
Research Article
Austin Chem Eng. 2022; 9(1): 1088.
Absorption of Carbon Dioxide into Glycine Activated Methyldiethanolamine (MDEA) Comparing with Diethanolamine (DEA)
Elhosane Y¹, Elhesain MHA², Muawia M¹ and Abdalla M²*
1Department of Chemical Engineering, Faculty of Engineering and Technical Studies, University of Al Imam Al Mahdi, Kosti, Sudan
2Department of Food Processing Engineering, Faculty of Engineering and Technical Studies, University of Al Imam Al Mahdi, Kosti, Sudan
*Corresponding author: Mohammed Abdallab, Department of Food Processing Engineering, Faculty of Engineering and Technical Studies, University of Al Imam Al Mahdi, Kosti, Sudan
Received: February 03, 2022; Accepted: March 21, 2022; Published: March 28, 2022
Abstract
It is widely accepted that increasing carbon dioxide (CO2) emissions to our atmosphere is the major contributor to global climate change, which pollutes environment. The chemical absorption using tertiary alkanolamines solution is one of most important method, which have been proposed and studied for the removal of carbon dioxide because the MDEA solvent has low cost, low corrosive tendencies, high stability, low viscosity, low tendency to foam, and low flammability, however it has low reaction rate. Therefore, we added glycine promoter to conventional solvents to increase the rate of the reaction because glycine is a primary amine compound which is reactive. Moreover, glycine has resistance to high temperatures; so, it will not easy to degrade making it not suitable for application in industry. The main purpose of this study was to provide reaction kinetics data of CO2 absorption into glycine promoted methyldiethanolamine (MDEA) by using laboratory scale wetted wall column equipment at the atmospheric pressure by varying temperature from 303.15 to 328.15 and glycine concentration from 1% to 3%, while the carbon dioxide absorption rate was measured by titration of liquid effluent. Based on the result of this study, we observed that by increasing temperature and concentration of glycine, the absorption rate of carbon dioxide in MDEA solution was increase. In addition, the reaction rate constant will be affected by the temperature and the concentration of promoter. The correlation of reaction rate constant (k glycine) was: k glycine = 5.3409E+13exp(-3251.9/T) with the activation energy for glycine promoter is 27.0363kJ/kmol, indicating it a very influencing, compared to diethanolamine (DEA).
Keywords: Reaction kinetic; Carbon dioxide absorption; Promoter; Wetted column
Introduction
Natural events and human activities are believed to be contributing to an increase in average global temperatures. This is caused primarily by increases in “greenhouse” gases, such as carbon dioxide and trace gases. Increasing of carbon dioxide (CO2) emissions from the chemical industry to our atmosphere is the major contributor to global climate change, which pollutes environment. That is why; removing carbon dioxide from the chemical industry field is very important things to mitigate the problem of global warming, wherefore we need to reduce CO2 emissions by using the optimized method [1].
This study focused on one of the solutions using reactive absorption technology to remove (CO2). Because absorption has such advantages as large capacity, high efficiency and good industrial performance, always has been favored by researchers. The selective chemical absorption of CO2 by a solvent is the most well-established method of CO2 capture in power plants and from the gas sources. High product yields and purities can be obtained with this method. Because the alkanolamines solution one of most important method which have been proposed and studied for the removal of carbon dioxide from natural gas sources and refinery gases or fossil fuel combustion [2]. We choose MDEA solvent as the absorbent because it has several advantages, such as a low vapour pressure, it is not easy degradation, low corrosive, low reaction heat, high selectivity to remove CO2, and more attractive. However, because of the poor reaction velocity, we added glycine promoters to conventional solvents to make the catalyst so fast, which hasn’t been done before with MDEA in privies investigations to ascertain kinetic data for CO2 absorption into glycine activated methyldiethanolamine (MDEA) solution.
Reaction Scheme and Mathematical Model
Carbon Capture and Storage (CCS) refers to the set of technologies developed to capture carbon dioxide CO2 gas from the exhausts of power stations and from other industrial sources, the infrastructure for handling and transporting to use as an energy source. There are several technologies that could be used for CO2 captures, such as absorption, adsorption, cryogenic recovery, membrane separation and chemical looping combustion. Because chemical absorption has three advantage of dealing with low concentration, low pressure and large flux exhaust gas in large scale industrial application; it has been regarded as one of the most promising method to capture CO2 from flue gas [3].
