
Mini Review
Austin Chem Eng. 2022; 9(1): 1087.
The In-Situ Spectroscopy to Investigate the Electrochemical Reduction of CO2
Meng Y1,2*, Feng X3,4, Hao C5, Sun Q6 and Javey A7
1State Key Laboratory of Advanced Optical Communications System and Networks, School of Electronics Engineering and Computer Science, Peking University, Beijing, China
2Center for Flexible Electronics Technology, Tsinghua University, Beijing, China
3Department of Engineering Mechanics, Tsinghua University, Beijing, China
4Interdisciplinary Research Center for Flexible Electronics Technology, Tsinghua University, Zhongguancun North First Street 2, Beijing, China
5State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, P.R. China
6Beijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences, No.8 Yangyan West One Road, Huairou District, Yanxi Economic Development Zone, Beijing, China
7Department of Electrical and Computer Engineering, Michigan State University, East Lansing, Michigan, USA
*Corresponding author: Yanfang Meng, State Key Laboratory of Advanced Optical Communications System and Networks, School of Electronics Engineering and Computer Science, Peking University, Beijing 100871, China; Center for Flexible Electronics Technology, Tsinghua University, Beijing, 100084, China
Received: January 28, 2022; Accepted: February 26, 2022; Published: March 05, 2022
Background
The in situ attenuated total reflection Fourier transform infrared spectroscopic (FTIR) [1-3], dating back to the 90s’ of last decades, is the most powerful approach to real time character the oxygen-containing group on the catalyst during the electrochemical reduction CO2 reaction. The FTIR offers platform for tackle the greenhouse effect steming form continuous increase of CO2 emissions. Though the monitoring real time the attenuated total reflection Fourier transform infrared spectroscopy of the catalyst and intermediate products separately, we can acquire the information of the intermediate products and variation condition of the reaction system and gain strong evident for further analysis. Therefore, the FTIR possesses the advantages of comprehensive, real-time and high sensitivity [4,5].
Research Gap
First, the interpretation of principle of relationship between the microstructure of the corresponding catalysts and the reaction product properties is not accessible by in situ attenuated total reflection Fourier transform infrared spectroscopy. Second, the optimum of synergistic interactions among the multiple factors is supposed to be unambiguitious by in situ attenuated total reflection Fourier transform infrared spectroscopy. Third, to meet the new demands of area of high efficiency, the comprehensive properties of the measurement are supposed to be explored urgently.
Research Question
The evaluating the setup of the in situ attenuated total reflection Fourier transform infrared spectroscopy (FTIR)
Scheme 1 illustrates the traditional of diagram of the electrochemical equipment utilized in the in situ FTIR measurement [6].
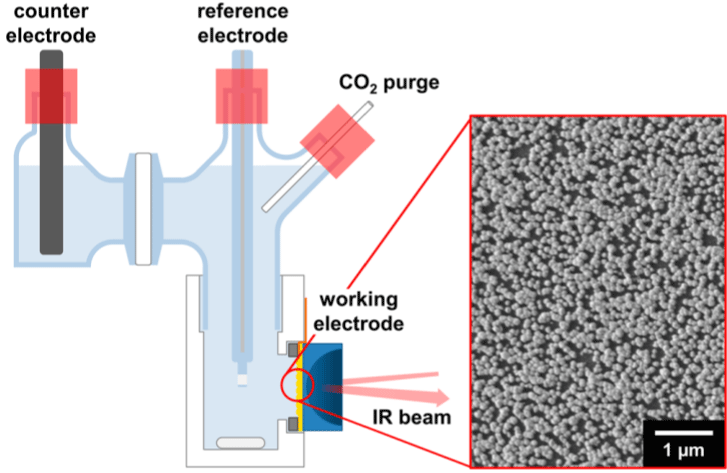
Scheme 1: The advanced scheme diagram of the electrochemical equipment
utilized in the in situ FTIR measurement [12]. Reproduced with permission
from Ref. [12]. Copyright 2018 American Chemical Society.
