Abstract
The individualized process of human aging occurs in the intersection of biological, cognitive, and psychological domains. Advances in the field of biochirality reveal new crucial determinants of aging at the molecular, cellular, and organism levels. All biologic macromolecules, including DNA, proteins, and lipids, exhibit a prevalence of chirality. Chirality and stereoselectivity are recognized as the fundamental properties of all life-supporting biomolecular systems. However, all homochiral molecules are vulnerable to multiple types of spontaneous aberrant modifications, including racemization. Indeed, several biomolecular structures, including amyloids, are known as the by-products of the major evolutionary pathway. Accumulation and aggregation of misfolded proteins are commonly recognized biomarkers of biological aging, neurodegenerative, immune, and psychiatric disorders.
Aging, accompanied by the decline in health, independence, perceptual, cognitive, and decision-making abilities, is closely associated with the negative impacts of most typical psychological stress. Both aging and psychological stress aversively affect the immune, hormonal, and neuro-transmitting systems. In our view, at the protein level, the primary determinants contributing to the crosstalk of genetic, epigenetic, and psychological pathways of aging are the spontaneous post-translational modifications.
Keywords: Spontaneous; Non-enzymatic; Post translational modifications; Racemization; Biological clock; Natural selection; Psychological aging
Abbreviations
AAs: Amino Acids; A-β: Amyloid-Beta; DNA: Deoxyribonucleic Acid; NS: Natural Selection; PTM: Post-Translational Modification
Introduction
Self-perception is contributed by the sense of embodiment [1] and conscious control of your own thoughts [2]. No doubt, the biological processes, running at the molecular and cellular levels, that underlie the humans’ perception of self-understanding, are influenced by the state of mind and psychological state of the subject [3]. Experimental evidence suggest that both aging and psychological stress affect the immune, hormonal, and neuro-transmitting systems [4-6]. However, the studies of bidirectional links between psychological and biological determinants of aging should be reconsidered in view of new results.
Contemporary concepts of prevalent biochirality and virtual reality brings new dimensions to the exploring mutual influence of biological and cognitive domains of self [1-3,7-9] and new meaning to Schopenhauer’s view on the world as the manifestation of “Will and Representations.”
Molecular Determinant of Neurodegenerative, Cognitive, And Psychiatric Pathways
The human’s mental state’s development, integrity, and decline are mediated by the multifactorial interplay of genetic and environmental factors at the molecular domains (Figure 1,2a).
Figure 1: Major determinant of biological aging.
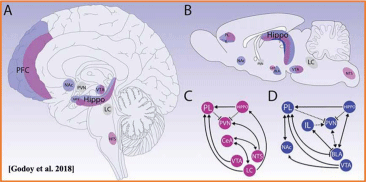
Figure 2a: Neuroanatomy of stress.
Schematic representation of primarily neuroanatomical substrates responsible for physical (pink) and psychological (blue) stressors processing. Upper panels show
that neural processing for different types of stressors detection and appraisal of the situation engage several structures, which may overlap at some instances on
human and rodent brain (A,B, respectively). Bottom panels represent how physical and psychogenic stressors require engagement of different networks (C, D,
respectively). Adapted from [Godoy et al. 2018].
Several types of biologic macromolecules, including DNA, proteins, and lipids, exhibit two interactive (co-existing) characters. First- prevalent chirality [10] and, second, vulnerability to multiple types of aberrant modifications [11]. Prevalent molecular chirality results from natural selection (NS), providing the functionality of DNA, proteins, and lipids along with perceptual and cognitive abilities to live organisms. However, several biomolecular structures, including amyloids, are known as the by-products of the main evolutionary pathway. Aberrant malfunctional structures containing D-amino acids (D-AAs) are the main contributors to the disregulation of neuronal signaling, biological aging, and various disease conditions [12].
Aging, as manifested in the neurodegenerative and psychiatric disorders, is associated with the progressive decline of functions at the molecular, cellular, organ, and organism levels [13-16].
