Research Article
Austin Chromatogr. 2014;1(3): 5.
Quantification of Phenolic Diterpenoids and Rosmarinic Acid in Salvia eremophila and Salvia santolinifolia by LC-DAD-MS
Jassbi AR1*, Miri R1, Alizadeh M1, Asadollahi M1,2, Massrorbabanari M1 and Baldwin IT3
1Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences, Iran
2Department of Biochemical Engineering, University of Debrecen, Hungary
3Department of Molecular Ecology, Max Planck Institute for Chemical Ecology, Germany
*Corresponding author: Jassbi AR, Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences, PO Box 71345-3388, Shiraz, Iran
Received: August 28, 2014; Accepted: October 16, 2014; Published: October 20, 2014
Abstract
Methanol and aqueous-methanol extracts of two species of sage, Salvia eremophila and S. santolinifolia, were analyzed by LC-DAD-MS to determine their antioxidant, antimicrobial and cytotoxic active constituents. Rosmarinic acid (1); carnosol (2) and carnosic acid (3) were found to be the major constituents of all extracts. The concentrations of these phenolic compounds (1-3) in the methanolic extracts of S. santolinifolia (6.72±0.26, 1.59±0.22 and 9.35±1.58 mg/ g dry plant material: PM) and S. eremophila (6.96±0.36, 4.27±0.4 and 20.16±0.74 mg/ g dry PM) as measured by reversed phase HPLC-UV, were significantly increased when 80% methanol was used for the extraction of S. eremophila (15.46±0.69, 17.64±1.05 and 39.05±0.64 mg/ g dry PM), but not for S. santolinifolia (7.44±0.48, 4.2±0.38 and 8.46±047 mg/ g dry PM). We propose that compounds 1-3 are valuable chemical markers for the natural antioxidant capacity of sage extracts.
Keywords: Phenolic diterpenoids; Rosmarinic acid; Salvia; LC-DAD-MS
Introduction
The plants of the genus Salvia are known for biologically active substances that make them interesting for people of different countries who use them in folk medicine and food industries [1,2]. They are represented by 61 species in Iran [3]. S. eremophila Boiss and S. santolinifolia Boiss are two desert species found in Iran of which the first is endemic to Iran and the second is in addition found in Afghanistan and Pakistan [4]. The two sages are rich sources of volatiles of similar composition, such as monoterpenes including camphene, limonene, borneol and 4-terpineol [5]. They are also morphologically similar and can be distinguished by micro-morphological characters (Figure 1) [4,6].
Figure 1: The two Salvia species growing wild in the deserts of Iran have similar morphology but can be differentiate via their calyx shapes.
Rosmanol, isorosmanol and carnosol together with four triterpenes and flavonoids have been identified from S. eremophila [7,8]. New triterpenoids, with dammarane, ursnane and amyrane type skeletons were isolated from the aerial parts of S. santolinifolia [9-11]. The chloroform and ethyl acetate extracts of S. santolinifolia showed antileischmanial activity [12]. Three new lingams, one of which with lipoxygenase inhibitory activity were reported from the plant, while Slavins A and B, the new amyrin type triterpenes inhibited butyrylcholinesterase enzyme [11,13,14].
Previously, we have determined the 2, 2-diphenyl-1- picrylhydrazyl (DPPH) radical scavenging potential, antibacterial and cytotoxic activity on three cancerous cell lines (HL60, K562 and MCF-7 cells) of different solvent extracts of eleven Salvia species from Iran [15]. We found the methanol and 80% methanol extracts of S. eremophila and S. santolinifolia as the most active plant extracts in the above bioassays [15]. In other experiments, the 80% methanol extract of S. santolinifolia showed significant neuroprotection against neuronal PC12 cells and inhibited intracellular Reactive Oxygen Species (ROS) and apoptosis [16]. In order to determine the bioactive substances of the above plants, we identify the chemical constituents of the above extracts by means of HPLC-DAD-ESIMS and quantify them by reversed-phase HPLC-UV.
Material and Methods
Reagents
All of the solvents were HPLC grade and purchased from Merck chemical company (Darmstadt, Germany).
