
Research Article
Austin Chem Eng. 2020; 7(3): 1081.
Facile Synthesis of Porous NiO/rGO Hybrid Material: Perspectives for Electrochemical CO2 Reduction
Geioushy RA¹*, Khaled M² and Fouad OA¹
¹Department of Nanomaterials and Nanotechnology, Advanced Materials Division, Central Metallurgical R & D Institute (CMRDI), 11421 Cairo, Egypt
²Department of Chemistry and Earth Sciences, Qatar University, Doha, Qatar
*Corresponding author: Geioushy RA, Department of Nanomaterials and Nanotechnology, Advanced Materials Division, Central Metallurgical R & D Institute (CMRDI), 11421 Cairo, Egypt
Received: August 05, 2020; Accepted: October 28, 2020; Published: November 04, 2020
Abstract
A porous nickel oxide/reduced graphene oxide (NiO/rGO) hybrid porous material was successfully synthesized via a simple wet-chemical approach followed by annealing at 400°C for 3 hours. XRD, FESEM, and Raman reveal the crystallinity and high purity of the prepared NiO/rGO. Linear Sweep Voltammetry (LSV) shows that in CO2- saturated solution, the measured current density of NiO and NiO/rGO is 3.9 and 6.1 mA/cm2 at -1.7 V vs. Ag/AgCl, respectively. The enhancement in cathodic current in CO2 than in N2 saturated solution is mainly originated from the CO2 reduction over NiO/rGO electrode surface. After 20 min CO2ER, ethanol (FE ~16%) was produced over NiO/rGO electrocatalyst at - 0.9 V vs. Ag/AgCl. These results imply the capacity of NiO/rGO as an electrocatalyst for energy conversion application.
Keywords: Porous Material; Nio/Rgo; Energy Conversion; CO2 Reduction
Abbreviations
NiO/rGO: Nickel oxide/reduced graphene oxide; XRD: X-ray Diffraction; FESEM: Field Emission Spectroscope; LSV: Linear Sweep Voltammetry; Ag/AgCl: Silver/ Silver Chloride; FE: Faradaic Efficiency
Introduction
Electrocatalysts have attracted enormous attention due to its potential application to solve energy and environmental crisis [1-2]. Catalytic CO2 conversion to fuels represents a suitable solution as new energy source and protects our environment at the same time [3-4]. CO2 electroreduction (CO2ER) to valuable chemicals and liquid fuels (methanol, formic acid,…etc.) is considered as the most attractive approach to mitigate the CO2 emission issue [5], besides it can be easily operated at room temperature and atmospheric pressure. Moreover, based on the applied potential and electrode material, variable products could be produced [6]. CO2ER has been extensively studied over various surfaces such as metal and metal oxides [7- 8]. Hydrogen Evolution Reaction (HER), high overpotential, and selectivity are the most common obstacles of CO2ER [9]. Therefore, more research efforts are still in demand for an efficient, selective, and nonprecious electrocatalyst design. Transition Metal Oxides (TMOs) have been extensively studied for CO2 electroreduction [2,5,10]. Among them, nickel oxide nanoparticles (NiO NPs) received much attention due to the low cost and its significant catalytic performance [11]. Because of the low specific surface area and active sites, NiO gives low hydrocarbon yield and low energy efficiency, which make its utilization for CO2ER is limited.
Due to the synergistic effect and geometric structures, carbonbased electrode performs a superior catalytic activity for CO2ER [12-13]. Reduced graphene oxide (rGO) as a new carbon form is a promising and exciting material for a variety of applications, owing to its extraordinary characteristics such as high surface area and excellent electron mobility. Recently, NiO/graphene hybrid found potential applications in catalysis, batteries, and H2 storage.
The electrochemical behavior of the synthesized NiO/rGO catalyst was evaluated using two-compartment electrochemical cell and 0.5 M NaHCO3 as an aqueous electrolyte. The results displayed the role of rGO as catalyst support for CO2ER. To the best of our knowledge, this is the first report for NiO/rGO evaluation as an electrocatalyst towards CO2 reduction.
Experimental
Materials
Nickel chloride hexahydrate (≥ 97%), hydroxylammonium chloride (NH2OH.HCl), sodium hydroxide (≥ 99.5%), sodium dodecyl sulfate (SDS, ≥ 99%), and sodium bicarbonate (≥ 99.5%). All chemicals procured from Sigma-Aldrich and used as received.