At the molecular level, aging is associated with the impact of physical, environmental, and psychological stressors [7-24]. Acute and chronic psychological stressors are characterized as complicated constructs capable to induce the changes at ANA and protein levels [25,26]. In particular, the neuropathology of schizophrenia is characterized by the convergence of morphological, cellular, and molecular correlates [27-30]. The most common pathways of schizophrenia and neurodegeneration include abnormalities in glutamatergic neurotransmission, altered synaptic plasticity, and elevated brain level of Amyloid-beta (A-b) [31,32]. During the last decade, the reciprocal influences of biology, psychology, behavioral, and social factors on health and illness gain significant attention. But the effect of psychological stressors on spontaneous biological events only in the embryonic state [29,30,33]. It is known that people exposed to major psychological stressors in early life, exhibit an elevated rates mortality from chronic diseases of aging [34]. The impact of psychological stressors on spontaneous, age-associated Post-Translational Modifications (PTMs) of protein remains unexplained. In our view, the critical mechanism underlying the link between PSs and lifespan is spontaneous PTMs.
Organism Level
At the organism level, the age-related decline in cognitive function includes compromised memory, language, critical thinking, and decision-making. Impaired cognition affects the psychologicalbehavioral functions, including social communicants and selfperception, leading to psychological stress associated with a range of psychopathologies. Psychological stress, in turn, is a significant determinant of cognitive decline. Age-associated deterioration of cognition and psychological functions correlates with neuronal degeneration, misfolding, disfunction, and aggregation of synapseassociated proteins and peptides, including NMDA receptors, A-β, TAU, neuroligins, neurexins, and many others [33]. An emerging concept proposes that molecular and sub-cellular structures “sense, integrate, and transduce psychosocial and behavioral factors”, suggesting the reciprocal causation between molecular, cognitive and psychological factors [34-41].
The psychological perspective of aging, seeing through molecular and cell biology prism, helps to understand the complex interrelationships among protein folding, cell signaling, brain structure, cognition, and behavior for both healthy and pathological trajectories [42,43].
Universal Biological Clocks of Aging
The perceptual and cognitive representations of the external world are associated with internal events at the different levels of biological organization.
At the cellular level, cognitive representations are reflected in neuronal representations [44] that are linked to many biomolecular events.
At the molecular level, the fundamental age-related biological clocks are associated with the alterations in the DNA [44-46] lipids [47], proteins [14,15], and interaction between them [46,48]. All molecular clocks contain chronological and biological information related to genetic and epigenetic impacts [49].
At the protein level, the age-related changes are documented in protein transcription, translation, and PTMs. At the same time, neuronal representations are linked to the dynamic system of enzymatic PTMs. It is well-documented that the dysregulation of PTMs leads to a range of neurological alterations (including intellectual disability, learning and memory impairments, autistic-like features, and seizures) [15,16,50] and aging [51]. Aberrant PTMs can potentially be used as the biological indicators of acute psychological and cognitive states. At PTMs domains, the significant age induced changes are observed in the enzymatic PTMs. Most dramatic agerelated changes occur in the level of protein phosphorylation (Figure 2b) [14].
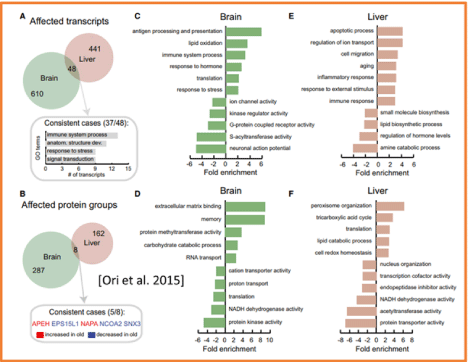
Figure 2b: Common and organ-specific alterations of the proteome in old rats.