Plant material
S. eremophila Boiss and S. santolinifolia Boiss were collected from surrounding of Shiraz-Darab road (about 35 Km to Darab from Shiraz: N 28°, 41', 33"; E 54°, 37', 45" at 1220 m altitude and 5 Km after Darab N 28°, 44', 38.5"; E 54°, 05', 20.6" at 1405 m altitude) in Fars Province, Iran and identified at MNCRC, Shiraz, Iran by MA. The voucher specimens, PC-87-92 and PC-87-98 were deposited for S. eremophila and S. santolinifolia respectively at herbarium of MNCRC. The aerial parts of the plants were dried at room temperature in the shade until further solvent extraction.
Solvent extraction of the plants
Fifty mg of the dried and ground aerial parts of each plant were separately extracted with 1.5 ml methanol and methanol: water (80:20) for 45 min in an ultrasonic bath and left for an additional 24 h at room temperature. The supernatant of the extracts were used for HPLC analyses after removing the particles by centrifugation at 21030g for 15 min.
The plant extracts HPLC analysis
To analyze the plant extracts reversed-phase HPLC analyses were performed using a Knauer analytical HPLC with a K-1001 pump. The HPLC column (Eurospher-100 C18, 250 X 4.6 mm, Knauer, Germany) was eluted with a gradient program beginning with 0.25 % H3PO4 in ultrapure water (solvent A) and increasing the power of the solvent with acetonitrile as solvent B as follows: 0-6 min, 0-12% of B; 6-10 min, 12-18% of B; 10-30 min, 18-58% of B; 30-35 min, 58- 80% of B; 35-45 min, 80% of B and 45-50 min, 80-0% of B. The four channels K-2600 UV detector was set at λ 210, 254, 320 and 365 nm. For quantification of the compounds different concentration of caffeic acid, a major metabolite in the sage plants [17], in 80% methanol were used to obtain calibration curve as the external standard, measured at λ 254 and 320 nm. However we could not detect traces of the standard compound in the extracts of our sample plants despite its presence in other species of the sages.
LC-DAD-ESIMS analysis
The LC-DAD-ESIMS analyses were performed on a Shimadzu LCMS-2010EV, coupled to a mass detector in ESI mode and a SPD-M20A diode array detector. The HPLC column was a Shim Pack XR-ODS 2 mm X 50 mm and the LC solvent program was set at a flow rate of 0.2 mL/min; with the following mobile phase program: solvent B and A were acetonitrile and 0.25% formic acid in water respectively. The injection volume of the 10-times diluted plant extract was 5 μL. The column was eluted with the program used for analytical HPLC except that the time program was divided by two and finally the LC column temperature was set at 40 °C. ESI was used as the ionization source for the MS in negative mode. The MS parameters were set as the following. The MS detector, interface, CDL and Q-array voltage were set at -1.5 kV, -4.5 kV, +10 V and-150 (Rf) respectively. The mass range in the scanning mode was 100-1000 mass unit, the nebulizer gas was N2 with a flow rate of 1.5 L/min and heat block and CDL temperatures were set at 230 and 275 °C respectively. The UV spectra were recorded on the DAD detector at λ 190-600 nm and the cell.
Results and Discussion
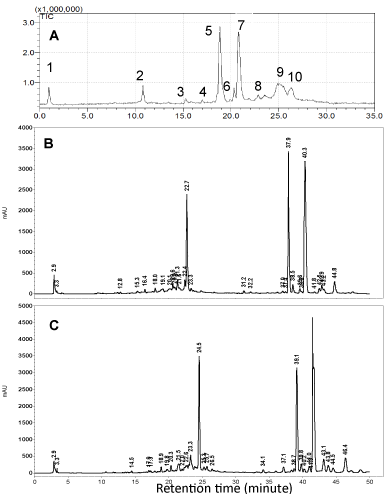
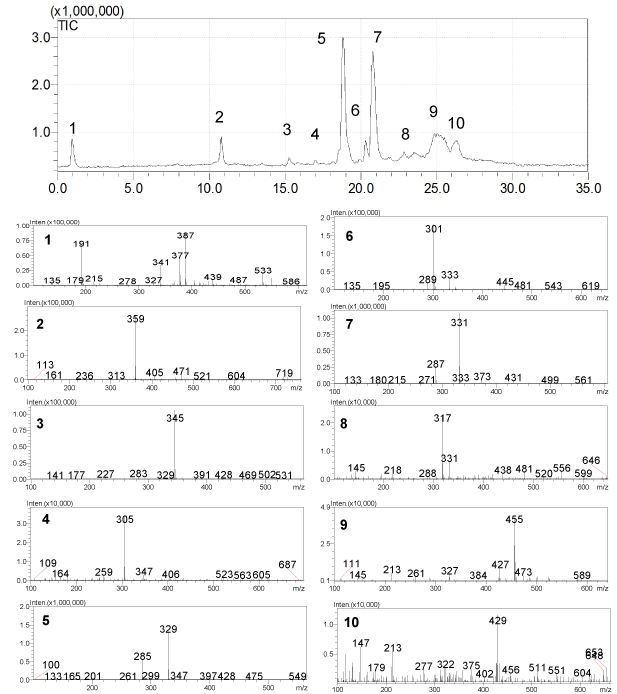
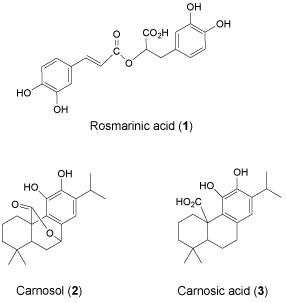
