
Review Article
Austin Chem Eng. 2021; 8(1): 1086.
Application of Metal-Organic Frameworks (MOFs) for Capturing CO2: Advancement and Challenges
Assen AH¹, Adil K² and Belmabkhout Y¹*
¹Technology Development Cell (TechCell), Technology Transfer Office (TTO), Mohammed VI Polytechnic University (UM6P), Ben Guerir, Morocco
²Le Mans Université, Institut Des Molécules Et Des Matériaux du Mans, Avenue Olivier Messiaen, Le Mans, France
*Corresponding author: Youssef Belmabkhout, Technology Development Cell (TechCell), Technology Transfer Office (TTO), Mohammed VI Polytechnic University (UM6P), Ben Guerir, Morocco
Received: April 17, 2021; Accepted: June 28, 2021; Published: July 05, 2021
Abstract
The development of suitable solutions for capturing CO2 is one of the leading technical scenarios, among others, to mitigate greenhouse effect. Among all splitting techniques that could potentially address this important challenge, membrane and swing adsorption technologies are recognized to be of great promise for a variety of CO2 containing gas streams in industry. Nevertheless, the deployment of such technologies requires advanced materials with excellent/suitable thermodynamic and kinetics features that are further married to an optimally engineered design. The ultimate purpose is to achieve the desired high CO2 capturing capacity and efficiency. The targeted materials should possess adequate affinity toward CO2, in addition to high CO2 uptake and excellent chemical stability toward impurities such as SOx and NOx. Metal-Organic Frameworks (MOFs), solid-sate materials consisting of metal ions or clusters coordinated to organic ligands, showed technically interesting capabilities for gas splitting in general and CO2 capture in particular. This review presents an overview about the perspective of applying MOFs for capturing CO2 from different sources. The authors offer multidisciplinary discussion about the different aspects that would be key elements in progressing or cutting the path of research, development and innovation to final deployment of MOF as adsorbent for capturing CO2. An overview about the main MOFs with reported studies on CO2 capture from different sources will be proposed. Some general direction on how to design MOFs in order to address the trade-off of capacity vs. selectivity, which is highly desirable for large-scale CO2 emitting industries, will also, be given.
Keywords: CO2 emission; CO2 capture; Metal-organic framework; Adsorption thermodynamics; Adsorption kinetics
Introduction
The fast-growing global energy demands, as a result of the urban and industrial development in recent years, brought serious environmental apprehensions due to the increasingly heavy dependence on fossil fuels combustion in various energy sectors. Accordingly, human being existence is now directly related to side effect of CO2 regeneration. The consequential elevated CO2 gas emissions are thus believed to be the prime cause for the global climate change. Therefore, strategies to alleviate production of such greenhouse gases are of important significance. While the quest for alternative clean renewable energy sources is on the horizon, a rather shorter-medium term resolution would be searching for feasible technologies towards efficient CO2 capture for different sources.
CO2 separation is not a recent problem; it has been widely explored in many areas related to “energy”, “health care” as well as “environment” (Figure 1). Selective CO2 removal from air, natural gas and syngas has been a common engraved practice in chemical, petrochemical, and refinery factories. It has also been, for years an important practice in rebreathers for scuba diving and military applications [1] as well as for air quality in confined spaces such as submarine [1] and space shuttles [2]. In contrast, CO2 separation in the global environmental context has sparked and became one of the main topics driving the research and development in CO2 separation field.
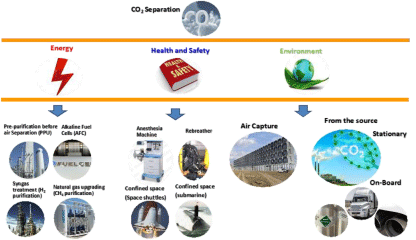
Figure 1: Schematic showing the wide use of CO2 separation at different scales and areas.
However, although quite mature, the solutions provided as defined for “energy” and “Health and Safety” related applications were not adaptable to the “environmental” problem because of the complex scale and cost factors. Nature’s free and fine handling of CO2 in our atmosphere has served the planet very well, however, the combination of increasing amount of CO2 emitted after the industrial revolution and the non-rational removal of trees from our planet over the last couple of hundred years made the natural carbon balance out of control. In their turns, oceans have absorbed so much CO2 and they are becoming saturated and acidic. Many tree-planting programs have been initiated over the years; however, it is going to take some time before the millions of trees planted in the last years become mature enough to provide sequestration benefits. Accordingly, a need of artificial CO2 sequestration-capture and storage/re-use is essential to offset the highly reduced natural sequestration potential.
CO2 Removal/Capture from Different Sources
Much smaller scale commercially available technologies for CO2 separation mainly use: cryogenic distillation, advanced solvents, solid sorbents, and membrane systems (Figure 2) [3]. Among these, cryogenic distillation is commonly used, however, its boiling point driving force concept represents a significant drawback for the technology to be implemented for CO2 capture. The common characteristic of other above-mentioned approaches is the use of separation agents such as solvent (absorbent) or solid adsorbent. Solid sorbents are particularly being explored for CO2 capture and can operate through weak physisorption or strong chemisorption interactions. Usually, solid adsorbents like activated carbons or zeolites are used in cyclic, multi-module adsorption-desorption processes, pressure (vacuum) swing (PSA or VSA) or Temperature Sing (TSA) and combination thereof (PTSA or VTSA).
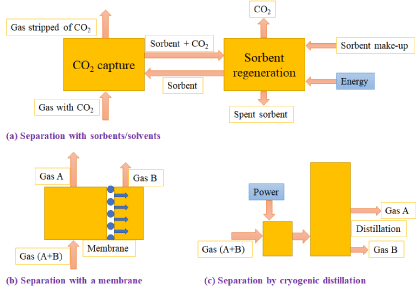
Figure 2: The main separation processes for the capture of CO2 [3].
Among the emerging CO2 separation agents, with a potential to handle the scale of CO2 capture (from stationary power plants and air), Metal-Organic Frameworks (MOFs) [4] have emerged as one of the most structurally/chemically modulable solid-state platform [5-9]. The versatility and the high degree of structural and functional control (as compared to zeolites for example) of most MOFs, offer to them the capacity to alter the selectivity/uptake tradeoff in very large ranges. These features situate MOFs as one of the potential adsorbents in the future to handle the capture of the CO2 emitted from 50 to 100 MW power plants as well for most methane (CH4) and hydrogen (H2) production/purification plants. Because of the dominant scale character of the CO2 capture problem, this review is mainly focused on discussing proven concepts and selective MOFs with capacity close to 10%wt, while having high selectivity, equivalent to the mature amine-based technology. Although the analysis of adsorbents in terms of CO2 energetic at the lab-scale is highly valuable and most of the time is conclusive at higher Technology Readiness Level (TRL), the real assessment of thermodynamics involved in the CO2 capture process is possible only after column studies with appropriate MOF formulation (beads, monoliths, etc.). Accordingly, the analysis made in this review is based on MOF performances assessed in powder or crystalline forms.
Large-scale emissions/removal
Electric-power generation remains the single largest source of CO2 emissions. Among the different high efficiency power generation plants, coal, oil and natural gas power plants in general and coal fired plants in particular emit large quantities of CO2 [10]. Today, despite the tremendous share growth of nuclear and renewable energy grid, the largely spread fossil fuel use and its associated carbon emissions continue to grow exponentially (Figure 3).
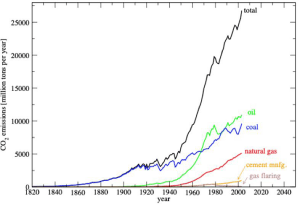
Figure 3: CO2 global concentration over time. Data from Marland et al. (2006) [11].
It is largely recognized that in addition to energy optimization and efficiency solutions, breaking the CO2 emissions escalation will inevitably require, in the medium- and long-terms, the deployment of CO2 Capture and Storage (CCS) solutions. Figure 4 shows a simplified schematic showing the CO2 flow management in the energy production sector which displays the bulk of the worldwide CO2 emissions. CCS is a promising method considering the huge worldwide energy demand and the feasibility of retrofitting existing plants.
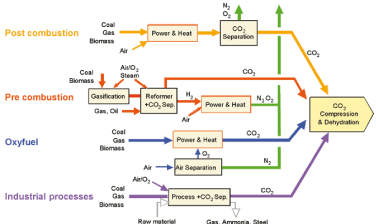
Figure 4: Overview of CO2 capture processes and systems for the energy sector [12].
CO2 capture and conversion to value-added products is a very important approach to maintain the sustainability of power generation technologies. However, in the currently employed CCS technology, more than two-third of the cost is devoted to CO2 capture making the conversion of the adsorbed CO2 to fine chemicals a very difficult task from cost point of view. One main strategy that can facilitate the economic implementation of CCS technologies in power plant industries is then to develop cost-efficient CO2 adsorbents which are the heart of CO2 mitigation processes. Currently, the capture of CO2 from the power plant industries mainly relies on amine scrubbing technique which uses alkanolamine solutions to capture CO2 from the combustion of fuel. However, the energy penalty associated with the regeneration of the alkanolamine makes this technique costly [13]. Solid-state porous physisorbents such as activated carbon and zeolites have been widely explored as alternative cost-effective adsorbents [14-17]. However, the quantity of CO2 generated from power plants is so high that those physisorbents cannot completely substitute the alkanolamine sorbents. Moreover, tuning the porosity and functionality attributes of those solid adsorbents is very difficult restricting their structural modifications for bulk CO2 capture. In this regard, MOFs [18], organic-inorganic hybrid materials with adjustable surface areas and modifiable pore structures for selective CO2 uptake, have shown great promise as future alternatives or complements for alkanolamines [19]. Regeneration of MOFs (i.e., release of CO2 from MOFs) can also be achieved with ease requiring only a little fraction of the energy that is required in amine scrubbing techniques, making MOFs very promising materials for development of energy efficient CCS process [20].