(A and B) We identified only 48 transcripts affected at the level of translation output (A) and eight proteins affected at the level of protein abundance (B) that were
altered in both brain and liver. The most represented gene ontology (GO) terms among consistently affected transcripts are shown in (A) while the five consistently
affected proteins are indicated in (B). (C-F) Functional enrichment was performed on the list of quantified transcripts and proteins that were ranked according to
the level of differential expression (fold change) using (Eden et al., 2009). Displayed GO terms are representative cases selected from among those significantly
enriched (cut-off for transcripts: q value < 0.05, minimum number of transcript > 4, fold enrichment ≥2; for proteins the same criteria were applied with the exception
of q value < 0.2). The fold enrichments are plotted using positive values for terms enriched in transcripts/proteins that are increased in old animals or using negative
values for terms enriched in transcripts/proteins that are decreased in old animals. Adopted from [14].
However, the role of spontaneous forms of PTMs remains mostly un-explored [13-16,52]. Notable that enzyme family serinethreonine protein kinases, known as major modulators of cellular transformations, target the most racemization-prone Amino Acids (AAs) residues [15,53]. Therefore, non-enzymatic racemization of serine and threonine are making the cell functions and survival vulnerable to spontaneous time dependent PTMs.
Many agreed that interaction between mental representations and PTMs of proteins are reciprocal. However, the answer to how and to what degree mental determinants of cognitive and psychological states can impact the system of PTMs requires additional experimental efforts.
Conclusion
It is not an accidental combination of events that (a) functions of the CNS are based in the molecular chirality [13,14], (b) numerous psychiatric drugs are chiral compounds [54-56], and (c) psychiatric disorders are strongly associated with the bilateral asymmetry of the brain [57]. But the link between seemingly unrelated events constitutes the challenging barrier for neuroscience and psychology. Psychological stressors, linked with the genetic and epigenetic modifications, are known as the influential causal events changing the physiology of many organs (including brain and gut [58]) and systems (including an immune system [59] and hormonal [5]) of the human body.
Aging, accompanied by the decline in health, independence, perceptual, cognitive, and decision-making abilities, is closely associated with the negative impacts of typical psychological stress [60,61]. Indeed, both psychological stress and aging show cumulative impact on most physiological systems [62-66] involving biological events at the molecular [67] and cellular [68] levels.
Epigenetic alterations are triggering by stressful or beneficial environmental input [69]. The excessive and chronic stresses are associated with the accelerated molecular and cellular aging. Timedependent accumulation of changes is a major molecular determinant of organism aging.
The chain of interlinked molecular, cellular, systemic and organism level of biological events allow to consider, the nonenzymatic racemization, time dependent and irreversible PTMs as the fundamental determinant of biological age. An organism’s aging and its counterpart spontaneous molecular events are inevitable processes. However, the body of evidence suggests that epigenetic aging could be if not preventable that treatable [70-73].
Acknowledgment
I gratefully thank Abel Lajtha for productive discussions and corrections.
References
- Kilteni K, Groten R, Slater M. The Sense of Embodiment in Virtual Reality Project: PhD project: Virtual Embodiment. Presence Teleoperators & Virtual Environments. 2012; 21.
- Guillot M. Thinking of oneself as the thinker: the concept of self and the phenomenology of intellection. Conscious Thinking and Cognitive Phenomenology. An International Journal for the Philosophy of Mind and Action. 2016; 19: 138-160.
- Brugada-Ramentol V, de Polavieja GG, Ángel-Carlos Román A-C. Toward a Molecular Profile of Self-Representation. Front Hum Neurosci. 2016; 10: 602.
- Jennifer E, Graham JE, Christian LM, Kiecolt-Glaser JK. Stress, Age, and Immune Function: Toward a Lifespan Approach. J Behav Med. 2006; 29: 389-400.
- Uhart M, Oswald L, McCaul M, et al. Hormonal Responses to Psychological Stress and Family History of Alcoholism. Neuropsychopharmacol. 2006; 31: 2255-2263.
- Clark BL, Thomas PG. A Cell for the Ages: Human γδ T Cells across the Lifespan. Int J Mol Sci. 2020; 21: 8903.