Absorption with chemical reaction
Mass transfer with chemical reaction takes place whenever two phases which are not at chemical equilibrium with one another are brought into contact. Such phenomena are made up of a number of elementary steps, which may be summarized as follows:
• Diffusion of one reactant from the bulk of gas phase to interface between the gas-liquid.
• Diffusion of the reactant from the interface towards the bulk of liquid phase.
• Chemical reaction within liquid phase.
• Diffusion of reactant initially presents within liquid phase, and/or of reaction product, within phase liquid itself, due to concentration gradients which are set up by the chemical reaction.
Mass transfer processes are coupled with a chemical reaction, in order to improve the rate and yield of the process.
In case of absorption of gas (A) into liquid, there is possibility that the dissolved gas (A) to reacts with other solvent/ reactant dissolved in liquid with rate of reaction of rA. Consider Figure 1, we make mass balance over volume element dv or (S.dx) (parameters in the question).
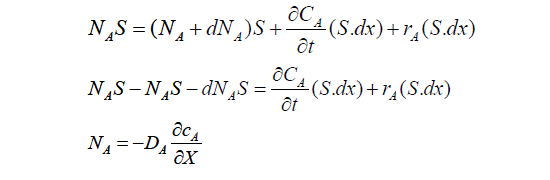
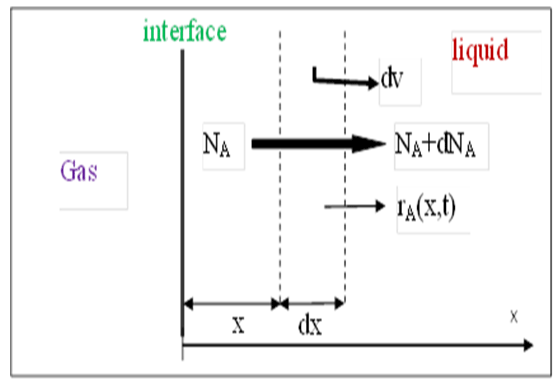
Figure 1: Mass Transfer of Gas into Liquid with Chemical Reaction.
Rate in = Rate out + Accumulation + Reaction rate.
Diffusion flux A in liquid (Fick’s first law). When the Equation (1) is divided by S.dx, and substitution value of NA get:
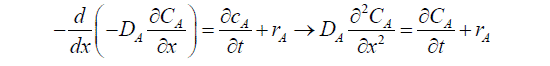
It becomes necessary to write such an equation (2) for each molecular species taking part in the reaction in order to completely describe the system.
Reaction kinetic of carbon dioxide absorption
The reactions will be occurring at the equilibrium when the carbon dioxide absorbed in the solution as follows:
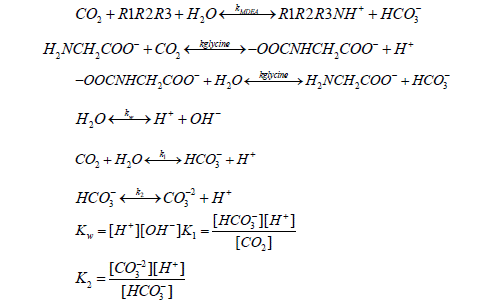
Carbon dioxide absorption in a wetted wall column
The wetted wall column was constructed from a tube with a wall defined surface area stand when the Liquid overflowed from the inside and formed a liquid film over the outer surface of the tube. Gas entered from the bottom of the wetted wall column and counter currently contacted the liquid film.
Experimental
This study was conducted to determine the reaction kinetics data of carbon dioxide absorption gas into glycine promoted MDEA using a laboratory scale wetted wall column at atmospheric pressure and the temperature in the interval 303.15K (30°C) - 328.15K (55°C) [4].
Materials
Mixture of gas a composition 20% of CO2 and 80% N2, MDEA, Glycine, NaOH, HCl, Oxalic acid (C2H2O4), Aqueduct, phenophalene, and methyl orange Indicator.
Equipment: WWC, water bath, MDEA solution tank, a tank of samples, burette, the stand, Conical flask, measuring cup, funnel glass, volume pipette, pipette.
Operating conditions and variables research
Pressure: 1) Atmosphere; 2) Temperature: 30, 35, 40, 45, 50, 55 °C.
Gas feed: 1) A mixture of 20% CO2 + 80% N2; 2) The gas flow rate: 6L/min.
Solvents: 1) Type of solvent: MDEA; 2) The concentration of solvent: 30% by weight; 3) The solvent flow rate: 200mL/min.
