
Review Article
Austin Chem Eng. 2022; 9(1): 1090.
A Review on Extraction of Bioactive Compounds from Moringa oleifera Leaves: Their Principle, Advantages, and Disadvantages
Paveanthan Mehganathan and Nur Ayshah Rosli*
School of Chemical Engineering, Engineering Campus, UniversitiSains Malaysia
*Corresponding author: Nur Ayshah Rosli, School of Chemical Engineering, Engineering Campus, UniversitiSains Malaysia, 14300 Nibong Tebal, Pulau Pinang, Malaysia
Received: May 24, 2022; Accepted: June 23, 2022; Published: June 30, 2022
Abstract
Moringa oleifera, belongs to the Moringaceae family, is an indigenous plant and native to the North India region. It has been used for centuries as traditional medicine and nutritional supplement. Moringa olefeira leaves contain high phenolics and flavonoid compounds as major constituents such as kaempferol, quercetin, caffeoylquinic acid, coumaroylquinic acid, and feruloylquinic acid. The extraction techniques play a critical role in the extraction outcome such as crude extracts yield, type and quantity of compound extracted. To date, there is a wide range of technologies for crude plant extraction such as ultrasoundassisted extraction, microwave-assisted extraction Soxhlet extraction, and dipping (maceration) technique. These extraction techniques employ various types of solvent which could enhance the efficiency of extraction and quality of the compound extracted. Hence, this review aims to describe and compare the conventional and novel extraction techniques methods of Moringa oleifera leaves based on the total phenolics content, flavonoid content, and antioxidant activity. The difference based on the extraction process principle, advantages, and disadvantages were further evaluated to show the suitability, environmentally friendly, the economic feasibility of the various extraction methods. From this review, ultrasound-assisted extraction (UAE) and microwave-assisted extraction (MAE) have minimized the processing time, which is useful for extracting thermolabile compounds, such as phenol compounds. In conclusion, novel extraction techniques could be effectively enhancing the total phenolic compound, flavonoid content, and antioxidant activities of crude extracts, which provides a theoretical basis for upgrading to alarge-scale applicationin the future.
Keywords: Moringa Oleifera; Extraction; Ultrasound-Assisted Extraction; Antioxidant
Introduction
Many tropical countries, including Malaysia, Cambodia, and the Philippines, have used Moringa oleifera as traditional medicine [1- 2]. Every part of the Moringa oleifera plant has its specific medicinal benefits. It is versatile in terms of its usage in combating malnutrition and medicinal properties, for instance, anti-microbial and antiinflammatory properties; hence it has been commercialized as a nutritional food and medicinal remedy. The Moringa oleifera plant can be considered a natural antioxidant, anti diabetic and anticancer agent because of its richness in presence of phenolic compounds such as flavonoids and phenolic acids [3-4].
Any medicinal research begins with pre-extraction and extraction procedures, which are critical steps in extracting bioactive constituents from plant materials. Solvent extraction is the process of extracting a bioactive compound from the plant material using a significant solvent. There are several factors such as solvent type, temperature, and agitation that can affect solid-liquid extraction (SLE) [5]. Temperature can increase the solubility of the bioactive component and lower the viscosity of the solvents which were used in the SLE method. Previously, maceration, Soxhlet, microwave-assisted extraction, and ultrasound-assisted extraction have all been used to extract Moringa oleifera leaves.
Conventional extractions such as maceration and Soxhlet extraction are routinely utilized in small research settings, however contemporary extraction methods such as microwave-assisted and ultrasound-assisted extraction have made considerable breakthroughs. Careful evaluation needs to be considered, especially the selection of a proper extraction method [6]. This review explains the principle, benefits, and drawbacks of extraction techniques to aid in the selection of the best technique for extracting bioactive chemicals from Moringa oleifera leaves in an easy, practicable, and quick manner.
Pre-Extraction Preparation of Plant Sample
The integrity of the biomolecules in the plant leaves requires sample preparation prior to extraction. Washing, drying, grinding, and sieving are all steps in the preparation process that affect phytochemical preservation in the final extracts and it is depending on the extraction procedures and the purpose of the extraction. Fresh samples are more delicate and decay more quickly than dried samples. However, there was no significant difference in total phenolic content between fresh and dried Moringa oleifera leaves, although the dried sample had a higher flavonoid concentration [2]. Primarily, the Moringa oleifera leaves were cleaned and dried under the shade. The dried sample was ground and sieved (20 mesh) to become powder. The powdered form is kept in a sealed container such as desiccators to prevent moisture trapped in the samples until it is used for the extraction. The presence of moisture could promote the growth of unwanted fungal [7].