Energy generation: Post-combustion capture: The flue gas stream from power plants generally comprises of several gases/ vapors with compositions of 15-16% CO2, 5-7% water vapor, 73- 77% N2, 3-4% O2 and traces of acidic gasses such as H2S, NOx, SOx [21]. Hence, to reduce or moderate the emissions of CO2 from flue gas, Post-Combustion Carbon Capture (PCC) is envisioned. It is generally relatively easy to reduce the CO2 in the flue gas stream to just below 5% using low selective cheap materials; however, the main problem in PCC is the scale of flue gas stream to be treated per hour or day. Hence, separation agents with much higher removal capacity are needed. At present, there is no single adsorption swing based technology that can handle the management of CO2 emissions from flue gas emitted from large-scale power plants.
Currently, liquid amine scrubbing is the state-of-the-art technique for removal of CO2 from flue gas streams, but this technique suffers from high cost and environmental pollution issues [13]. Owing to their structural tunability, targeting the development of MOFs as CO2/N2 separating agents for PCC holds the greatest promise. Besides the high CO2/N2 selectivity, MOFs to be used as sorbents should have significant CO2 adsorption capacity and stability under flue gas conditions. To demonstrate the usefulness of MOFs for the targeted application, Pai et al. [22] studied diamine-appended MOFs using a vacuum swing adsorption process in the PCC from dry flue gas. The MOFs, denoted as mmen-Mg2 (dobpdc), and mmen-Mn2(dobpdc) (Figure 5), were found to exhibit an S-shaped CO2 isotherm and have achieved a CO2 recovery ≥90% and purity ≥95%. The low affinity of the MOFs for N2 and the distinct shape of the CO2 isotherm were hypothesized to be the main reasons for the lower energy consumption. In another example, Hu et al. [23] studied MIL-101(Cr) MOFs that contain post-synthetically tethered distinct alkylamine molecules to the unsaturated Cr(III) centers for PCC. Owing to the interaction between CO2 molecules and amine groups, the MOFs were found to exhibit very high CO2 uptakes with almost no N2 adsorption under ambient conditions. Besides the remarkable CO2 uptake and very high CO2/N2 selectivity of MIL-101-diethylenetriamine, the very high stability and the mild regeneration energy make it very promising for PCC [23]. In general, tailoring the structure of MOFs by employing different approaches such as targeting MOFs with high density of coordinative unsaturated metal ions as CO2 binding sites, frameworks with their pore environment decorated with heteroatoms that maximize framework-CO2 interactions, MOFs with controlled pore metrics and hydrophobicity to exclude competing gas/vapor molecules such as water, etc. have shown great promise in the development of several MOFs for PCC under practical applications [24-28].
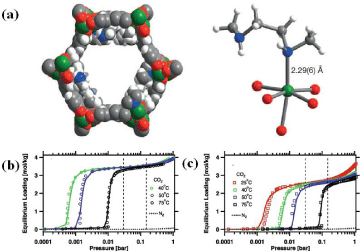
Figure 5: (a) Crystal structures of mmen-Mg2(dobpdc) (M = Mg or Mn), with portions of the crystal structure displayed on the right side (Green, grey, red, blue and
white spheres represent M, C, O, N and H atoms, respectively; with some H atoms omitted for clarity); CO2 and N2 adsorption isotherms for (b) mmen-Mg2(dobpdc)
and (c) mmen-Mn2(dobpdc) [22,24].
Fuel production: Natural gas/Biogas upgrading: Methane is the lightest and the cleanest hydrocarbon fuel with the lowest carbon to hydrogen ratio. Most of the sources of CH4 such as natural gas and biogas contain significant amount of CO2 together with other impurities such as H2S and humidity that could be emitted into the atmosphere [29-31]. As a result, capturing of CO2 impurities is very crucial for upgrading of natural gas and biogas and for reducing emissions into the atmosphere. Owing to the disadvantages associated with the currently employed amine scrubbing technology [13], the development of alternative cost and energy-efficient processes and materials, such as adsorption-based technology, that could offer great prospects to address this challenge is a necessity. The key in this regard is the choice of appropriate adsorbent with high stability towards humidity and H2S, with good CO2 adsorption capacity and selectivity.
Owing to their tunable porous structures, MOFs are considered to be suitable candidates to overcome the challenges in natural gas and biogas sweetening processes. In the same way as that of MOFs for post-combustion CO2 capture, targeting structures with high density of CO2 binding sites can help in the assembly of MOFs relevant for natural gas/biogas upgrading. Additionally, MOFs with adjusted pore aperture sizes to distinguish CO2 and CH4 based on their different molecular sizes (kinetic diameters of CO2 and CH4 are 3.3Å and 3.8Å, respectively), can be suitable targets for the targeted application. The stability of the frameworks towards moisture and H2S is also additional parameter to be envisaged. Among the different MOFs that showed good performance for CO2/CH4 separation, UTSA-49 (Zn(mtz)2; mtz = 5-methyl-1H-tetrazolate), showed CO2/ CH4 selectivity of 33.7 at 298 K for 1:1 mixture of CO2 and CH4 [32]. The presence of uncoordinated nitrogen atoms on the structure and two types of small pore openings (2.9Å×3.6 Å and 3.6 Å×4.0 Å), are supposed to play role for preferential adsorption of CO2 over CH4. The mixed-metal ZIF, ZIF-204 (Zn2Cu3(Im)10; Im = imidazolate), was also explored for its CO2 capture potential from a ternary gas mixture of CO2, CH4 and H2O with the MOF demonstrating selective adsorption of CO2 from the mixture without being affected by the presence of water [33]. Similarly, two polymorphic MOFs, Qc-5-Cu-a and -β [Cu(quinoline-5-carboxylate)2], that exhibit pore sizes suitable for size selective CO2/CH4 separation, were shown to have potential for molecular sieving of CO2 from CH4 in a humid environment [34]. Even though it is at its infancy, the development of H2S stable MOFs that can simultaneously capture CO2 and H2S from natural gas and biogas streams is a recent research interest. In this regard, the Gasoc- MOF [35] and the rare earth cluster based fcu-MOFs [36] gave interesting results for selective capture of acid gases from methane with good H2S/CO2 selectivity as well. Recently, Ultra-microporous fluorinated MOFs showed an exception with highly tunable/stable removal capabilities for both CO2 and H2S [37]. However, there remains a lot to be done in the design and preparation of MOFs suitable for selective removal of CO2 from CH4 in the presence of other impurities such as H2S. To have full practical implementation of MOFs for natural gas/biogas upgrading, further research works that target to overcome hurdles related to MOF stability towards such impurities need to be addressed.
H2 production: Pre-combustion capture: The other process that requires the capture of CO2 at large-scale is the pre-combustion C-capture, which mainly involves the capture of CO2 from hydrogenrich synthesis gas prior to combustion. A typical pre-combustion mixture might contain 15-60% CO2 mixed with H2 and other impurities at relatively high pressures. In the quest for highly selective and efficient adsorbent materials for the targeted application, as a substitute for the commonly used energy-intensive liquid amine adsorption, MOFs have attracted intense research interest owing to their tailorable structures [38]. Compared to the CO2/N2 and CO2/ CH2 separations, the number of studies on CO2/H2 separations using MOFs is very limited. UTSA-16 [39], a microporous MOF that has been explored for capture of CO2 using PSA unit from steam-methane reforming off-gases (76% H2, 17% CO2, 3% CH4 and 4% CO); Cu-BTTri [40,41], H3[(Cu4Cl)3(BTTri)8] (BTTri³- = 1,3,5-benzenetristriazolate) that displays high CO2/H2 separation capability (IAST selectivity up to 860) for 80:20 and 60:40 H2/CO2 gas mixtures due to its exposed cation sites (adsorbs 4.5 CO2 molecules per Cu at saturation); Mg- MOF-74 (dobdc4- = 1,4-dioxido-2,5-benzenedicarboxylate) [42], another MOF with open metal sites which adsorbs 1.8 CO2 molecules per Mg at saturation; and Ni-4PyC [43], an ultra-microporous (3.5 and 4.8 Å pores) Ni-(4-pyridylcarboxylate)2 MOF that exhibits CO2/ H2 selectivity of 285 for 20:80 and 230 for 40:60 mixtures at 10 bar and 40°C with facile adsorption-desorption cycles and CO2 selfdiffusivity (~3×10-9 m2/s), are among the well-known MOFs that have been experimentally tested for CO2/H2 separation under precombustion CO2 capture conditions with significant improvements in performances over zeolites and activated carbons. Due to the high pressure of the pre-combustion gas mixture, MOF-based membranes could be targeted for better efficiency besides the necessity to work on the development of MOFs with high CO2 capacity.
Small-scale emissions/removal
Compared to the traditional CO2 capture processes from large point sources, little attention has been given to CO2 emissions from small-scale sources such as buildings, aircrafts, cars, trucks, and other confined air spaces mainly due to their expected smaller contribution to greenhouse gas emissions (about 10% to 15%) and the higher cost of the process than the larger-scale CO2 capture [44]. However, the CCS from small and mobile source provides a means to adjust/stabilize the atmospheric CO2 concentrations in confined air spaces and in other places where mitigation efforts fall short of targets [45]. As a result, development of feasible and economical CCS technologies for small and medium-scale CO2 emissions must receive more attention. In this section, we make a perspective analyses on the application of small-scale CCS for medical applications and highlight some works on the CO2 capture from confined air spaces.