- Schneiderman N, Gail Ironson G, Siegel SD. Stress and health: Psychological, Behavioral, and Biological Determinants. Annu Rev Clin Psychol. 2005; 1: 607-628.
- Dziechciaż M, Filip R. Biological psychological and social determinants of old age: bio-psycho-social aspects of human aging. Ann Agric Environ Med. 2014; 21: 835-838.
- Kohda M, Hotta T, Takeyama T, Awata S, Tanaka A, Asai J, et al. If a fish can pass the mark test, what are implications for consciousness and selfawareness testing in animals? PLOS Biol. 2019; 17: e3000021.
- Inaki M, Liu J, Matsuno K. Cell chirality: its origin and roles in left–right asymmetric development. Philos Trans R Soc Lond B Biol Sci. 2016; 371: 20150403.
- Ogrodnik M, Salmonowicz H, Gladyshev VN. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell. 2019; 18: e12841.
- Díaz-Villanueva JF, Díaz-Molina R, Victor García-González V. Salvador Ventura, Academic Editor. Protein Folding and Mechanisms of Proteostasis. Int J Mol Sci. 2015; 16: 17193-17230.
- Bulvik BE, Berenshtein E, Konijn AM, Grinberg L, Vinokur V, Eliashar R, et al. Aging is an organ-specific process: changes in homeostasis of iron and redox proteins in the rat. Age (Dordr). 2012; 34: 693-704.
- Ori A, Toyama BH, Harris MS, Ingolia NT, Hetzer MW, Beck M, et al. Integrated Transcriptome and Proteome Analyses Reveal Organ-Specific Proteome Deterioration in Old Rats. Cell Syst. 2015; 1: 224-237.
- Dyakin VV, Wisniewski TW, Lajtha A. Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration. Symmetry. 2020; 12: 585.
- Dyakin VV, Wisniewski TM, Lajtha A. Racemization in Post-Translational Modifications - Relevance to Protein Aging, Aggregation and Neurodegeneration: Tip of the Iceberg. Symmetry. 2021; 13: 455.
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001; 14: 1143-1152.
- Bonneau RH, Hunzeker JT. Stress-induced Modulation of the Immune Response to Herpes Simplex Virus Infections. 2. Animal Model Studies. In Psychoneuroimmunology (Fourth Edition). 2007
- Bashour H, Salam AA. Psychological stress and spontaneous abortion. Int J Gynaecol Obstet. 2001; 3: 179-181.
- Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty L, et al. Review. Effects of Psychological, Environmental and Physical Stressors on the Gut Microbiota. Front. Microbiol. 2018; 9.
- Lehman BJ, David DM, Gruber JA. Rethinking the biopsychosocial model of health: Understanding health as a dynamic system. Social Personality Psychology Compass. 2017; 11: e12328.
- Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. Review. Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front Behav Neurosci. 2018; 3: 127.
- Alexis K, Hayes SN, Sawchuk C, Johnson MP, Best PJ, Gulati R, et al. Analysis of Posttraumatic Stress Disorder, Depression, Anxiety, and Resiliency within the Unique Population of Spontaneous Coronary Artery Dissection Survivors. J. of the American Heart Association. 2020; 9: e014372.
- Miller GE, Chen E, Parker KP. Psychological Stress in Childhood and Susceptibility to the Chronic Diseases of Aging: Moving Towards a Model of Behavioral and Biological Mechanisms. Psychol Bull. 2011; 137: 959-997.
- Haghighi F, Xin Y, Chanrion B, O’Donnell AH, Ge Y, Dwork AJ, et al. Increased DNA methylation in the suicide brain. Dialogues in Clinical Neuroscience. 2014; 16: 430-438.
- Ising M, Holsboer F. Genetics of stress response and stress-related disorders. Dialogues Clin Neurosci. 2006; 8: 433-444.
- Harrison P, Weinberger D. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005; 10: 40-68.