Promoter: 1) Type of promoter: glycine; 2) The concentration of promoter: 1-3% (wt. %).
Glycine Analysis of concentration content of carbonate and bicarbonate will conducted by titration method.
Operation procedure
Fill water into the water bath as a regulator of temperature in the WWC jacket. Creating Methyldiethanolamine solution of 30% by weight. Each adding of glycine (wt. %) in accordance with the variable to a solution of MDEA 30% by weight. MDEA solution with the promoter enters into the reservoir tank. Setting the operating temperature in accordance with the variable. Circulate hot water in the annulus side column of WWC and returned to the water bath [5].
Circulating MDEA solution with promoter by using the pump from the reservoir tank to the overflow tank until the solution overflow. Adjusting the flow rate of MDEA solution with the promoter, so the solution flows from the bottom to up through the inside of the tube to form a thin film on the tube surface. When the temperature has reached the desired system and the flow has stabilized, a mixture of carbon dioxide and nitrogen gas flowed through the saturator tank. Then a mixture of carbon dioxide and nitrogen gas flows from the bottom of the column to the top of the column outer stainless steel pipe so that it contacts between carbon dioxide and nitrogen gas with a thin layer of fluid around the outer surface of the tube until steady state conditions. If achieved steady state conditions, taking samples of MDEA solution with promoter from the sample reservoir tank. For analysis using the content of carbonate and bicarbonate using a titration method by using phenolphthalein and a methyl orange indictor.
Data evaluation
Based on data from experimental results and some of the literature can be calculated to determine the reaction rate constants of promoter with the following stages:

Results and Discussion
Based on the experimental data, the result which I got from this study as the reaction kinetics data of carbon dioxide absorption into glycine activated methyldiethanolamine (MDEA) by using laboratory scale wetted column equipment at atmospheric pressure by varying temperatures from 303.15 to 328.15, the solvent content from 30% wt. MDEA, and glycine concentration (1%-3% wt), the mixer of gas content from 20% carbon dioxide and 80% nitrogen [6-8].
The effect of the temperature and the concentration of promoter on the carbon dioxide absorption rate
In Figure 2a and 2b, where the carbon dioxide absorption rate tends to increase with increasing temperature at a glycine concentration. Absorption rate is due to the increasing temperature, because the kinetic energy of the substances molecules which react will increase so that the reaction will be faster and the temperature is increase the diffusivity [9]. And also by increasing promoter concentration from (1-3% wt.) the reaction rate will be faster because we already increase the amount of catalyst.
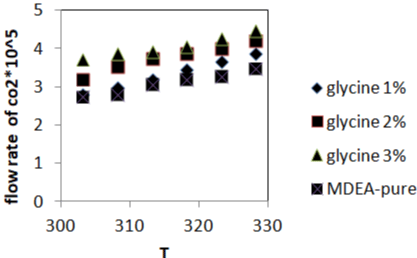
Figure 2a: The effect of the temperature and the concentration of promoter
on the carbon dioxide absorption rate.
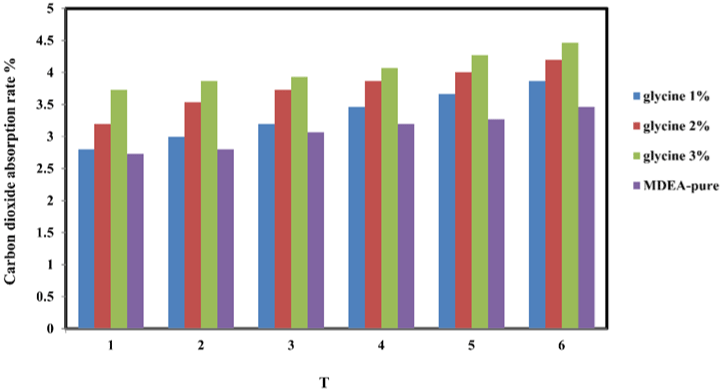
Figure 2b: The effect of the temperature and the concentration increase of promoter towards carbon dioxide absorption rate.
The effect of the temperature and the concentration of promoter on carbonate final concentration
In Figure 3 show that the increase of temperature at increase promoter concentration from (1%-3%wt) cause increase in carbonate concentration [10].