Medical applications: CO2 is known for a number of its medical applications. To mention a few, a liquefied CO2, supplied in cylinders filled to a high pressure, is used as an insufflations gas during bodily investigations and for minimal invasive surgery (laparoscopy, endoscopy, and arthroscopy). Medical CO2 also provides respiratory stimulation during and after anesthesia. In a solid form (dry ice), it serves as a cryotherapy agent to achieve a temperature of -76 °C, which in turn enables freezing of tissues and removal of wart, moles, and skin tags. For effective utilization of CO2 for the clinical applications with minimum cost and improved patient care, there is a critical need to develop practical and economical way of selective capturing and, hence storing the gas. This could be achieved using fully recyclable CO2 sorbents where CO2 can be effectively extracted and properly used. The CO2 sorbents to be utilized for this purpose should have highly efficient and selective CO2 separation potential and high gravimetric CO2 uptake capacity. To date, there are no welldocumented CO2 sorbents that have been practically used for medical purposes [46].
Applications in confined spaces: Environments involving occupants in sealed air spaces such as aircrafts, submarines, vehicles, buildings, etc. lead to build-up of CO2 concentrations over time due to respiration. The humans inside the confined spaces can then be affected by the rising levels of CO2. In such environment, the safe exposure limit is about 1% CO2 since higher concentration can lead to adverse side effects such as headaches and lethargy [47]. The high level of CO2 in such environment is also linked to operational issues due to dry ice formation [48] and hence it must be removed from air prior to liquefaction. As a result, the development of appropriate techniques and materials that can maintain safe levels of CO2 by efficiently adsorbing such low concentration of CO2 is essential. Among the different techniques, direct air capture [49] has been implemented for the past a few decades to capture small-scale CO2 emissions in submarines [1] and spaceships [2]. However, the technique is expensive due to its large energy consumption. Porous materials to be used for this application require suitable binding sites that possess sufficiently strong interactions with CO2 at low coverage but at the same time being easier for regeneration without the need for a high energy input [2]. In recent years, MOFs have shown very promising potential to tackle such challenge. Among the different examples of MOFs, NbOFFIVE-1-Ni [NiNbOF5(pyrazine)2•2H2O], due to its suitable structural features such as appropriate sized square channels, pore walls with polar functional groups and the lower energy requirement for regeneration, has been shown to be a viable material for this application [50]. Other MOFs, functionalized with polar groups, have also shown attractive features for lowconcentration CO2 uptake [51,52]. Given the possibility to tune their structures for a particular application, a lot of room is then available for further design of new MOFs with more enhanced performance.
State-of-the-Art in Research, Development, and Innovation for MOF Deployment in CO2 Capture
Although most of CO2 capture adsorbents studies at the labscale are based on purely equilibrium thermodynamics where the main separation driving force is the difference in the interaction between CO2 and the less absorbable products/secondary products, few recent studies demonstrated disruptive advanced concepts that allow the combined maximization of CO2 uptake and selectivity while intrinsically reducing the level of interaction with adsorbents [20,53]. The benefit of such endeavor is to maximize the reduction of adsorptive cycling’s cost via optimal adsorbent regeneration, primarily linked to MOF structural and functionality features. Such control could have a dramatic effect on the main metrics for the separation while affording mild conditions for cycling. With this regard, it is obvious that CO2-MOF interactions with physical nature are more preferred to achieve such objective. In this section, we will go over the main works highlighting the use of MOF as chemical adsorbents and the recent works that allowed making dissimilarity between physical adsorption mechanisms as function of the extent of thermodynamics and kinetics involved in the CO2 capture process. Several types of MOFs have been proposed for CO2 capture, including (i) MOFs with open metal sites [7,42,54-63]; (ii) MOFs without open metals sites [64-80]; (iii) MOFs with narrow pore size via interpenetration [53,81,82] or shortening the size of the ligands [53,67] and (iv) MOFs decorated with specific functional groups, including (NH2, OH, etc.) [76,83-87]. From these types of materials, we discuss selected ones that offer the maximized uptake-selectivity performances at the lab-scale. For a more comprehensive account on CO2 adsorbents, we direct the reader to reviews by Sumida et al. 2012 [88] and Sayari et al. 2011 [89].
Chemical adsorption
Chemisorption, which relies on chemical reaction between CO2 and sorbent, is the most widely employed and well-developed CO2 capture technique. Among the state-of-the-art chemical adsorption techniques, liquid amine scrubbing is the most popular approach for bulk-scale carbon capture [45]. In an attempt to improve the performance of amine-based sorbents, solid chemisorbents were developed from amine-modified porous materials. Amine-grafted or functionalized MOFs are among those materials that have been explored for CO2 capture applications [24,90]. Compared to the unmodified porous materials, the amine-functionalized counterparts were found to exhibit significantly improved CO2 affinity. The regeneration costs are also relatively lower than that of liquid aminebased techniques. However, problems associated with the use of such chemisorbents such as the high temperature requirement for regeneration (even though it is lower than that of liquid chemisorbents), the difficulty in the preparation of the materials, the high cost of the precursors, the poor chemical and thermal stabilities, etc. hamper the further development of such materials for CO2 capture applications [90].
Physical adsorption
The use of physisorbent materials that selectively bind CO2 at low concentrations is an alternative to chemical absorption. However, it was reported in different works that physical adsorption is too weak to handle low concentration CO2 removal in highly or average diluted gas streams [12,91]. Even at lab-scale, the use of physical adsorbents, from the family of porous materials available commercially, for CO2 capture from diluted gas streams was not recognized as a viable promising solution. In this subsection, we will shed light on this topic to break this old, prolonged misconception. For this purpose, the revealed advanced concepts involving kinetics will be discussed only macroscopically with the link to the structural and functional features of the MOF adsorbents.
CO2 capture based on adsorption equilibrium thermodynamics: CO2 adsorption based on equilibrium thermodynamics represents the bulk of the studies for CO2 capture in wide range of conditions (pressure, temperature, and compositions). The thermodynamic system based on this mechanism is diffusion resistance free, as most the porosity features of the CO2 separation agents involved are optimal (enough large aperture size and pores diameter) to allow to achieve high self and transport diffusion parameter toward and from the highly homogeneous pore network.
Various examples of MOFs have been reported to be suitable for thermodynamically driven separation of CO2 from CH4 and N2. One of the earlier example that has been explored for such application is the interpenetrated microporous MOF, Zn(BDC)(4,4’-Bipy)0.5 (MOF- 508) [92]. The MOF can allow diffusion of all the three molecules with little or no restriction since the size of its one-dimensional channels (~4.0 Å x 4.0 Å) (Figure 6) is higher than the kinetic diameters of CO2, CH4 and N2 (3.30 Å, 3.64 Å and 3.80 Å, respectively). The preferential adsorption of CO2 over N2 or CH4 (breakthrough selectivity of 3 and 6 for CO2/CH4 and CO2/N2, respectively) together with a higher adsorption enthalpy for CO2 with respect to the other two gases can then prove the enthalpic-driven mechanism [92]. Due to the absence of specific functional groups that can interact with CO2, the adsorption selectivity is not high. However, this is one of the first examples of MOFs that permitted further practice of pore size reduction and interpenetration as design principles for the targeted application.
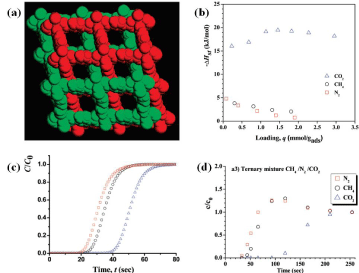
Figure 6: Crystal structure of MOF-508 showing its one-dimensional channel (a); Isosteric heat of adsorption for N2, CH4, and CO2 (b); Single-component at 323 K and 1 bar (c) and Multicomponent (1:1:1 mixture of CH4/N2/CO2) at 303 K and 4 bar (d) breakthrough curves for N2 (square), CH4 (circle), and CO2 (triangle) [92].
The other examples of MOFs that showed potential for enthalpicdriven separation of CO2 are SIFSIX-2-Cu and the self-interpenetrated analogue SIFSIX-2-Cu-i, which contain square grid layers from the connection of Cu2+ cations and 4,4’-dipyridylacetylene linkers and are intercalated by (SiF6)2- pillars [53,93,94]. Both materials exhibited CO2/N2 separation with a selectivity ranging from 140 to 13.7 (298K, 1 bar, gas mixture CO2/N2: 10/90) based on Ideal Adsorbed Solution Theory (IAST). Similar results were obtained for CO2/CH4 separation with a selectivity increasing from 5.3 to 33 (298 K, 1 bar, gas mixture CO2/CH4: 50/50) upon using SIFSIX-2-Cu-i. The pore dimensions of SIFSIX-2-Cu and SIFSIX-2-Cu-i are 13.5 Å and 5.15 Å, respectively, which are above the molecular sizes of the three molecules. These results and the enhanced CO2-MOF interactions as reflected in the higher isosteric heat of adsorption evidenced the thermodynamic equilibrium adsorption mechanism. These and other examples [68,95-97] demonstrate the importance of interpenetration and pore size constriction to induce MOF-CO2 interactions and hence better enthalpy-driven separation performances. MOFs without interpenetration but with reduced pore apertures such as UTSA-16 also gave interesting results in terms of thermodynamic separation of CO2 from gas streams [98,99].