- Bettens K, Sleegers S, Van Broeckhoven C. Genetic insights in Alzheimer’s disease. Lancet Neurol. 2013; 12: 92-104.
- Gan L, Cookson MR, Petrucelli L, et al. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat Neurosci. 2018; 21: 1300-1309.
- Fyfe I. Genetic insights into dementia disorders. Nat Rev Neurol. 2021; 17: 193.
- Religa D, Laudon H, Styczynska M, Winblad B, Näslund J, Haroutunian V. Amyloid beta pathology in Alzheimer’s disease and schizophrenia. Am J Psychiatry. . 2003; 160: 867-872.
- Longinetti E, Mariosa D, Larsson H, Ye W, Ingre C, Almqvist C, et al. Neurodegenerative and psychiatric diseases among families with amyotrophic lateral sclerosis. Neurology. 2017; 89: 578-585.
- Südhof TC. Neuroligins and Neurexins Link Synaptic Function to Cognitive Disease. Nature. 2008; 16: 903-911.
- Albert B. Social Foundations of Thought and Action: A Social Cognitive Theory. Prentice-Hall. ISBN 978-0-13-815614-5.
- Bandura A. Toward a Psychology of Human Agency. Perspect Psychol Sci. 2006; 1: 164-180.
- van Bokhoven H. Genetic and epigenetic networks in intellectual disabilities Annu Rev Genet. 2011; 45: 81-104.
- Toulopoulou T, van Haren N, Zhang X, et al. Reciprocal causation models of cognitive vs volumetric cerebral intermediate phenotypes for schizophrenia in a pan-European twin cohort. Mol Psychiatry. 2015; 20: 1386–1396.
- Martin P, McEwen BS. Review. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosomatic Medicine. 2018; 80: 126-140.
- Martino G, Langher V, Cazzato V, Vicario CM. Editorial: Psychological Factors as Determinants of Medical Conditions. Front. Psychol. 2019; 10: 2502.
- Tao Y, Kang B, Petkovich DA, Bhandari YR, In J, Genevieve Stein-O’Brien G. et al. Aging-like Spontaneous Epigenetic Silencing Facilitates Wnt Activation, Stemness, and Braf V600E-Induced Tumorigenesis. Cancer Cell. 2019; 35: 315-328.e6.
- Nollet M, Wisden W, Franks NP. Review. Sleep deprivation and stress: a reciprocal relationship. Interface Focus. 2020; 10: 20190092.
- Park DP, Farrell ME. Handbook of the Psychology of Aging-The Aging Mind in Transition: Amyloid Deposition and Progression toward Alzheimer’s Disease. Chapter 5 in Handbook of the Psychology of Aging (Eighth Edition). 2016; 87-103.
- Hullinger R, Puglielli L. Molecular and cellular aspects of age-related cognitive decline and Alzheimer’s disease. Behav Brain Res. 2017: 322: 191-205.
- Pearson J, Naselaris T, Holmes EA, Kosslyn SM. Mental Imagery: Functional Mechanisms and Clinical Applications. Trends Cogn Sci. 2015; 19: 590-602.
- Melzer D, Pilling LC, Luigi Ferrucci L. The genetics of human ageing. Nat Rev Genet. 2020; 1: 88-101.
- Jylhävä J, Pedersen NL, Hägg S. Biological Age Predictors. [Jylhävä ] Juulia Jylhävä 1, Nancy L Pedersen, N.L.; Hägg, S. Biological Age Predictors EBioMedicine. 2017; 21: 29-36.
- Collino S, Montoliu I, Martin FP, Scherer M, Mari D, Salvioli S, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One. 2013; 8: e56564.
- Slagboom PE, van den Berg N, Deelen J. Phenome and genome-based studies into human ageing and longevity: An overview. Biochim Biophys Acta Mol Basis Dis. 2018; 1864: 2742-2751.