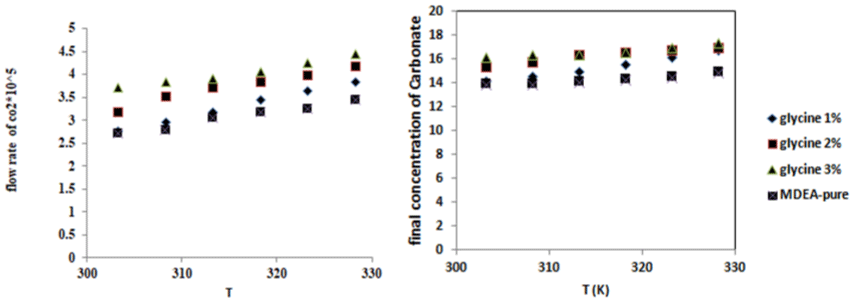
Figure 3: The effect of the temperature and the concentration of promoter on carbonate final concentration.
The effect of promoter concentration increase with time contact between gas and liquid will be slow that is why due the increase of carbonate concentration.
Reaction rate constant
The equation of reaction rate constant obtained from the results of this research and is featured in two models that is glycine promoter reaction rate constant (kglycine) and reaction constant rate apparent (kapp).
From experiment results, literature and calculation we obtained overall reaction rate constant as show in the Figure 4 we got it by using trial method for equation 3.9 and 3.10. In this figure we observed when the promoter concentration and temperature increase; the overall constant rate will be increase too. This case because reaction rate constant is affected by the temperature and promoter constant [11].
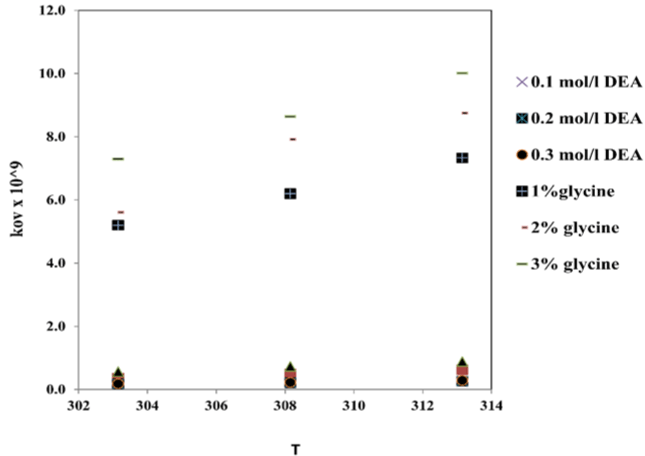
Figure 4: The effect of the temperature and the concentration of promoter on
overall constant rate reaction comparing with DEA prompter.
Reactivity glycine as a promoter in the absorption of carbon dioxide can be determined from the reaction rate constants were calculated by Arrhenius equation k =A*exp(-E/RT) [12].
In Figure 5, we obtained intercept for glycine that is ln A = 31.609 and the slope is (-E/R) = 3251.9, so the equation kglycine = 5.3409E+13exp (-3251.9/T).
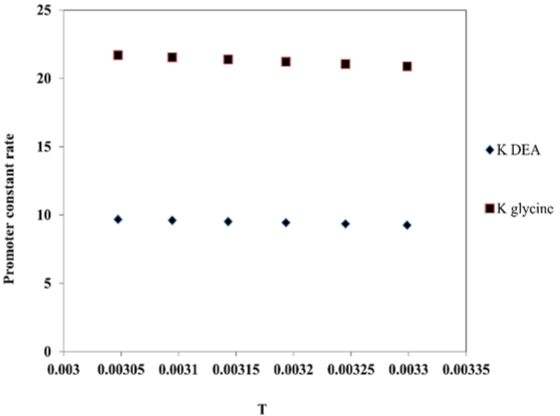
Figure 5: The effect of the temperature on promoter constant rate reaction
comparing with DEA promoter using MDEA solvent.
Where,
K = Reaction rate constant
A = Pre exponential factor
E = Activation energy
R = Gas constant 8.314 (kJ/kmol.k)
T = Temperature
From above equation obtained the regression value 0.9964 for glycine promoter and the activation energy is 27.0363kJ/kmol.
Figure 6 is showed the low value of ln k, at the high value of 1/T, this case proves that at the higher temperature, the value of activation energy will be smaller and the reaction time which is needed will be fast wherefore will increase reaction rate constant [13-15].