The other most practiced strategy in the design of MOFs for thermodynamic separation of CO2 is the incorporation of high charge density (CO2-philic groups) such as amine functional groups, coordinative unsaturated metal sites, etc. The use of functionalized linkers to assemble MOFs suitable for enthalpic-driven CO2 separation is exemplified by the isoreticular series of RE-fcu-MOFs, constructed from the assembly of the 12-c RE hexanuclear cluster MBBs, [RE6(μ3-OH)8(O2C-)12], with a series of ditopic heterofunctional as well as homo-functional linkers of various lengths and functionalities [7]. Despite the triangular pore aperture diameters of the MOFs (5-6 Å) being above the kinetic diameters of CO2 and N2, a high CO2/N2 adsorption selectivity (ca. 370) was predicted by IAST calculation. These results together with the high value of the calculated isosteric heats of adsorption for CO2 (58.1 kJ mol-1 at low loading for Tb-FTZB-fcu-MOF)) evidenced thermodynamic mechanism for the observed selective separation. The high charge density within the frameworks due to the presence of tetrazolate moieties, fluorine groups and coordinative unsaturated sites is responsible for the enhanced MOF-CO2 interaction. The other very popular MOFs that have been widely investigated for equilibriumbased CO2/CH4, CO2/N2 and CO2/H2 separations are Mg-MOF-74 and its derivatives [24,42,51,55] made from 2,5-dihydroxybenzene- 1,4-dicarboxylic acid (H2DOBDC) or elongated form of the linker and magnesium salt sources. The presence of high density of open metal sites and the amenability of the MOF for post-synthetic grafting of functionalized groups that can interact with CO2 made it to be one of the well-studied MOFs for selective CO2 capture.
CO2 Capture based on Kinetics: Although important and critical metric in the selection of adsorbents in general and CO2 adsorbents in particular, selective kinetics CO2 adsorption is rarely studied as the main driving force for separating CO2 from N2, CH2 and H2. At the exception for H2, CO2 is expected to diffuse much faster than N2 and CH4 into and from any pore network. The desirable cap enhancement on the diffusion properties could be achieved by using MOF with reduced pore aperture size that will potentially enhance the kinetics selectivity toward CO2. Due to the difficulty in measuring experimentally the diffusion of gases into the pores, only few works reported kinetic gas-based separation. Among the early examples of MOFs, [Cu2(bza)4(pyz)]n (bza = benzoic acid; pyz = pyrazine), which possesses two types of confined 1D pore channels with apertures of 2 Å, has been shown to be promising for possible kinetic separation of CO2 from N2 or CH2 [100,101]. In spite of the fact that it is difficult to extract adsorption kinetics data, gas mobilities were demonstrated using single-crystal membrane of the MOF with high permeabilities for CO2 as compared to N2 and CH4 (1.5×10-12, 1.1×10-13 and 5.9×10-14 mol m m-2s-1Pa-1 for CO2, N2 and CH4, respectively) (Figure 7) [102].
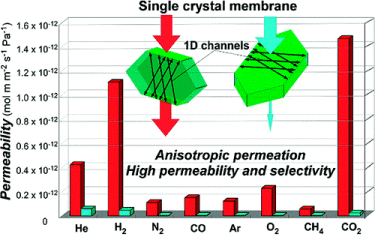
Figure 7: Comparison of gas permeabilities along the channels (red) and vertical to the channels (blue) of the single crystal membrane [102].
CO2/CH4 kinetics-driven separation was also demonstrated using a layered doubly-interpenetrated Cu-MOF, Cu(hfipbb)(H2hfipbb)0.5 (H2hfipbb = 4,4'-(hexafluoroisopropylidene) bis(benzoic acid)) [103]. The narrow window (~3.5×3.5 Å) of the MOF, that are comparable to the molecular dimensions of CO2 and CH2, make it a suitable candidate for kinetic separation of the gases [103]. Despite the slight differences in heats of adsorptions for both gases at zero loading (29.7 and 21.4 kJ mol-1 for CO2 and CH4, respectively), high overall CO2/ CH4 selectivity of 25 (Figure 8) was observed due to the significantly faster diffusion of CO2 (2.97×10-3 s-1) than CH4 (1.14×10-4 s-1) [103].
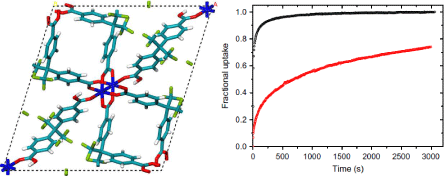
Figure 8: Left: crystal structure of Cu(hfipbb)(H2hfipbb)0.5; Right: Fractional adsorption uptake of CO2 (black) and CH4 (red) at 298K [103].
CO2 Capture based on cooperative effect between Thermodynamics and Kinetics: The synergy between thermodynamics and kinetics was also found to play role in the selective capture of CO2 using MOFs. In this regard, one peculiar platform is the M-SIFSIX MOFs [53]. Among the these family of MOFs, the Zn-pyrazine (pyr) MOF, SIFSIX-3-Zn or [Zn(pyr)2(SiF6)] n, with square pore channels of ~3.84 Å, exhibited highly selective CO2/N2 (selectivity = 495) and CO2/CH4 (selectivity = 109) separation potential as evidenced by low-pressure pure-component CO2, N2 and CH4 adsorption data, column breakthrough studies using CO2/ N2:10/90 and CO2/CH2:50/50 gas mixtures at 298K and atmospheric pressure, IAST calculations to predict adsorption equilibria for CO2/CH4:05/95, CO2/CH4:50/50, CO2/N2:10/90 and CO2/H2:30/70 mixtures and competitive adsorption kinetic studies of the aforementioned gas mixtures (Figure 9). The contracted pore aperture of the MOF, due to the short ligand employed to assemble it, was asserted to provide sufficient diffusion barriers to differentiate the adsorption rates of CO2 and N2. Moreover, the high charge density within the structure favors thermodynamic separation. The adsorption properties of this 1st generation of fluorinated-MOF demonstrated the interplay of both kinetics and thermodynamics for selective capture of CO2 from gas mixtures.
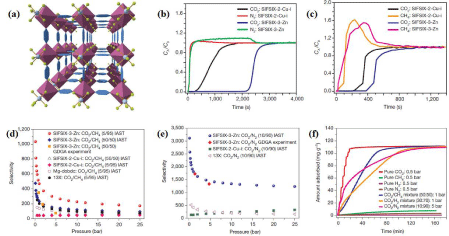
Figure 9: (a) Crystal structure of SIFSIX-3-Zn; Column breakthrough experiment for CO2/N2:10/90 (b), for CO2/CH4:50/50 (c) carried out on SIFSIX-2-Cu-i and
SIFSIX-3-Zn (298 K, 1 bar); Calculated (using IAST) CO2 adsorption selectivity for two different CO2/CH4 mixtures (d) and for CO2/N2:10:90 (e) on SIFSIX-3-Zn
compared to SIFSIX-2-Cu-I, Mg-dobdc and 13X zeolite at 298 K; Kinetics of adsorption of SIFSIX-3-Zn for pure gases and gas mixtures containing various
compositions of CO2 (f) [53].
One major weakness in the 1st generation of fluorinated-MOFs is the limited stability of the adsorbents in the presence of moisture. To circumvent such problem, the 2nd generation of hydrolytically stable CO2 selective fluorinated-MOFs, such as AlFFIVE-1-Ni and NbOFFIVE-1-Ni, 3-periodic pcu-MOFs based on the 2-periodic Ni- (pyrazine)2 square grid, bridged by (AlOF5)2- or (NbOF5)2- pillars, were developed (Figure 10) [50,104]. The NbOFFIVE-1-Ni was found to exhibit high gravimetric and volumetric CO2 uptake with very high selectivity, the uptake being very similar to the capacities obtained for the 1st generation fluorinated-MOFs.
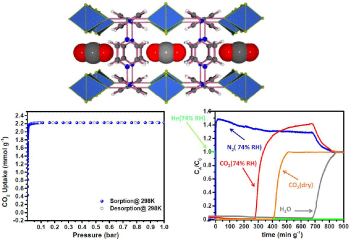
Figure 10: Crystal structure of NbOFFIVE-1-Ni showing the interaction with CO2 (Top), CO2 adsorption isotherm for NbOFFIVE-1-Ni up to 1 bar and 298 K (Bottom
left), and breakthrough adsorption for the NbOFFIVE-1-Ni with CO2/N2 (1%/99%) mixed-gas at 1 bar and 298 K in both dry and humid conditions (Bottom right) [50].
Advanced concept to develop CO2 adsorbents with high selectivity and capacity: The successful practice of CO2 capture using MOFs lies in the structural tunability of the adsorbents that paves the way for the design of materials with improved CO2- framework interactions. The possibility to introduce appropriate pore dimensions and suitable CO2 binding sites in the frameworks have played very important role in the design and preparation of various CO2 adsorbent MOFs with remarkable uptake capacities and selective adsorptions of the gas in the presence of other gases/vapors such as N2, CH4, H2O, etc. For example, the fluorinated-MOFs proved the power of favorable CO2-MOF thermodynamics and kinetics to develop CO2 selective MOFs. Even though the 2nd generation fluorinated MOFs showed high CO2/N2 separation performance with very high thermoand chemical stability, one common drawback associated with those MOFs is their low CO2 uptakes necessitating the development of advanced concepts to further enhance the capacity via enhanced packing of the adsorbed molecules. Hence, the 3rd generation of fluorinated-MOFs were then envisaged from 3,6-di(4-pyridyl)-1,2,4,5-tetrazine (dptz) as the ligand and either SiF6 2- or TiF62- as the pillar [20]. Among that family of MOFs, dptz-CuTiF6, was found to exhibit the highest CO2 uptakes at 10% CO2 and 298 K, with the gravimetric and volumetric uptakes being superior to the first- and second-generation MOFs. The MOF also requires significantly lower energy input for regeneration making it a better replacement for the common aqueous amine scrubbing technique (38 kJmol-1 vs. 105 kJmol-1) [20]. The crystal structure of dptz-CuTiF6 and the evolution of the fluorinated-MOFs as CO2 adsorbents are displayed in Figure 11.