Maceration
Maceration is previously used in wine-making techniques and has become extensively used in plant extraction studies. The plant materials (coarse or powdered) were soaked for at least three days at room temperature in solvents such as methanol, acetone, or ethanol, with regular agitation [8]. The maceration technique is based on the diffusion and osmosis phenomena. This process assists in the release of phytochemicals from the softened plant’s cell wall. Filtration was used to filter the mixture after three days. Vongsak et al., (2013) used the maceration procedure on Moringa leaves, which involved macerating the dried powdered leaves with 70% ethanol (1:40, w/v) for 72 hours at ambient temperature with intermittent shaking [2]. The extract was filtered, and the marc (extraction residue) was extracted again using the same method and solvent until the extraction was finished. This maceration procedure yielded the largest extract yield (40.50%, w/w) with maximum total phenolic contents of 13.23 g CAE/100 g extract and total flavonoid contents of 6.20 g IQE/ 100 g extract, respectively. At effective concentrations, this extract has a high DPPH scavenging activity, with an IC50of 62.94g/mL. The maceration technique requires longer extraction duration to obtain a high yield of total phenolic content. Although maceration is one of the traditional techniques, this method appears simple and easy to handle [9]. Suitable solvent type and strength can help to enhance the extraction efficiency to produce high yield of crude extract. Besides, a high amount of solvent used in the extraction process also requires proper management of waste.
Soxhlet Extraction
Soxhlet extraction has been a standard technique for extraction for over a century [10]. The ground material is placed in a thimble filled with solvent for extraction purposes in this protocol. When the liquid reaches the overflow level, a siphon aspirates it from the thimble and unloads it back into the distillation flask with the extracted phytochemical. As this process is continuous, the method will be continued until the extraction is complete Furthermore, when the sample is continually brought into touch with fresh sections of the extractants, the mass transfer equilibrium is displaced. Moringa oleiferacrude has been previously extracted using the Soxhlet process which involved placing dried leaves on a thimble and extracting with 70% ethanol and solvent ratio of 1:50, w/v [2]. The extraction was done five times until it was exhausted. The combined extract from each extraction process id filtered in the last step, and the filtered are dried at 50°C under decreased pressure. The crude yield obtained from the Soxhlet method is 35.87% w/w, which is lower than the maceration method. Using the Soxhlet technique, the total phenolics and total flavonoids contents were 12.47 g CAE/ 100 g and 6.71 g IQE/100 g, respectively [2]. Soxhlet method requires a smaller quantity of solvent compared to maceration [11]. Similar with maceration technique, Soxhlet extraction requires a longer extraction duration which is 16 to 20 hours for extraction and also produces a high volume of solvent as wastes which can cause environmental problems if not treated properly before discharge. The ideal sample for Soxhlet extraction is also limited to a dry and finely divided solid. Additionally, temperature, solvent-sample ratio, and agitation speed were among the important factors influencing the Soxhlet extraction efficiency [12].
Microwave-Assisted Extraction (MAE)
Microwave-assisted extraction (MAE) is a modern method that has become interest to researchers for its capability. The MAE uses microwave energy to help analytes from plant material partition into the solvent [13]. MAE improves extraction kinetics and minimizes solvent consumption for more effective extraction [14]. Figure 1 shows the extraction mechanism of MAE in which the solute in the plant matrix undergoes desorption under high pressure and temperature condition. Subsequently, the solutes will separate from the plant matrix and diffuse in the solvent. The transfer of the analytes from the matrix to solvent is achieved by the diffusion and convection process. The microwave oven works at a frequency of 2.45 Hz with a wavelength of 12.2 cm and extracts phenolic chemicals from Moringa oleifera leaves [15]. There were several parameters investigated such as temperature, time, sample-to-solvent ratio, ethanol concentration, and microwave power. After the irradiation of the microwave, the mixture of the extract was cooled before the filtration. The antioxidant and phenolic content of the components were quantified after the ethanolic extract was purified and lyophilized to dryness under ideal circumstances. Under the optimum conditions with 35% ethanol solvent, the total phenolic content was 16.5 mg GAE per g of the dry Moringa oleifera leaves [16]. In another research by C. Rodríguez-Pérezthe optimum MAE conditions were at temperature of 158°C, solvent concentration 42% ethanol, and 20 min extraction, has produced 25.75% crude yield, and total phenolic content of 86 ± 4 (mg Eq GAE/ g dry leaf) respectively [17]. They concluded, extraction temperature and the solvent-sampleratioplay an important role in the extraction of polyphenols using MAE. MAE has various advantages over traditional methods, including increased extraction rates, automation, and the ability to create multiples samples at once [15]. By contrast, MAE may also cause high pressure and localized heating, thus may lead to an explosion risk and a limited number of samples in the microwave space.