Synthesis of NiO/rGO Hybrid Structure
Starting with graphite, we have synthesized Graphene Oxide (GO) using modified Hummers, methods [14]. GO stock solution (1 mg/mL) was prepared. After that, 0.3 mL of the prepared GO stock solution dissolved in 18.1 mL distilled water under sonication for 30 min. This was followed by, adding 0.3 mL 0.1 M NiCl2.6H2O and 0.01g SDS under vigorous stirring for 2h. Then, rapidly 0.54 mL 1 M NaOH and 1.2 mL 0.1 M NH2OH.HCl are added and stirred for 30 min. After that, the formed solid was centrifuged, washed with H2O and ethanol, and dried at 40°C for 18h. Finally, the collected powder was annealed at 400°C for 3h. Moreover, pure NiO powder was prepared via the same procedure without adding GO solution.
Characterization Tools
Powder X-ray diffraction (XRD, Rigaku MiniFlex) was used to identify the phase structure of the as-synthesized materials. The samples morphologies were investigated by a field emission scanning electron microscope (FESEM, Lyra 3, Tescan). EDX was used for elemental composition analysis. Raman spectra of the prepared NiO/ rGO hybrid structure were collected in the range from 500 to 2000 cm-1.
Electrode Fabrication and Electrochemical Measurements
Cu metal pieces (fisher scientific company) were mechanically and chemically treated before used as substrate. For catalyst electrode fabrication, 1.0 mg of the prepared material was dispersed in 1.0 ml acetone and 60 μl nafion (5 wt. %), then ultrasonicated until ink solution was formed. A 200 μl from the ink solution was dropped on the Cu substrate and dried in air. To evaluate the catalyst/electrode behavior towards CO2ER, LSV measurements and CO2 reduction experiments were carried out under ambient conditions (see supplementary data).
Results and Discussion
Materials Characterization
The XRD patterns of the as-synthesized NiO and NiO/rGO are shown in Figure 1. XRD shows the diffraction peaks at 37.3°, 43.4°, 62.9°, 75.5°, and 79.5° which corresponding to the crystal orientations (111), (200), (220), (311), and (222), respectively. These peaks are indexed for NiO crystal planes which are in excellent agreement with JCPDS No. 04-0835. Moreover, the broadening peaks of NiO/ rGO pattern than those of NiO associated with the contribution of graphene. No other peaks could be detected, revealing the purity and well crystallinity of the synthesized NiO powder. For more investigations, Raman scattering was employed to characterize carbon materials. As can be seen form Figure 2, the characteristic D and G bands of reduced graphene oxide appeared at 1345 cm-1 and 1590 cm-1, respectively. Furthermore, the intensity ratio of D: G bands for NiO/rGO sample is higher than that of GO (inset). This difference in intensity ratio reveals the removal of functional groups-containing oxygen during the reduction of graphene oxide [2].
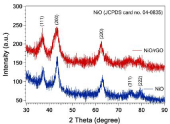
Figure 1: XRD patterns of NiO and NiO/rGO hybrid structure.
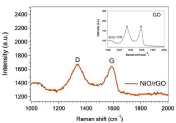
Figure 2: Raman spectra of NiO/rGO and graphene oxide (inset).
The surface morphologies of the synthesized NiO and NiO/rGO samples were shown in Figure 3. As seen from Figure 3(a, b), NiO has a porous and belt-like structure consists of small particles. The porous structure of NiO nanoparticles is clearly revealed by SEM image Figure 3(b). Furthermore, the decoration of NiO with reduced graphene oxide layers is observed as shown in Figure 3(c, d). The porous NiO/rGO hybrid structure shows no big changes. Actually, it is clear from SEM images that some macropores up to ~250 nm and micropores are generated on the surfaces. EDX analysis confirmed the existence of Ni, O, and C (not shown here).

Figure 3: FE-SEM images of NiO (a, b) and NiO/rGO hybrid structure (c, d).
Electrochemical Behavior
To evaluate the catalytic activity of NiO/rGO electrocatalyst towards CO2 reduction, Linear Sweep Voltammetry (LSV) measurements were carried out with and without CO2-saturated 0.5 M NaHCO3 aqueous solution at scan rate of 20 mV/s. Figure 4 shows the LSV of NiO and NiO/rGO electrodes under N2 and CO2 atmosphere. It is clear that NiO/rGO exhibited cathodic current density higher than that of NiO. Moreover, the measured current density in CO2 saturated electrolyte is larger than that in N2 saturated electrolyte over both NiO and NiO/rGO electrode surfaces. At -1.7 V vs. Ag/AgCl and in CO2 saturated solution, the recorded current over NiO and NiO/rGO was 3.9 and 6.1 mA/cm2, respectively. It is well known that the recorded current in N2 saturated electrolyte is mainly conducted with hydrogen evolution reaction [12,15]. The enhanced current density in CO2 saturated electrolyte is due to the CO2 reduction reaction over electrode surface. LSV curves show a cathodic peak at - 1.2 V. This may be attributed to the reduction of Ni+2 to Ni0. The results confirmed the catalytic activity of NiO/ rGO as an electrocatalyst and implying the role of rGO as supporting catalyst towards CO2ER. The reduction experiment was carried out using chronoamperometry method. After 20 min reduction process, liquid sample was withdrawn and injected into GC-MS. Actually, ethanol was found to be produced over NiO/rGO electrode surface at - 0.9 V (see supplementary data Figure S1). It is well known that applied potential, material loading, electrolyte, pH, and temperature are significant key factors in CO2 electroreduction. Although the Faradaic efficiency of ethanol formation calculated over NiO/rGO is ~16%, these results are promising indeed and future work is needed for better performance and understanding the reaction mechanism.