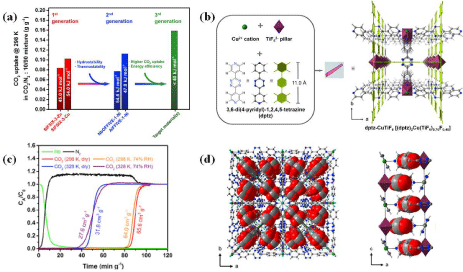
Figure 11: (a) The evolution of fluorinated-MOFs as CO2 adsorbent for flue gas treatment, (b) Crystal structure description for dptz-CuTiF6, (c) Dynamic column
breakthrough tests for dptz-CuTiF6 with CO2-N2 (10:90) at 298 or 328 K and 1 bar under both dry and humid conditions, and (d) structure depicting the packing
arrangement of CO2 molecules within the channels [20].
Despite the numerous opportunities the structural features of MOFs provide, there are certain challenges associated with the successful implementation of MOFs for CCS applications, the challenges in the incorporation of some CO2 binding functional groups during synthesis, selective adsorption of CO2 in the presence of water and acid gases, and the large-scale application of the adsorbents for the intended target. To fabricate MOFs that possess all the necessary attributes for the desired capacity and selectivity, the development of advanced concepts with respect to design, synthesis, characterization, and applications should remain to be the subject of further research.
Challenges Facing MOFs in the Process of Transition to High Technology Readiness Levels for CO2 Capture
Compared to many other porous materials, MOFs have shown outstanding CO2 capture potentials and selective adsorptions in the presence of other gases/vapors. There are, however, several challenges regarding the synthesis and CO2 capture applications of MOFs that need to be overcome to place this class of adsorbents at high Technology Readiness Levels (TRLs). Among the different concerns that need to be addressed prior to commercialization, the problems associated with MOF stability and scale-up will be assessed here.
Formulation and stability
One of the MOF formulation challenge with respect to CO2 capture applications is the difficulty to introduce multiple binding sites within the same structure. The presence of several CO2-philic groups is particularly important to improve the selective adsorption of CO2 at low partial pressures. The strategy of amine grafting has already shown some promising potential for enhancing CO2 adsorption [51,105]. There are still lots of rooms to increase the density of grafted molecules for enhanced MOF-CO2 interactions. Related to this, another challenge while attempting to introduce multiple binding sites is the dramatic decrease in surface areas and pore volumes and hence the resultant CO2 adsorption capacities. A balance between functionalization and porosity must be maintained while designing and formulating MOFs for the targeted application. Adsorbent regeneration energies need also to be considered while formulating MOFs with multiple CO2 binding sites. An increase in the regeneration energies is obviously expected upon incorporation of several CO2-philic groups [106].
As already mentioned, many of the process conditions for CO2 capture consist of other competing gases/vapors such as moisture/ water and acidic gasses such as SO2 and H2S. Different MOFs showed great promise as sorbents for CO2 capture [19,107] but many MOFs that have been explored for CO2 capture lack the requisite stability in the presence of these chemicals due to structure collapse and irreversible binding of the sulfur contaminants [36,108-116]. This is usually reflected in the reduction of adsorption uptakes upon cyclic measurements due to the continuous decrease in the materials’ surface areas and/or pore volumes. The design of MOFs to fully alleviate emissions of such corrosive and anthropogenic molecules then remains a fundamental challenge. As a result, it is extremely important to continue work in areas coupled to rational design and controlled crystallization. So far, pre- and post-synthetic MOF synthesis strategies have been considered and a significant improvement has been achieved regarding water and acid stabilities of MOFs [117-120, 125]. But the suits of acid and base stable MOFs for CO2 capture applications are still underexplored.
The formulation of MOF adsorbents that preferentially binds CO2 over the other competing molecules is another challenge. Owing to their higher polarity, H2O or acidic gasses are preferentially adsorbed over CO2 on the binding sites of MOFs. One option that is implemented to alleviate this challenge is to dehydrate and remove the sulfur contaminants prior to the CO2 capture step. Although mature technologies are implemented for this purpose, it incurs, however, extra cost to the process and makes it more expensive. This is then one of the research opportunities to be considered when formulating a MOF for CO2 capture in the presence of water and/or acid gases [37,104,114].
Scale-UP
MOFs, at small scale, have shown to be promising candidates for future development of alternative CO2 capture technologies. However, they are yet to be transitioned for bulk-scale CO2 capture applications at industry level. One of the main obstacles that slow down the transition of MOFs to TRLs is the problem associated with the scale-up in the synthesis of MOFs [121]. Pilot-scale production of MOFs and their CO2 capture performance testing are currently under investigation, but the large-scale production of MOFs is still underexplored. MOFs are usually prepared in a lab-scale by employing solvo(hydro)thermal syntheses approaches. However, transformation of the lab-scale synthetic conditions to large-scale production through either direct mixing of reagents in solvents or solvent-free mechanochemical methods is not always practical [122]. Efforts must be made towards the development of MOF synthetic protocols that are amenable to scale-up. In fact, there are some ongoing MOF scale-up approaches with promising results for further development [123]. For example, a continuous solvent-free solidstate grinding synthesis with a kg per hour-scale MOF production has been achieved by twin-screw extrusion [124]. To position MOFs at high TRLs, the development of synthesis protocols for scaledup production in general and methods amenable for continuous production in particular should then be a subject of further research.
Lack of multidisciplinary research approaches
The other challenge that slows down the generation of CO2 capturing MOFs at industrial scale and their transition towards TRLs is lack of multidisciplinary research approach. The scientific community with significant mass of expertise (chemists, crystallographers, computational experts, chemical/ process engineers, etc.) should create extensive collaborations. The basis for the development of tailored-MOFs that can satisfy the requirements of CO2 capture processes for various technologies lies on the understanding of structure-function relationships. Besides designing and preparing the adsorbents at lab-scale, the mechanisms how the materials perform in an actual CO2 capture process should be elucidated. Consequently, the different experts must work handin- hand to address the structure-property relationships. Large-scale screening of MOF for their adsorption properties is unlikely to be achieved through experimental studies. Therefore, the development of computational modeling tools that can successfully predict the MOFs’ CO2 capture performance must be further developed. Besides the material design and testing, engineering economics models must be established to evaluate lifecycle, scale-up and capture costs of adsorbents for various processes.
Conclusion
Remarkable advances have been made toward the development MOFs for CO2 capture. Some of the achievements that have been made in the design and preparation of MOFs for CO2 capture include the design and synthesis of various tunable MOFs at lab-scale that possess high CO2 adsorption capacities at low partial pressures (~1- 10 % CO2 concentration) with suitable CO2/N2 selectivity, and in the presence of humidity. In order to achieve the above-mentioned features, the optimal pore structures (size and functional decoration) are key elements for selective adsorption of CO2 with sufficient selectivity over N2/CH4 under humid conditions, which are the requirements for post-combustion CCS process, and could be also reworked for other applications such as natural gas/biogas upgrading or confined space applications. Even though the aforementioned achievements indicate an important step in moving forward, there are still several other issues that need to be addressed including long-term chemical and mechanical stabilities, selective CO2 capture in the presence of contaminants such as H2S and SO2, assembly of MOFs that combine high CO2 uptake capacity with high selectivity particularly in the presence of humidity and acid gases, large-scale production and deployment of MOFs and assessment of the stability and adsorption properties of MOFs under real industrial conditions. Most of the challenges associated with the applicability of MOFs for CO2 capture stem from the little research carried out in the area and lack of multidisciplinary approaches. Given the promise of MOF structures for tunability of their chemical structures and their crystalline nature that allows researchers to experimentally probe structure-property relationships or mechanism of CO2-framework interactions, performing application-oriented research in the area can guarantee to overcome the challenges. Most of the previous research reports in the area are based on evaluation of MOFs’ CO2 overall uptake capacities under static adsorption tests (i.e., from single component isotherms) rather than on the actual industry relevant conditions (dynamic adsorptions using mixtures of gases/ vapors). Future research must then be centered on testing/assessment of MOFs’ CO2 capture performance in dynamic settings at elevated technology readiness levels.
Acknowledgement
The authors acknowledge the financial support of Mohammed VI polytechnic University.
References
- Carey R, Gomezplata A, Sarich A. An overview into submarine CO2 scrubber development. Ocean Eng. 1983; 10: 227-233.
- DallBauman LA, Finn JE. Adsorption and its Applications in Industry and Environmental Protection: Applications in Environmental Protections, Part B. In: Dabrowski A, editor. 1999; 120; 455-471.
- Leila F. Post-Combustion Capture of CO2 from Fossil Fueled Power Plants. Kgs. Lyngby, Denmark: Technical University of Denmark. 2010.
- Zhou H-CJ, Kitagawa S. Metal-Organic Frameworks (MOFs). Chem Soc Rev. 2014; 43: 5415-5418.