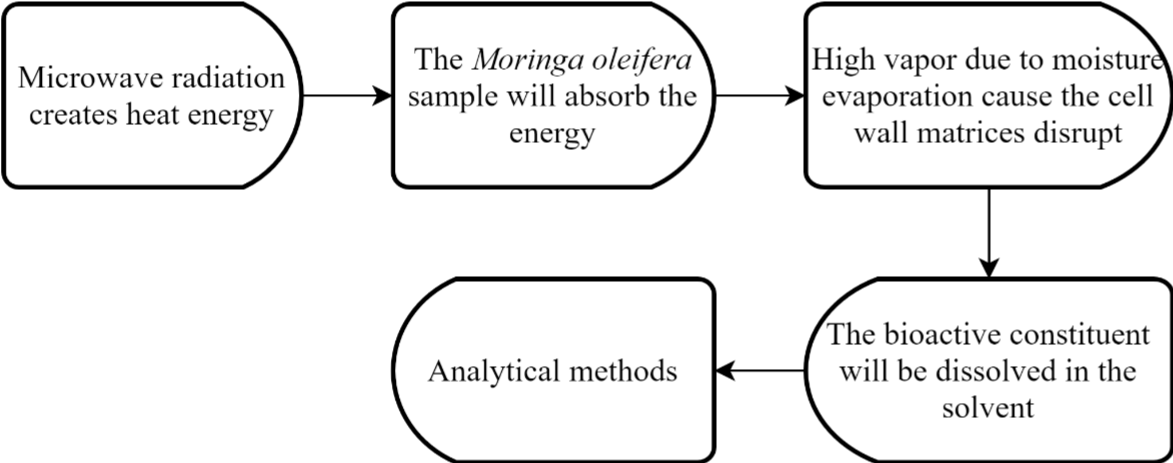
Figure 1: Mechanism of the microwave-assisted (MAE) method.
Ultrasound-Assisted Extraction (UAE)
In recent years, ultrasound-assisted extraction (UAE) has been used in extracting bioactive compounds from plants on a laboratory scale and industrial scale. Ultrasound frequencies ranging from 20 to 2000 kHz are used in UAE [11]. UAE has extracted a variety of bioactive chemicals using water and ethanol-water as solvents. Mechanic effect of acoustic cavitation from ultrasound in the mechanism of UAE methods displayed in (Figure 2), increases the area of contact between solvents and the plant sample. The mechanical energy will form cavities in the liquid. The collapse of biological cell walls occurs when bubbles expand due to energy absorption, facilitating the release of chemicals and increasing mass transit of solvents into plant cells. A study by Rodríguez-Pérez reportedthat the extraction of crude Moringa oleifera extracts using 25 mL of solvents for 15 minutes extraction at room temperature producing higher phenolic content using UAE technique compared to the maceration technique [18]. Besides, Lin et al.,reported 52% of ethanol was used as a solvent and obtained higher flavonoid content values which were 47.04 mg QE/ g MOLs dried weight [19]. Similar to MAE, the UAE technique has successfully obtained higher phenolic content and flavonoid content with shorter extraction duration and less amount of solvent [15]. Patist et al., analyzed current instances of ultrasonic uses in industry and concluded that this approach has a large economic potential [20- 21]. Ultrasound-assisted extraction appears as low cost in smaller and large-scale applications. However, the ultrasound power should not exceed 20 kHz as it will induce the formation of free radicals, thus affecting the active polyphenols available in the crude extracts [22].
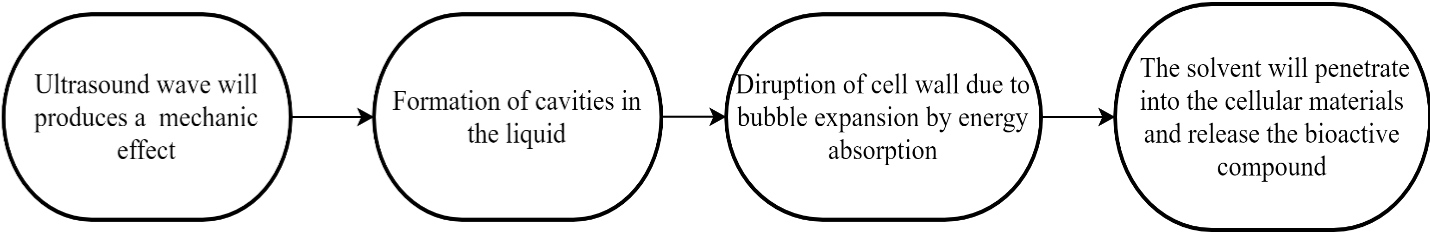
Figure 2: Mechanism of Ultrasound-assisted extraction.