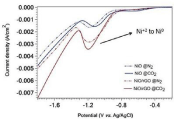
Figure 4: LSV of NiO and NiO/rGO- based electrodes in N2 and CO2 saturated
0.5 M NaHCO3 electrolyte at scan rate of 20 mV/s.
Conclusions
Here in, the successful synthesis of NiO nanoparticles decorated with rGO layers has been done via simple wet-chemical approach followed by annealing at 400°C for 3h. By using very low catalyst loading, Cu-modified NiO/rGO displayed the highest cathodic current density (6.1 mA/cm2) compared to Cu-modified NiO electrode (3.9 mA/cm2) in CO2-saturated 0.5 M NaHCO3 at -1.7 V vs. Ag/AgCl. This current enhancement attributed to the role of rGO contribution. LSV results indicate the initiation of CO2 reduction reaction over NiO/ rGO electrode surface. The electrocatalytic performance of NiO/rGO is mainly attributed to the porous structure and the synergistic effect of NiO and rGO.
References
- Beheshti M, Kakooei S, Ismail MC, Shahrestani S. Investigation of CO2 electrochemical reduction to syngas on Zn/Ni-based electrocatalysts using the cyclic voltammetry method. Electrochim. Acta. 2020; 341: 135976.
- Geioushy RA, Khaled MM, Hakeem AS, Alhooshani K, Basheer C. High efficiency graphene/Cu2O electrode for the electrochemical reduction of carbon dioxide to ethanol. J. Electroanal. Chem. 2017; 785: 138-143.
- Kandy MM, Gaikar VG. Photocatalytic reduction of CO2 using CdS nanorods on porous anodic alumina support. Mater. Res. Bull. 2018; 102: 440-449.
- Oh Y, Hu X. Organic molecules as mediators and catalysts for photocatalytic and electrocatalytic CO2 reduction. Chem Soc Rev. 2013; 42: 2253-2261.
- Geioushy RA, Khaled MM, Alhooshani K, Hakeem AS, Rinaldi A. Graphene/ ZnO/Cu2O electrocatalyst for selective conversion of CO2 into n- propanol. Electrochim. Acta. 2017; 245: 456-462.
- Kuhl KP, Cave ER, Abram DN, Jaramillo TF. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012; 5: 7050-7059.
- Malik K, Rajbongshi BM, Verma A. Syngas production from electrochemical reduction of CO2 at high current density using oxide derived Zn/Cu nanocomposite. J. CO2 Utili. 2019; 33: 311-319.
- Lu Q, Jiao F. Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy. 2016; 29: 439-456.
- Xie JF, Chen JJ, Huang YX, Zhang X, Wang WK, Huang GX, et al. Selective electrochemical CO2 reduction on Cu-Pd heterostructure. App. Cata. B: Environ. 2020; 270: 118864.
- Luo W, Zhang Q, Zhang J, Moioli E, Zhao K, Züttel A. Electrochemical reconstruction of ZnO for selective reduction of CO2 to CO. App. Catal. B: Environ. 2020; 273: 119060.
- Lu S, Shang Y, Ma S, Lu Y, Liu QC, Li ZJ. Porous NiO nanofibers as an efficient electrocatalyst towards long cycling life rechargeable Li–CO2 batteries. Electrochim. Acta. 2019; 319: 958-965.
- Zhang R, Lv W, Li G, Le L. Electrochemical reduction of CO2 on SnO2/ nitrogen-doped multiwalled carbon nanotubes composites in KHCO3 aqueous solution. Mater. Lett. 2015; 141: 63-66.
- Bashir SM, Hossain SS, Rahman S, Ahmed S, Hossain MM. NiO/MWCNT Catalysts for Electrochemical Reduction of CO2. Electrocatal. 2015; 6: 544- 553.
- Ismail AA, Geioushy RA, Bouzid H, Al-Sayari SA, Al-Hajry A, Bahnemann DW. TiO2 decoration of graphene layers for highly efficient photocatalyst: Impact of calcination at different gas atmosphere on photocatalytic efficiency. App. Catal. B: Environ. 2013; 129: 62-70.
- Chang TY, Liang RM, Wu PW, Chen JY, Hsieh YC. Electrochemical reduction of CO2 by Cu2O-catalyzed carbon clothes. Mater. Lett. 2009; 63: 1001-1003.