The reaction tank equipped with counter electrode, reference electrode, gas inlet and gas outlet. The infrared laser is fixed in the bottom of the device and companied with a detector. The real time in situ attenuated total reflection Fourier transform infrared spectroscopy measurement is conducted using each Cu film, which is plated on a hemispherical Si ATR prism. Spectra can be read out via the Kretschmann attenuated total reflection (ATR) configuration using a JASCO FT/IR4200 Fourier transform IR spectrometer equipped with the HgCdTe (MCT) detector with a resolution of 4 cm-1. However the traditional setup of the in situ FTIR had the drawbacks of unsatisfying sensitivity and stability.
The mechanism of the in situ attenuated total reflection Fourier transforms infrared spectroscopy (FTIR)
To describe the mechanism of the in situ ATR- FTIR, the experiments are carried out in two custom spectroelectro-chemical instruments. Spectroscopic measurements are collected in a cell described in their previous work. Experiments were performed in a newly designed spectroelectrochemical cell, which allows for stirring of the electrolyte using a magnetic stir bar. Practically, both cells are the same when not stirred to guarantee that we can directly make comparison between the data from experiments at each concentration from these two cells. The ability to monitor reactions under convection represents a significant step forward in the operando investigation of electrochemical processes.
The IR spectra were calculated using the following equation [7]:
ΔR/R= [R(Ea)-R(Eb)]/R(E ) …… Equation 1
Where, the parameters of R(Ea) and R(Eb) are the reflectivities from the electrode surface recorded at the applied electrode potential E1 (+0.1V vs. RHE) and E2, respectively.
The analysis procedure of the in situ ATR- FTIR
The in situ ATR-FTIR displays the real time IR spectra of the catalyst and intermediate products respectively. In the process of electrochemical reduction, the in situ attenuated total reflection Fourier transform infrared spectroscopy monitor the real time variation of the spectroscopy of the catalyst and intermediate products of during the potential stepwise sweeping from 0 to -0.8 V in a CO2-saturated 0.5m KHCO3 solution. Figure 1 show the realtime ATR-IR spectra recorded during stepping the Pd/C loaded on an Au-coated Si prism from 0 to -0.8V in a CO2-saturated 0.5m KHCO3 solution. It is can be observed several absorbance bands between 2400 and 1600 cm-1 for all the samples. Among these bands, the reversed band centered at 2343 cm-1 should be ascribed to the consumption of CO2 in the solution [8]. The band with positive orientation between 2010 and 1960 cm-1 can be assigned to the linear-bonded CO (COL). The most evident band sit between 1890 and 1830 cm-1 is ascribed to the vibration feature of bridgebonded CO (COB) [9,10]. Another weak band around 1800 and 1780 cm-1 locates at the high potential region involved triple-bonded (COT) or multibonded CO (COM) [11]. However, the efficiency of above analysis procedure of the in situ ATR- FTIR is not desired.
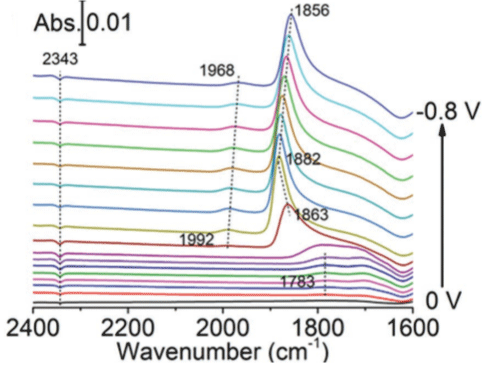
Figure 1: The real-time ATR-IR spectra recorded during stepping the Pd/C
loaded on an Au-coated Si prism from 0 to -0.8V in a CO2-saturated 0.5m
KHCO3 solution. Reproduced with permission from Ref. [16]. Copyright 2018
WILEY-VCH.
Methods
The evaluating the setup of ATR-FTIR
To overcome the unsatisfying sensitivity and stability of traditional setup of the in situ attenuated total reflection Fourier transform infrared spectroscopy (FTIR), the evaluating the setup of the in situ attenuated total reflection Fourier transform infrared spectroscopy (FTIR) is proposed.
Scheme 2 illustrates the advanced scheme diagram of the electrochemical equipment utilized in the in situ FTIR investigation.