- Eddaoudi M, Moler DB, Li H, Chen B, Reineke TM, O’Keeffe M, et al. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal-Organic Carboxylate Frameworks. Acc Chem Res. 2001; 34: 319-330.
- Eddaoudi M, Kim J, Rosi N, Vodak D, Wachter J, Keeffe M, et al. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science. 2002; 295: 469.
- Xue D-X, Cairns AJ, Belmabkhout Y, Wojtas L, Liu Y, Alkordi MH, et al. Tunable Rare-Earth fcu-MOFs: A Platform for Systematic Enhancement of CO2 Adsorption Energetics and Uptake. J Am Chem Soc. 2013; 135: 7660- 7667.
- Assen AH, Belmabkhout Y, Adil K, Bhatt PM, Xue DX, Jiang H, et al. Ultra- Tuning of the Rare-Earth fcu-MOF Aperture Size for Selective Molecular Exclusion of Branched Paraffins. Angew Chem Int Ed. 2015; 54: 14353- 14358.
- Assen AH, Virdis T, De Moor W, Moussa A, Eddaoudi M, Baron G, et al. Kinetic separation of C4 olefins using Y-fum-fcu-MOF with ultra-fine-tuned aperture size. Chem Eng J. 2021; 413: 127388.
- Aaron D, Tsouris C. Separation of CO2 from Flue Gas: A Review. Sep Sci Technol. 2005; 40: 321-438.
- Marland G, Boden T, Andres. RJ. Global, Regional, and National CO2 Emissions. Technical report, Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, TN, USA. 2006.
- Li J-R, Ma Y, McCarthy MC, Sculley J, Yu J, Jeong H-K, et al. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord Chem Rev. 2011; 255: 1791-1823.
- Veawab A, Tontiwachwuthikul P, Chakma A. Corrosion behaviour of carbon dioxide steel in the CO2 absorption process using aqueous amine solutions. Ind Eng Chem Res. 2006; 38: 3917-3924.
- Lee A, Xiao G, Xiao P, Joshi K, Singh R, Webley PA. High temperature adsorption materials and their performance for pre-combustion capture of carbon dioxide. Energy Procedia. 2011; 4: 1199-1206.
- Schäffer A, Brechtel K, Scheffknecht G. Comparative study on differently concentrated aqueous solutions of MEA and TETA for CO2 capture from flue gases. Fuel. 2012; 101: 148-153.
- Korelskiy D, Ye P, Fouladvand S, Karimi S, Sjöberg E, Hedlund J. Efficient ceramic zeolite membranes for CO2/H2 separation. J Mater Chem A. 2015; 3: 12500-12506.
- Stocker K, Ellersdorfer M, Lehner M, Raith JG. Characterization and Utilization of Natural Zeolites in Technical Applications. BHM. 2017; 162: 142-147.
- Zhou H-C, Long JR, Yaghi OM. Introduction to Metal-Organic Frameworks. Chem Rev. 2012; 112: 673-674.
- Ding M, Flaig RW, Jiang H-L, Yaghi OM. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem Soc Rev. 2019; 48: 2783-2828.
- Liang W, Bhatt PM, Shkurenko A, Adil K, Mouchaham G, Aggarwal H, et al. A Tailor-Made Interpenetrated MOF with Exceptional Carbon-Capture Performance from Flue Gas. Chem. 2019; 5: 950-963.
- Liang L, Liu C, Jiang F, Chen Q, Zhang L, Xue H, et al. Carbon dioxide capture and conversion by an acid-base resistant metal-organic framework. Nat Commun. 2017; 8: 1233.
- Pai KN, Baboolal JD, Sharp DA, Rajendran A. Evaluation of diamineappended metal-organic frameworks for post-combustion CO2 capture by vacuum swing adsorption. Sep Purif Technol. 2019; 211: 540-550.
- Hu Y, Verdegaal WM, Yu S-H, Jiang H-L. Alkylamine-Tethered Stable Metal- Organic Framework for CO2 Capture from Flue Gas. ChemSusChem. 2014; 7: 734-737.
- McDonald TM, Mason JA, Kong X, Bloch ED, Gygi D, Dani A, et al. Cooperative insertion of CO2 in diamine-appended metal-organic frameworks. Nature. 2015; 519: 303-308.
- Drisdell WS, Poloni R, McDonald TM, Pascal TA, Wan LF, Pemmaraju CD, et al. Probing the mechanism of CO2 capture in diamine-appended metalorganic frameworks using measured and simulated X-ray spectroscopy. Phys Chem Chem Phys. 2015; 17: 21448-21457.
- Queen WL, Brown CM, Britt DK, Zajdel P, Hudson MR, Yaghi OM. Site- Specific CO2 Adsorption and Zero Thermal Expansion in an Anisotropic Pore Network. J Phys Chem C. 2011; 115: 24915-24919.
- Lee JS, Vlaisavljevich B, Britt DK, Brown CM, Haranczyk M, Neaton JB, et al. Understanding Small-Molecule Interactions in Metal-Organic Frameworks: Coupling Experiment with Theory. Adv Mater. 2015; 27: 5785-5796.
- Adil K, Bhatt PM, Belmabkhout Y, Abtab SMT, Jiang H, Assen AH, et al. Valuing Metal-Organic Frameworks for Postcombustion Carbon Capture: A Benchmark Study for Evaluating Physical Adsorbents. Adv Mater. 2017; 29: 1702953.
- Mukherjee S, Kumar A, Zaworotko MJ. Metal-organic framework based carbon capture and purification technologies for clean environment. In: Ghosh SK, editor. Metal-Organic Frameworks (MOFs) for Environmental Applications: Elsevier. 2019; 5-61.
- Cavenati S, Grande CA, Rodrigues AE. Removal of Carbon Dioxide from Natural Gas by Vacuum Pressure Swing Adsorption. Energy Fuels. 2006; 20: 2648-2659.
- Férey G, Serre C, Devic T, Maurin G, Jobic H, Llewellyn PL, et al. Why hybrid porous solids capture greenhouse gases? Chem Soc Rev. 2011; 40: 550-562.
- Xiong S, Gong Y, Wang H, Wang H, Liu Q, Gu M, et al. A new tetrazolate zeolite-like framework for highly selective CO2/CH4 and CO2/N2 separation. Chem Commun. 2014; 50: 12101-12104.
- Nguyen NTT, Lo TNH, Kim J, Nguyen HTD, Le TB, Cordova KE, et al. Mixed-Metal Zeolitic Imidazolate Frameworks and their Selective Capture of Wet Carbon Dioxide over Methane. Inorg Chem. 2016; 55: 6201-6207.
- Chen K-J, Madden DG, Pham T, Forrest KA, Kumar A, Yang Q-Y, et al. Tuning Pore Size in Square-Lattice Coordination Networks for Size-Selective Sieving of CO2. Angew Chem Int Ed. 2016; 55: 10268-10272.
- Belmabkhout Y, Pillai RS, Alezi D, Shekhah O, Bhatt PM, Chen Z, et al. Metal-organic frameworks to satisfy gas upgrading demands: fine-tuning the soc-MOF platform for the operative removal of H2S. J Mater Chem A. 2017; 5: 3293-3303.
- Bhatt PM, Belmabkhout Y, Assen AH, Weselinski LJ, Jiang H, Cadiau A, et al. Isoreticular rare earth fcu-MOFs for the selective removal of H2S from CO2 containing gases. Chem Eng J. 2017; 324: 392-396.
- Belmabkhout Y, Bhatt PM, Adil K, Pillai RS, Cadiau A, Shkurenko A, et al. Natural gas upgrading using a fluorinated MOF with tuned H2S and CO2 adsorption selectivity. Nat Energy. 2018; 3: 1059-1066.
- Li J-R, Sculley J, Zhou H-C. Metal-Organic Frameworks for Separations. Chem Rev. 2012; 112: 869-932.
- Grande CA, Blom R, Andreassen KA, Stensrød RE. Experimental Results of Pressure Swing Adsorption (PSA) for Pre-combustion CO2 Capture with Metal Organic Frameworks. Energy Procedia. 2017; 114: 2265-2270.
- Herm ZR, Swisher JA, Smit B, Krishna R, Long JR. Metal-Organic Frameworks as Adsorbents for Hydrogen Purification and Precombustion Carbon Dioxide Capture. J Am Chem Soc. 2011; 133: 5664-5667.
- McDonald TM, D’Alessandro DM, Krishna R, Long JR. Enhanced carbon dioxide capture upon incorporation of N,N'-dimethylethylenediamine in the metal-organic framework CuBTTri. Chem Sci. 2011; 2: 2022-2028.
- Herm ZR, Krishna R, Long JR. CO2/CH4, CH4/H2 and CO2/CH4/H2 separations at high pressures using Mg2 (dobdc). Microporous Mesoporous Mater. 2012; 151: 481-487.
- Nandi S, De Luna P, Daff TD, Rother J, Liu M, Buchanan W, et al. A singleligand ultra-microporous MOF for precombustion CO2 capture and hydrogen purification. Sci Adv. 2015;1: e1500421.
- Hendriks C, de Visser E, Jansen D, Carbo M, Ruijg GJ, Davison J. Capture of CO2 from medium-scale emission sources. Energy Procedia. 2009; 1: 1497-1504.
- Boot-Handford ME, Abanades JC, Anthony EJ, Blunt MJ, Brandani S, Mac Dowell N, et al. Carbon capture and storage update. Energy Environ Sci. 2014; 7: 130-189.
- Assen AH, Belmabkhout Y, Adil K, Lachehab A, Hassoune H, Aggarwal H. Advances on CO2 storage. Synthetic porous solids, mineralization and alternative solutions. Chem Eng J. 2021: 129569.