Conclusion
Comparing the extraction techniquesof polyphenols from Moringa oleifera leaves and considering their advantages as shown in the (Table 1), the ultrasound-assisted extraction and microwaveassisted extraction techniques appears as the most promising technique in term of yield and compound extracted. Besides, solvent type is important factor in all extraction techniques reviewed. In addition, the solvent volume utilized in the four procedures has no significant influence. As a comparison, only total phenolic content, flavonoid content, and total yield were considered in this study. Ultrasound-assisted extraction and microwave-assisted extraction are more applicable and could require less cost for small and large-scale applications. When compared to MAE and UAE, which are known as the “Green extraction method”, the large volume of chemical waste created by the maceration technique has been a serious issue [23]. Aside from that, characteristics such as solvent types, solvent strength, extraction time, agitation speed, sample-solvent ratio, and temperature were explored using factorial design experiments; the most influencing factor in Moringa oleifera extraction solvent strength utilizing 50% ethanol [17,18]. On the other hand, 70% ethanol solvent is the suitable extraction for maceration and Soxhlet extraction [2]. Draw to close, the solvent types and strength give a significant effect on the extraction methods. The temperature, solvents, and agitation also need to be considered at the same time because these influences have possibilities to enhance the extraction. Thus, extraction with few influential factors can be a better extraction method.
Characteristic
Maceration
Microwave-assisted extraction
Soxhlet extraction
Ultrasound-assisted extraction
Driving force
Solvent contact
Microwave power
Heat
Acoustic cavitation
Extraction time
Several hours
3-30 min
6-24 hours
10-60 min
Sample size
1-30 g
1-10 g
1-30 g
1-30 g
Solvent amount
Large-volume
10-40 mL
150-500 mL
50-200 mL
Power amount
High
High
High
Moderate
Advantages
- Not use of sophisticated equipment
- Simple and cheap- Fast
- Easy to handle
- Moderate use of solvent- Not use of sophisticated equipment
- Easy to handle
- Safe (atmospheric and ambient temperature)
- Moderate use of solvent
- Reproducible
- Cheap
- Rapid if a probe is usedDisadvantages
- Risk of spills and exposure to organic vapours
- Required filtration step- Risk of explosion (solvent must absorb microwave power)
- Expensive
- Required filtration step
- Possible degradation of thermolabile compounds (higher pressure)
- Sample process is limited- Exposure risk to organic vapours
- Degradation of thermolabile compounds- Required filtration
- Possible degradation of the compound at high frequenciesCompounds extracted
-Phenolic compound
- Flavonoid compound-Phenolic compound
- Flavonoid compound-Phenolic compound
- Flavonoid compound-Phenolic compound
- Flavonoid compound
Table 1: Comparison of various solid-liquid extraction techniques for extraction of bioactive compound from plants.
Acknowledgement
This work was financially supported by the Ministry of Science, Technology & Innovation (MOSTI) Malaysia under Fundamental Research Grant Scheme Malaysia and all the research work conducted in UniversitiSains Malaysia (USM).
References
- H I Muhammad, M Z Asmawi, NAK Khan. A review on promising phytochemical, nutritional and glycemic control studies on Moringa oleifera Lam. in tropical and sub-tropical regions. Asian Pacific Journal of Tropical Biomedicine. Hainan Medical University. 2016; 6(10): 896-902. doi: 10.1016/j. apjtb.2016.08.006.
- B. Vongsak, P. Sithisarn, S. Mangmool, S. Thongpraditchote, Y. Wongkrajang, et al. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Industrial Crops and Products. 2013; 44: 566–571. doi: 10.1016/j.indcrop.2012.09.021.
- Pilaipark Chumark 1, Panya Khunawat, Yupin Sanvarinda, Srichan Phornchirasilp, Noppawan Phumala Morales, et al. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. Leaves. Journal of Ethnopharmacology. 2008; 116(3): 439-446. doi: 10.1016/j.jep.2007.12.010.
- K Poobalan, V Lim, N Nur Syazni Nik Mohamed Kamal, N AdlinYusoff, K Zi Khor, et al. Effects of Ultrasound Assisted Sequential Extraction (UASE) of Moringa oleifera Leaves Extract on MCF 7 Human Breast Cell Line. Malaysian Journal of Medicine and Health Sciences. 2018; 14(1): 102-106.
