, Duan P, Xu Y. Catalytic hydropyrolysis of microalgae. Influence of operating variables on the formation and composition of bio-oil. Bioresour. Technol. 2015; 184: 349-54.
- Machado A, He S, Davies TE, Seshan K, Da Silva VT. Renewable fuel production from hydropyrolysis of residual biomass using molybdenum carbide-based catalysts. An analytical Py-GC/MS investigation. Catalysis Today. 2018; 302: 161-8.
- Melligan F, Hayes MHB, Kwapinski W, Leahy JJ. A study of hydrogen pressure during hydropyrolysis of Miscanthus x giganteus and online catalytic vapour upgrading with Ni on ZSM-5. J Anal Appl Pyrol. 2013; 103: 369-77.
- Marker TL, Felix LG, Linck MB, Roberts MJ. Integrated hydropyrolysis and hydrocoversion for the direct production of gasoline and diesel fuels or blending components from biomass Proof of principle testing. AICHEJ. 2012: 31: 191-9.
- Stummann MZ, Hoj M, Schandel CB, Hansen AB, Wiwe lP, Gabrielsen J, et al. Hydrogen assisted catalytic biomass pyrolysis. Effect of temperature and pressure. Biomass and Bioenergy. 2018; 115: 97-107.
- Stummann MZ, Hoj M, Schandel CB, Hansen AB, Hansen LP, Wiwe lP, et al. Effect of the catalyst in fluid bed catalytic hydropyrolysis. Catalysis Today. 2019.
- Dayton DC, Carpenter J, Farmer J, Turk B, GuptaR. Biomass Hydropyrolysis in a Pressurized Fluidized Bed Reactor. Energy and Fuels . 2013; 27: 3778- 85.
- Dayton DC, Hlebak J, Carpenter JR, Wang K, Mante OD, Peters JE. Biomass Hydropyrolysis in a Fluidized Bed Reactor. Energy and Fuels. 2016; 30: 4879-87.
- Wang K, Dayton DC, Peters JE , Mante OD. Reactive catalytic fast pyrolysis of biomass toproduce high-quality bio-crude. Green Chem. 2017; 19: 3243- 51.
- Pinjari S, Kumaravelan MK, Peddy VC, Gandham S, Patruni J, Kumar P. Maximizing the production of hydrogen and carbon nanotubes: Effect of Ni and reaction temperature. Int J. Hydrogen Energy . 2018; 43: 2781-93.
- Chandler DS, Resende FLP. Comparison between catalytic fast pyrolysis and catalytic hydropyrolysis for the production of liquid fuels in a fluidized bed reactor. Energy Fuels . 2019; 33: 3199-209.
- Chen Y, Zhang L, Zhang Y, Li A. Pressurized pyrolysis of sewage sludge Process performance and products characterization. J. Anal Appl Pyrol. 2019; 139: 205-2012.
- Raymundo LM, Mullen CA, Strahan GD, Boateng AA, Trierweiler JO. Deoxygenation of biomass pyrolysis vapors via in-situ and ex-situ thermal and biochar promoted upgrading. Energy Fuels. 2019; 33: 2197-207.