- Law J, Watkins S, Alexander D. In-flight carbon dioxide exposures and related symptoms: associate, susceptibility, and operational implications. NASA. 2010.
- Castle WF. Fifty-Years’ Development of Cryogenic Liquefaction Processes. In: Timmerhaus KD, Reed RP, editors. Cryogenic Engineering. New York, NY: Springer New York. 2007; 146-160.
- Goeppert A, Czaun M, Surya Prakash GK, Olah GA. Air as the renewable carbon source of the future: an overview of CO2 capture from the atmosphere. Energy Environ Sci. 2012; 5: 7833-7853.
- Bhatt PM, Belmabkhout Y, Cadiau A, Adil K, Shekhah O, Shkurenko A, et al. A Fine-Tuned Fluorinated MOF Addresses the Needs for Trace CO2 Removal and Air Capture Using Physisorption. J Am Chem Soc. 2016; 138: 9301-9307.
- McDonald TM, Lee WR, Mason JA, Wiers BM, Hong CS, Long JR. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal- Organic Framework mmen-Mg2 (dobpdc). J Am Chem Soc. 2012; 134: 7056-7065.
- Fracaroli AM, Furukawa H, Suzuki M, Dodd M, Okajima S, Gándara F, et al. Metal-Organic Frameworks with Precisely Designed Interior for Carbon Dioxide Capture in the Presence of Water. J Am Chem Soc. 2014; 136: 8863-8866.
- Nugent P, Belmabkhout Y, Burd SD, Cairns AJ, Luebke R, Forrest K, et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature. 2013; 495: 80-84.
- Llewellyn PL, Bourrelly S, Serre C, Vimont A, Daturi M, Hamon L, et al. High Uptakes of CO2 and CH4 in Mesoporous Metal-Organic Frameworks MIL- 100 and MIL-101. Langmuir. 2008; 24: 7245-7250.
- Kong X, Scott E, Ding W, Mason JA, Long JR, Reimer JA. CO2 Dynamics in a Metal-Organic Framework with Open Metal Sites. J Am Chem Soc. 2012; 134: 14341-14344.
- Mason JA, Sumida K, Herm ZR, Krishna R, Long JR. Evaluating metalorganic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ Sci. 2011; 4: 3030-3040.
- Sumida K, Horike S, Kaye S, Herm ZR, Queen WL, Brown C, et al. Hydrogen storage and carbon dioxide capture in an iron-based sodalite-type metal organic framework (Fe-BTT) discovered via high-throughput methods. Chem Sci. 2010; 1: 184-191.
- Hamon L, Heymans N, Llewellyn PL, Guillerm V, Ghoufi A, Vaesen S, et al. Separation of CO2-CH4 mixtures in the mesoporous MIL-100(Cr) MOF: experimental and modelling approaches. Dalton Trans. 2012; 41: 4052- 4059.
- Soubeyrand-Lenoir E, Vagner C, Yoon JW, Bazin P, Ragon F, Hwang YK, et al. How Water Fosters a Remarkable 5-Fold Increase in Low-Pressure CO2 Uptake within Mesoporous MIL-100(Fe). J Am Chem Soc. 2012; 134: 10174-10181.
- Ramsahye NA, Maurin G, Bourrelly S, Llewellyn PL, Devic T, Serre C, et al. Adsorption of CO2 in metal organic frameworks of different metal centres: Grand Canonical Monte Carlo simulations compared to experiments. Adsorption. 2007; 13: 461-467.
- Li J-R, Yu J, Lu W, Sun L-B, Sculley J, Balbuena PB, et al. Porous materials with pre-designed single-molecule traps for CO2 selective adsorption. Nat Commun. 2013; 4: 1538.
- Yang S, Sun J, Ramirez-Cuesta AJ, Callear SK, David WIF, Anderson DP, et al. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nat Chem. 2012; 4: 887-894.
- Britt D, Furukawa H, Wang B, Glover TG, Yaghi OM. Highly efficient separation of carbon dioxide by a metal-organic framework replete with open metal sites. Proc Natl Acad Sci. 2009; 106: 20637.
- Nugent P, Rhodus V, Pham T, Tudor B, Forrest K, Wojtas L, et al. Enhancement of CO2 selectivity in a pillared pcu MOM platform through pillar substitution. Chem Commun. 2013; 49: 1606-1608.
- Bae Y-S, Hauser BG, Farha OK, Hupp JT, Snurr RQ. Enhancement of CO2/CH4 selectivity in metal-organic frameworks containing lithium cations. Microporous Mesoporous Mater. 2011; 141: 231-235.
- Ramsahye NA, Maurin G, Bourrelly S, Llewellyn PL, Serre C, Loiseau T, et al. Probing the Adsorption Sites for CO2 in Metal Organic Frameworks Materials MIL-53 (Al, Cr) and MIL-47 (V) by Density Functional Theory. J Phys Chem C. 2008; 112: 514-520.
- Burd SD, Ma S, Perman JA, Sikora BJ, Snurr RQ, Thallapally PK, et al. Highly Selective Carbon Dioxide Uptake by [Cu(bpy-n)²(SiF6)] (bpy-1 = 4,4'-Bipyridine; bpy-2=1,2-Bis(4-pyridyl)ethene). J Am Chem Soc. 2012; 134: 3663-3666.
- Mohamed MH, Elsaidi SK, Wojtas L, Pham T, Forrest KA, Tudor B, et al. Highly Selective CO2 Uptake in Uninodal 6-Connected “mmo” Nets Based upon MO42– (M = Cr, Mo) Pillars. J Am Chem Soc. 2012; 134: 19556-19559.
- Noro S-i, Hijikata Y, Inukai M, Fukushima T, Horike S, Higuchi M, et al. Highly Selective CO2 Adsorption Accompanied with Low-Energy Regeneration in a Two-Dimensional Cu(II) Porous Coordination Polymer with Inorganic Fluorinated PF6– Anions. Inorg Chem. 2013; 52: 280-285.
- Llewellyn PL, Bourrelly S, Vagner C, Heymans N, Leclerc H, Ghoufi A, et al. Evaluation of MIL-47(V) for CO2-Related Applications. J Phys Chem C 2013; 117: 962-970.
- Yang Q, Guillerm V, Ragon F, Wiersum AD, Llewellyn PL, Zhong C, et al. CH4 storage and CO2 capture in highly porous zirconium oxide based metalorganic frameworks. Chem Commun. 2012; 48: 9831-9833.
- Wiersum AD, Soubeyrand-Lenoir E, Yang Q, Moulin B, Guillerm V, Yahia MB, et al. An Evaluation of UiO-66 for Gas-Based Applications. Chem Asian J. 2011; 6: 3270-3280.
- Pirngruber GD, Hamon L, Bourrelly S, Llewellyn PL, Lenoir E, Guillerm V, et al. A Method for Screening the Potential of MOFs as CO2 Adsorbents in Pressure Swing Adsorption Processes. ChemSusChem. 2012; 5: 762-776.
- Yang W, Davies AJ, Lin X, Suyetin M, Matsuda R, Blake AJ, et al. Selective CO2 uptake and inverse CO2/C2H2 selectivity in a dynamic bifunctional metalorganic framework. Chem Sci. 2012; 3: 2993-2999.
- Tan C, Yang S, Champness NR, Lin X, Blake AJ, Lewis W, et al. High capacity gas storage by a 4,8-connected metal–organic polyhedral framework. Chem Commun. 2011; 47: 4487-4489.
- Phan A, Doonan CJ, Uribe-Romo FJ, Knobler CB, O’Keeffe M, Yaghi OM. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc Chem Res. 2010; 43: 58-67.
- Wang B, Côté AP, Furukawa H, O’Keeffe M, Yaghi OM. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature. 2008; 453: 207-211.
- Banerjee R, Phan A, Wang B, Knobler C, Furukawa H, Keeffe M, et al. High- Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science. 2008; 319: 939.
- Millward AR, Yaghi OM. Metal-Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J Am Chem Soc. 2005; 127: 17998-17999.
- Furukawa H, Ko N, Go YB, Aratani N, Choi SB, Choi E, et al. Ultrahigh Porosity in Metal-Organic Frameworks. Science. 2010; 329: 424.
- Rowsell JLC, Yaghi OM. Effects of Functionalization, Catenation, and Variation of the Metal Oxide and Organic Linking Units on the Low-Pressure Hydrogen Adsorption Properties of Metal-Organic Frameworks. J Am Chem Soc. 2006; 128: 1304-1315.
- Kesanli B, Cui Y, Smith MR, Bittner EW, Bockrath BC, Lin W. Highly Interpenetrated Metal-Organic Frameworks for Hydrogen Storage. Angew Chem Int Ed. 2005; 44: 72-75.
- Devic T, Salles F, Bourrelly S, Moulin B, Maurin G, Horcajada P, et al. Effect of the organic functionalization of flexible MOFs on the adsorption of CO2. J Mater Chem. 2012; 22: 10266-10273.
- Zheng B, Bai J, Duan J, Wojtas L, Zaworotko MJ. Enhanced CO2 Binding Affinity of a High-Uptake rht-Type Metal-Organic Framework Decorated with Acylamide Groups. J Am Chem Soc. 2011; 133: 748-751.
- Cui P, Ma Y-G, Li H-H, Zhao B, Li J-R, Cheng P, et al. Multipoint Interactions Enhanced CO2 Uptake: A Zeolite-like Zinc–Tetrazole Framework with 24-Nuclear Zinc Cages. J Am Chem Soc. 2012; 134: 18892-18895.