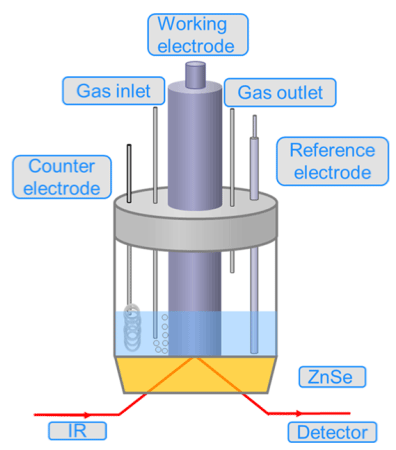
Scheme 2: The traditional of diagram of the electrochemical equipment
utilized in the in situ FTIR measurement. Reproduced with permission from
Ref. [6]. Copyright 2019 American Chemical Society.
Marco Dunwell et al. [12] proposed an advanced scheme diagram of the electrochemical equipment utilized in the in situ FTIR measurement with Au film working electrode, Ag/AgCl reference electrode, and graphite rod counter electrode. The spectroscopic measurement during the whole process is under stirred to guarantee the stability in 0.5 M NaHCO3. The scanning electron microscope image of the Au film on Si is inserted in Scheme 2. The in-situ measurement experiments were conducted in two custom spectroelectrochemical cells. In 0.25 and 1.0 M NaHCO3 solution, the Spectroscopic measurements were carried out in a cell described in their previous work [13]. Experiments in 0.5M NaHCO3 aqueous solution were performed in a newly designed spectroelectrochemical cell with the Si ATR crystal is mounted on the side of the cell, enables for stirring of the electrolyte using a magnetic stir bar (Scheme 2). Practically, the measurements are adopted the identical cells when not stirred, such that data from experiments at each concentration from these two cells can be directly compared. The capacity to monitor reactions under convection demonstrates a significant step forward in the operando investigation of electrochemical processes. In traditional interfacial Fourier Transform infrared spectroscopy, transport is severely hindered by the thin-layer electrolyte configuration. Although the use of ATR-SEIRAS enhances transport significantly, transport was still determined solely by diffusion in all previous spectroelectrochemical studies [14].
The mechanism of the in situ ATR-FTIR
To further clarify the mechanism of the ATR-FTIR, blank experiments are supposed to be conducted. The catalyst species and intermediates can also be labeled by isotope and can be real-time detect by luminance from isotope. As an example, Song Jin et al. [15] have dramatically elevated the efficient of the electrochemical reduction carbon dioxide by incorporating the oxygen-containing species onto the copper electrode. They carried out the in situ spectroscopy combining with the isotope exchanging strategy to verify that the new peck was ascribed to the a kind of intermediatessurface hydroxyl group.
The analysis procedure of the in situ ATR-FTIR
Apart from the basis experiment, control experiments were also carried out using FTIR in an Ar-saturated 0.5M KHCO3 solution. For example, Shangqian Zhu et al. conducted FTIR by control experiments were also carried out using FTIR. They found that CO bands could still be observed even no external CO2 source was provided. Nevertheless, the CO band intensities were drastically decreased and the onset potentials for the observation of CO were more negative as compared with the spectra recorded in the CO2-saturated solution. They anticipated that this phenomenon could be explained by CO2 molecules in equilibrium with bicarbonate anions could still promote the reaction, and was consistent with their previous surface enhanced IR spectroscopic investigation on Cu surfaces [16]. Besides, they also detected that all the CO bands were absent in the spectra recorded in the saturated phosphate buffered saline solution, suggesting they were generated from CO2 reduction in the Ar-saturated KHCO3 solution.
The in-situ spectroscopy to investigate the electrochemical reduction of CO2 provides the information of the intermediate products and variation condition of the reaction system and gain the evidence for further analysis. Koga Osamu et al. employed in situ attenuated total reflection Fourier transform infrared spectroscopy to studies the adsorbed species on Ni and Fe electrode in electrodechemical reduction of CO2. They detected that the two species, ascribe to linear and bridged CO molecule were also formed on Ni electrode [5]. The ATR-IR analysis provided deep insight for them to processing optimization to promote the conversion efficiency of CO2.
Significance
With the growing awareness towards environmental protection, the electrochemical reduction of CO2 have been given considerable attention to fulfill the demands of essential environmental protection and utilization of source. The great progresses have been made to improve the efficient of electrochemical reduction of CO2. Through systematically introducing the fundamental knowledge of mechanism of the electrochemical reduction of CO2 in situ attenuated total reflection Fourier transform infrared spectroscopy, our review offer systematical information for the corresponding scientists and researchers to exploit he electrochemical reduction CO2 by the in situ attenuated total reflection Fourier transform infrared spectroscopy (FTIR). However, there are still many challenges for the development of exploitation he electrochemical reduction CO2 by the in situ attenuated total reflection Fourier transform infrared spectroscopy (FTIR).