- G. Baskar, G. Kalavathy, R. Aiswarya, I. Abarnaebenezer Selvakumari. Advances in bio-oil extraction from nonedible oil seeds and algal biomass. Advances in Eco-Fuels for a Sustainable Environment. 2019; 187–210. doi: 10.1016/b978-0-08-102728-8.00007-3.
- A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Medicinal & Aromatic Plants. 2015; 4(3): 196. doi: 10.4172/2167-0412.1000196.
- AM M San, S Thongpraditchote, P Sithisarn, W Gritsanapan. Total phenolics and total flavonoids contents and hypnotic effect in mice of Ziziphus mauritIana Lam. seed extract. Evidence-based Complementary and Alternative Medicine. 2013. doi: 10.1155/2013/835854.
- D M Colvin. A Review on Comparison of the Extraction Methods Used in Licorice Root: Their Principle, Strength and Limitation. Medicinal & Aromatic Plants. 2018; 7(6): doi: 10.4172/2167-0412.1000323.
- N Cujic, K Šavikin, T Jankovic, D Pljevljakušic, G Zdunic, et al. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chemistry. 2016; 194: 135-142. doi: 10.1016/j. foodchem.2015.08.008.
- M A López-Bascón-Bascon, M D Luque de Castro. Soxhlet extraction. Liquid- Phase Extraction Elsevier. 2019; 327–354. doi: 10.1016/B978-0-12-816911- 7.00011-6.
- Extraction Technologies for Medicinal and Aromatic Plants.
- F EghbaliBabadi, P Boonnoun, K Nootong, S Powtongsook, M Goto, et al. Identification of carotenoids and chlorophylls from green algae Chlorococcumhumicola and extraction by liquefied dimethyl ether. Food and Bioproducts Processing. 2020; 123: 296–303. doi: 10.1016/j.fbp.2020.07.008.
- B Trusheva, D Trunkova, V. Bankova. Different extraction methods of biologically active components from propolis; a preliminary study. Chemistry Central Journal. 2007; 1(1): doi: 10.1186/1752-153X-1-13.
- Lavilla, C Bendicho. Fundamentals of Ultrasound-Assisted Extraction. Water Extraction of Bioactive Compounds: From Plants to Drug Development, Elsevier. 2017; 291–316. doi: 10.1016/B978-0-12-809380-1.00011-5.
- Ivanovs and D. Blumberga. Extraction of fish oil using green extraction methods: A short review. Energy Procedia. 2017; 128: 477–483. doi: 10.1016/j.egypro.2017.09.033.
- P-A D Gaspillo, K Sin, W A Baraoidan. Microwave-Assisted Extraction of Phenolic Compounds from Moringa oleifera Lam. Leaves Using Response Surface Methodology as Optimization Tool. 2018. [Online]. Available: https:// www.researchgate.net/publication/293335942
- C Rodríguez-Pérez, B Gilbert-López, J A Mendiola, R Quirantes-Piné, A Segura-Carretero, et al. Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from moringa oleifera leaves by multiresponse surface methodology. Electrophoresis. 2016; 37(13): 1938–1946. doi: 10.1002/elps.201600071.
- C. Rodríguez-Pérez, R. Quirantes-Piné, A. Fernández-Gutiérrez, and A. Segura-Carretero. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Industrial Crops and Products. 2015; 66: 246–254. doi: 10.1016/j.indcrop.2015.01.002.
- X. Lin, L. Wu, X. Wang, L. Yao, and L. Wang. Ultrasonic-assisted extraction for flavonoid compounds content and antioxidant activities of India Moringa oleifera L. leaves: Simultaneous optimization, HPLC characterization and comparison with other methods. Journal of Applied Research on Medicinal and Aromatic Plants. 2020; 20. doi: 10.1016/j.jarmap.2020.100284.
- A Patist, D Bates. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innovative Food Science and Emerging Technologies. 2008; 9(2): 147–154. doi: 10.1016/j.ifset.2007.07.004.
- Chaoting Wen, Jixian Zhang, Haihui Zhang, Courage Sedem Dzah, Manyakara Zandile, et al. Advances in ultrasound assisted extraction of bioactive compounds from cash crops – A review. Ultrasonics Sonochemistry. 2018; 48: 538–549. doi: 10.1016/j.ultsonch.2018.07.018.
- N. Medina-Torres, T. Ayora-Talavera, H. Espinosa-Andrews, A. Sánchez- Contreras, N. Pacheco. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy. 2017; 7(3): 47. doi: 10.3390/agronomy7030047.
- S Kumar, A K Pandey. Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal. 2013; 2013. doi: 10.1155/2013/162750.