- Vaesen S, Guillerm V, Yang Q, Wiersum AD, Marszalek B, Gil B, et al. A robust amino-functionalized titanium (iv) based MOF for improved separation of acid gases. Chem Commun. 2013; 49: 10082-10084.
- Luebke R, Eubank JF, Cairns AJ, Belmabkhout Y, Wojtas L, Eddaoudi M. The unique rht-MOF platform, ideal for pinpointing the functionalization and CO2 adsorption relationship. Chem Commun. 2012; 48: 1455-1457.
- Sumida K, Rogow DL, Mason JA, McDonald TM, Bloch ED, Herm ZR, et al. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem Rev. 2012; 112: 724-781.
- Sayari A, Belmabkhout Y, Serna-Guerrero R. Flue gas treatment via CO2 adsorption. Chem Eng J. 2011; 171: 760-774.
- Sayari A, Heydari-Gorji A, Yang Y. CO2-Induced Degradation of Amine- Containing Adsorbents: Reaction Products and Pathways. J Am Chem Soc. 2012; 134: 13834-13842.
- Ben-Mansour R, Habib MA, Bamidele OE, Basha M, Qasem NAA, Peedikakkal A, et al. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations – A review. Appl Energy. 2016; 161: 225-255.
- Bastin L, Bárcia PS, Hurtado EJ, Silva JAC, Rodrigues AE, Chen B. A Microporous Metal-Organic Framework for Separation of CO2/N2 and CO2/CH4 by Fixed-Bed Adsorption. J Phys Chem C. 2008; 112: 1575-1581.
- Subramanian S, Zaworotko MJ. Porous Solids by Design: [Zn(4,4'- bpy)²(SiF6)]n•xDMF, a Single Framework Octahedral Coordination Polymer with Large Square Channels. Angew Chem Int Ed. 1995; 34: 2127-2129.
- Shekhah O, Belmabkhout Y, Chen Z, Guillerm V, Cairns A, Adil K, et al. Made-to-order metal-organic frameworks for trace carbon dioxide removal and air capture. Nat Commun. 2014; 5: 4228.
- Elsaidi SK, Mohamed MH, Schaef HT, Kumar A, Lusi M, Pham T, et al. Hydrophobic pillared square grids for selective removal of CO2 from simulated flue gas. Chem Commun. 2015; 51: 15530-15533.
- Chen X-Y, Zhao B, Cheng P, Ding B, Liao D-Z, Yan S-P, et al. Multi- Dimensional Systems Built from Dichromate Anions-Syntheses, Crystal Structures, and Magnetic Properties. Eur J Inorg Chem. 2004; 2004: 562- 569.
- Scott HS, Ogiwara N, Chen K-J, Madden DG, Pham T, Forrest K, et al. Crystal engineering of a family of hybrid ultramicroporous materials based upon interpenetration and dichromate linkers. Chem Sci. 2016; 7: 5470- 5476.
- Xiang S, He Y, Zhang Z, Wu H, Zhou W, Krishna R, et al. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat Commun. 2012; 3: 954.
- Xiang S, Wu X, Zhang J, Fu R, Hu S, Zhang X. A 3D Canted Antiferromagnetic Porous Metal-Organic Framework with Anatase Topology through Assembly of an Analogue of Polyoxometalate. J Am Chem Soc. 2005; 127: 16352- 16353.
- Takamizawa S, Nakata E-i, Yokoyama H. Synthesis of novel copper (II) benzoate pyrazine and its phase transition induced by CO2 adsorption. Inorg Chem Commun. 2003; 6: 763-765.
- Takamizawa S, Akatsuka T, Miyake R. Switch of channel geometry by 1D component slide responding to slight structural stimuli of adsorbed guest. CrystEngComm. 2010; 12: 82-84.
- Takamizawa S, Takasaki Y, Miyake R. Single-Crystal Membrane for Anisotropic and Efficient Gas Permeation. J Am Chem Soc. 2010; 132: 2862-2863.
- Bao Z, Alnemrat S, Yu L, Vasiliev I, Ren Q, Lu X, et al. Kinetic separation of carbon dioxide and methane on a copper metal-organic framework. J Colloid Interface Sci. 2011; 357: 504-509.
- Cadiau A, Belmabkhout Y, Adil K, Bhatt PM, Pillai RS, Shkurenko A, et al. Hydrolytically stable fluorinated metal-organic frameworks for energyefficient dehydration. Science. 2017; 356: 731-735.
- McDonald TM, D’Alessandro DM, Krishna R, Long JR. Enhanced carbon dioxide capture upon incorporation of N,N'-dimethylethylenediamine in the metal-organic framework CuBTTri. Chem Sci. 2011; 2: 2022-2028.
- Liao P-Q, Chen X-W, Liu S-Y, Li X-Y, Xu Y-T, Tang M, et al. Putting an ultrahigh concentration of amine groups into a metal–organic framework for CO2 capture at low pressures. Chem Sci. 2016; 7: 6528-6533.
- Trickett CA, Helal A, Al-Maythalony BA, Yamani ZH, Cordova KE, Yaghi OM. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion. Nat Rev Mater. 2017; 2: 17045.
- Savage M, Cheng Y, Easun TL, Eyley JE, Argent SP, Warren MR, et al. Selective Adsorption of Sulfur Dioxide in a Robust Metal-Organic Framework Material. Adv Mater. 2016; 28: 8705-8711.
- Martínez-Ahumada E, López-Olvera A, Jancik V, Sánchez-Bautista JE, González-Zamora E, Martis V, et al. MOF Materials for the Capture of Highly Toxic H2S and SO2. Organometallics. 2020; 39: 883-915.
- Carter JH, Han X, Moreau FY, da Silva I, Nevin A, Godfrey HGW, et al. Exceptional Adsorption and Binding of Sulfur Dioxide in a Robust Zirconium- Based Metal-Organic Framework. J Am Chem Soc. 2018; 140: 15564- 15567.
- Smith GL, Eyley JE, Han X, Zhang X, Li J, Jacques NM, et al. Reversible coordinative binding and separation of sulfur dioxide in a robust metalorganic framework with open copper sites. Nat Mater. 2019; 18: 1358-1365.
- Zárate JA, Sánchez-González E, Jurado-Vázquez T, Gutiérrez-Alejandre A, González-Zamora E, Castillo I, et al. Outstanding reversible H2S capture by an Al(iii)-based MOF. Chem Commun. 2019; 55: 3049-3052.
- Tan K, Canepa P, Gong Q, Liu J, Johnson DH, Dyevoich A, et al. Mechanism of Preferential Adsorption of SO2 into Two Microporous Paddle Wheel Frameworks M(bdc)(ted)0.5. Chem Mater. 2013; 25: 4653-4662.
- Tchalala MR, Bhatt PM, Chappanda KN, Tavares SR, Adil K, Belmabkhout Y, et al. Fluorinated MOF platform for selective removal and sensing of SO2 from flue gas and air. Nat Commun. 2019; 10: 1328.
- Belmabkhout Y, Pillai RS, Alezi D, Shekhah O, Bhatt PM, Chen Z, et al. Metal-organic frameworks to satisfy gas upgrading demands: fine-tuning the soc-MOF platform for the operative removal of H2S. J Mater Chem A. 2017; 5: 3293-3303.
- Ethiraj J, Bonino F, Lamberti C, Bordiga S. H2S interaction with HKUST-1 and ZIF-8 MOFs: A multitechnique study. Microporous Mesoporous Mater. 2015; 207: 90-94.
- Ding N, Li H, Feng X, Wang Q, Wang S, Ma L, et al. Partitioning MOF-5 into Confined and Hydrophobic Compartments for Carbon Capture under Humid Conditions. J Am Chem Soc. 2016; 138: 10100-10103.
- Zhang W, Hu Y, Ge J, Jiang H-L, Yu S-H. A Facile and General Coating Approach to Moisture/Water-Resistant Metal–Organic Frameworks with Intact Porosity. J Am Chem Soc. 2014; 136: 16978-16981.
- Nguyen JG, Cohen SM. Moisture-Resistant and Superhydrophobic Metal- Organic Frameworks Obtained via Postsynthetic Modification. J Am Chem Soc. 2010; 132: 4560-4561.
- Howarth AJ, Liu Y, Li P, Li Z, Wang TC, Hupp JT, et al. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat Rev Mater. 2016; 1: 15018.
- Rubio-Martinez M, Avci-Camur C, Thornton AW, Imaz I, Maspoch D, Hill MR. New synthetic routes towards MOF production at scale. Chem Soc Rev. 2017; 46: 3453-3480.
- Ren J, Dyosiba X, Musyoka NM, Langmi HW, Mathe M, Liao S. Review on the current practices and efforts towards pilot-scale production of metalorganic frameworks (MOFs). Coord Chem Rev. 2017; 352: 187-219.
- Khabzina Y, Dhainaut J, Ahlhelm M, Richter H-J, Reinsch H, Stock N, et al. Synthesis and Shaping Scale-up Study of Functionalized UiO-66 MOF for Ammonia Air Purification Filters. Ind Eng Chem Res. 2018; 57: 8200-8208.
- Crawford D, Casaban J, Haydon R, Giri N, McNally T, James SL. Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent. Chem Sci. 2015; 6: 1645-1649.
- Moumen E, Assen AH, Adil K, Belmabkhout Y. Versatility vs stability. Are the assets of metal–organic frameworks deployable in aqueous acidic and basic media?. Coord Chem Rev. 2021; 443: 214020.