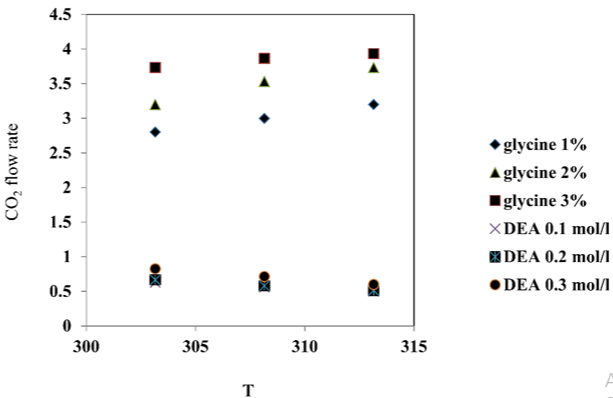
Figure 6: The effect of the temperature and the concentration of glycine
promote on CO2 flow rate comparing with DEA.
Conclusion
Based on the collation date we observed that:
When we increase the temperature the absorption rate will be increase, and also by increasing promoter concentration from (1-3% wt.) the reaction rate will be faster.
The overall reaction rate constant is affected by the temperature and promoter concentration and it is so big comparing with anther promoter in some pervious works.
The correlation of reaction rate constant (kglycine) expressed by the following equation:
Kglycine = 5.3409E+13exp (-3251.9/T) with activation energy for glycine promoter is 27.0363kJ/kmol.
When we used diethanolamine (DEA) as a promoter instead of glycine the carbon dioxide Absorption rate also increase when the concentration of the promoter increase however decrease when the temperature increase.
References
- Aboudheir A, Ramachandran N, Raphael I, Paitoon T. Kinetics of the Absorption of CO2 into Mixed Aqueous Loaded Solutions of Monoethanolamine and Methyldiethanolamine, A-I. 2006; 45.
- Ahuja DA. Relative contributions of greenhouse gas emission to global warming. 1990; 344: 529-531.
- Altway A. Mas transfer with chemical reaction, Bee Marketer Institute, Jakarta. 2009.
- Amalia R. Kinetics of Carbon dioxide Absorption into Aqueous Potassium Carbonate Solution Promoted by Methyldiethanolamine and Diethanolamine Mixture. Thesis, ITS, Surabaya. 2014.
- Cullinanae JT and Rochelle GT. Carbon dioxide Absorption with Aqueous Potassium Carbonate Promoted by Piperazine. Chem. Eng. Sci. 2004; 59: 3619-3630.
- Dang H and Rochelle GT. CO2 Absorption Rate and Solubility in Monoethanolamine/Piperazine/Water. Dissertation. The University of Texas at Austin. 2001.
- Danckwerts PV. Gas-Liquid Reactions, McGraw-Hill, New York. 1970.
- Geankoplis CJ. Transport Process and Unit Operations, 3th Edition, Prentice- Hall International. 1993.
- Lin ChW, Allan N Soriano, Meng H. Kinetics study of carbon dioxide absorption into aqueous solutions containing N-methyldiethanolamine + diethanolamine. 2009; 40: 403-412.
- Rozi M. Simulation of Absorption CO2 and H2S with Aqueous MDEA in Valve- Tray column. Thesis, ITS, Surabaya. 2013.
- Paul SA, Ghoshal AK, Mandal B. Kinetics of absorption of carbon dioxide into aqueous blends of 2-(1-piperazinyl)-ethyalamine (PZEA) and N-Methyldiethanolamine (MDEA). Chemical Engineering Science. 2009; 64: 1618-1622.
- Penders NJC, Hamborg ES, Huttenhuis PGE, Fradette S, Carley GA, Versteeg GF. Kinetics of Absorption of Carbon Dioxide in Aqueous Amine and Carbonate Solution with Carbonic Anhydrase. International Journal of Greenhouse Gas Control. 2013; 12: 259-268.
- Wilke CR and Chang P. Correlation of Diffusion Coefficient in Dilute Solutions. ALChE Journal. 1955; 1: 264-270.
- Xu S, Wang Y, Otto FD, Mather AE. Kinetic of the Reaction of Carbon dioxide with 2-Amino-2-Methyl-1-Propanol Solutions. 1995; 51: 841-850.
- Yi Fei, Zou Hai-Kui, Chu Guang-Wen, Shao Lei. Modeling and Experimental Studies on Absorption of CO2 by Benfield Solution in Rotating Packed Bed. Chemical Engineering Journal. 2009; 145: 377-384.