References
- Bai HC, Cheng T, Li SY, et al. Controllable CO adsorption determines ethylene and methane productions from CO2 electroreduction. Science Bulletin. 2021; 66: 62-68.
- Hori Y, Koga O, Yamazaki H, et al. Infrared Spectroscopy of Adsorbed CO and Intermediate Species in electrochemical reduction of CO2 to Hydrocarbons on a Cu electrode. Electrochimca Acta. 1995: 2617-2622.
- Yoshio H, Osamu K, Akiko A, Michio E. Infrared Spectroscopic Observation of Adsorbed CO Intermediately Formed in the Electrochemical Reduction of CO2 at a Nickel Electrode. Bulletin of the chemical society of Japan. 1992: 3008-3010.
- Jin S, Hao Z. M, Zhang K, et al. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Inter. Edition. 2021; 38: 20627-20648.
- Osamu K, Tadanori M, Hiroki Y, et al. Infrared spectroscopic observation of intermediate species on Ni and Fe electrodes in the electrochemical reduction of CO2 and CO to hydrocarbons. Bulletin of the chemical society of Japan. 1998: 7292-7301.
- Chen S, Chen A. Electrochemical Reduction of Carbon Dioxide on Au Nanoparticles: An in Situ FTIR Study. J. Phys. Chem. C. 2019; 123: 23898- 23906.
- Adams BD, Asmussen RM, Ostrom CK, et al. Synthesis and comparative study of nanoporous palladium-based bimetallic catalysts for formic acid oxidation. J. Phys. Chem. C. 2014; 118: 29903-29910.
- Nikolic B, Huang H, Gervasio D, et al. Electroreduction of carbon dioxide on platinum single crystal electrodes: electrochemical and in situ FTIR studies. J. Electroanal. Chem. Interfacial Electrochem. 1990; 295: 415.
- Wang JY, Zhang HX, Jiang K, et al. From HCOOH to CO at Pd Electrodes: A Surface-Enhanced Infrared Spectroscopy Study. J. Am. Chem. Soc. 2011; 133: 14876.
- Jiang K, Xu K, Zou S, et al. B-Doped Pd Catalyst: Boosting Room- Temperature Hydrogen Production from Formic Acid-Formate Solutions. J. Am. Chem. Soc. 2014; 136: 4861.
- Zhu S, Jiang B, Cai WB, et al. Direct Observation on Reaction Intermediates and the Role of Bicarbonate Anions in CO2 Electrochemical Reduction Reaction on Cu Surfaces. J. Am. Chem. Soc. 2017; 139: 15664.
- Dunwell M, Yang X, Setzler BP, et al. Examination of Near-Electrode Concentration Gradients and Kinetic Impacts on the Electrochemical Reduction of CO2 using Surface Enhanced Infrared Spectroscopy. ACS Catal. 2018; 8: 3999-4008.
- Dunwell M, Lu Q, Heyes JM, et al. The Central Role of Bicarbonate in the Electrochemical Reduction of Carbon Dioxide on Gold. J. Am. Chem. Soc. 2017; 139: 3774-3783.
- Osawa M. Surface-Enhanced Infrared Absorption. In Near-Field Optics and Surface Plasmon Polaritons. Kawata S, Ed.; Springer: Berlin, Heidelberg. 2001; 81: 163-187.
- Lu Q, Li CS, Zhang HC, et al. Oxygen induced promotion of electrochemical reduction of CO2 via co-electrolysis. Nat. Commun. 2020; 11: 3844.
- Zhu SQ, Wang Q, Qin XP, et al. Tuning Structural and Compositional Effects in Pd-Au Nanowires for Highly Selective and Active CO2 Electrochemical Reduction Reaction. Adv. Energy Mater. 2018; 8: 1802238.
- Osamu K, Tadanori M, Hiroki Y, Yoshio H, et al. Infrared spectroscopic observation of intermediate species on Ni and Fe electrodes in the electrochemical reduction of CO2 and CO to hydrocarbons. Bulletin of the chemical society of Japan. 1998: 7292-7301.