- Levine M.E. Assessment of Epigenetic Clocks as Biomarkers of Aging in Basic and Population Research. Gerontol A Biol Sci Med Sci. 2020; 75: 463- 465.
- Prieto M, Folci A, Martin S. Post-translational modifications of the Fragile X Mental Retardation Protein in neuronal function and dysfunction. Mol Psychiatry. 2020; 25: 1688-1703.
- Cloos P, Christgau S. Post-Translational Modifications of Proteins: Implications for Aging, Antigen Recognition, and Autoimmunity. Article in Biogerontology. 2004; 5: 139-158.
- Bell CG, Lowe R, Adams PD, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019; 20: 249.
- Aoki M, Blazek E, Peter K, A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. PNAS USA. 2001; 98: 136-141.
- Baker GB, Prior TI, Coutts RT. Chirality and drugs used to treat psychiatric disorders. J Psychiatry Neurosci. 2002; 27: 401-403.
- Howland RH. Clinical implications of chirality and stereochemistry in psychopharmacology. J Psychosoc. Nurs. Ment. Health Serv. 2009; 47: 17- 21.
- Saganuwan SA. Chirality of Central Nervous System (CNS) Acting Drugs: A Formidable Therapeutic Hurdle Against CNS Diseases. Central Nervous System Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry - Central Nervous System Agents). 2019; 9: 171-179.
- Kustubayeva A, Kamzanova A, Kudaibergenova S, Pivkina V, Matthews G. Major Depression and Brain Asymmetry in a Decision-Making Task with Negative and Positive Feedback. Symmetry. 2020, 12: 2118.
- Gur TL, Worly BL, Bailey MTF. Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Front. Psychiatry. 2015; 6: 5.
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Rev. 1998; 105: 83-107.
- Velten J, Lavallee KL, Scholten S, Meyer AH, Zhang X-C, Schneider S, et al. Lifestyle choices and mental health: a representative population survey. BMC Psychol. 2014; 2: 58.
- Lavretsky H, Newhouse PA. Stress, Inflammation and Aging. J Geriatr Psychiatry. 2012; 20: 729-733.
- Loriena Y, Carolyn A. Does psychosocial stress accelerate the aging process? Aging America. 2010; 2: 100-118.
- Simm A, Klotz L-O. Stress and biological aging: A double-edged sword. Gerontol. Geriatr. 2015; 48: 505-510.
- Robert L, Labat-Robert J. Stress in biology and medicine, role in aging. Pathol Biol (Paris). 2015; 63: 230-234.
- Morey JN, Boggero IA, Scott AB, Suzanne C. Current Directions in Stress and Human Immune Function. SegerstromCurr Opin Psychol. 2015; 5: 13-17.
- Kashetti S. Psychosocial Stress: A Cause towards Ageing. J. of Gerontology & Geriatric Research. 2020; 9: 6.
- Moreno-Villanueva M, Bürkle A. Molecular consequences of psychological stress in human aging. Experimental Gerontology. 2015; 68: 39-42.
- Hayashi T. Conversion of psychological stress into cellular stress response: Roles of the sigma-1 receptor in the process PCN. Psychiatry and Clinical Neuroscience. Frontier Review. 2015; 69: 179-191.
- Tsankova T, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007; 8: 355-367.
- Zannas AS. Editorial Perspective: Psychological stress and epigenetic agingwhat can we learn and how can we prevent? Journal of Child Psychology and Psychiatry. 2016; 57: 674-675.
- Schiele MA, Gottschalk MG, Domschke K. The applied implications of epigenetics in anxiety, affective and stress-related disorders - A review and synthesis on psychosocial stress, psychotherapy and prevention. Clinical Psychology Rev. 2020; 77: 101830.
- Pedersen JB. The psychological process of ageing. What does it mean to get old? Nordic Journal of Psychiatry. 2009; 47: 49-51.
- Ferracioli NGM. Psychological aspects of aging and psychology’s contributions to gerontology: theoretical and technical interface. MOJ Gerontol Ger. 2018; 3: 115-116.