S. eremophila and S. santolinifolia were the two most interesting bioactive plants among eleven sage plants tested in a previous bioassay study quantifying total phenol contents, cytotoxic, antioxidant and antibacterial activities [15]. The Reversed Phase (RP) HPLC analyses of the polar solvent extracts of the plants showed very similar HPLC chromatograms (Figure 2) as we found very similar GC-MS chromatogram patterns for the essential oils of these two plants previously [5]. We have detected three major compounds: rosmarinic acid (1), carnosol (2) and carnosic acid (3) in both bioactive methanol and 80% methanol extract using LC-DAD-ESIMS (Figures 2-4). The identification of compounds was based on interpretation of ESI-MS and UV spectra of each peak. For rosmarinic acid (1) the UV peaks at λ max 254, 290 and 328 nm together with a quasi-molecular ion [M-1] - at m/z=359 and a week peak at m/z=161 in the ESI mass spectrum were compatible with those data previously reported for this compound (Figure 3) [18]. Carnosol (2) and carnosic acid (3) exhibited quasi-molecular ions [M-1] - at m/z=329 and m/z=331. The peaks at m/z=285 and m/z=287 [M-CO2] - in the ESI mass spectrum (Figure 3) presented the fragment ions of [M-CO2] - in the mass spectra of 2 and 3 which is compatible with the presence of a free acid and a lactones ring functions in the molecules respectively [19]. Both of compounds 2 and 3 showed λ max at 283 and 246 nm in the UV spectra respectively which are compatible for those reported for the compounds in the literature [19].
Figure 2: LC-ESI-MS chromatogram of S. santolinifolia (A), HPLC Chromatograms of 80% methanol extract of Salvia eremophila (B) and S. santolinifolia (C) at 210 nm.2: rosmarinic acid 5: carnosol, 7: carnosic acid.
Figure 3: LC-ESI-MS chromatogram; and ESI mass spectra of constituents, 2): rosmarinic acid, 5): carnosol, 7): carnosic acid, from the Salvia species.
Figure 4: Chemical constituents of the methanol and 80% methanol extracts of S. santolinifolia and S. eremophila.
HPLC-UV analyses of the methanol and 80% methanol extracts of the two sage plants were performed using RP18 reversed phase column (Figure 2). The concentrations of the phenolic compounds 1-3 in the methanol and 80% methanol extracts of S. santolinifolia were measured and found to be statistically similar (p> 0.05) (Table 1). While the HPLC analyses showed that the amounts of the respective compounds were higher in 80% methanol extract of S. eremophila (15.46±0.69, 17.64±1.05 and 39.05±0.64 mg/ g dry PM) compared to those extracted by methanol (6.96±0.36, 4.27±0.4 and 20.16±0.74 mg/ g dry PM). The ratio percentage of carnosic acid to carnosol was lower when an 80% methanol extract was used, but the ratio of rosmarinic acid to the total diterpenoids contents was higher in both methanol and 80% methanol extracts of S. santolinifolia (37.0: 63.0% and 37.2: 62.8% ) compared to those obtained for S. eremophila (21.4: 78.6 % and 22.2: 77.8% ) respectively (Table 1).
Plants
Extracting solvent
Rosmarinic acid a
Carnosol
Carnosic acid
Carnosic acid/ carnosol ratio %
Rosmarinic acid/ total diterpenoids ratio %
S. santolinifolia
80% methanol
7.44±0.48 b
4.2±0.38 d
8.46±047 f
66.8: 33.2
37.0: 63.0
S. santolinifolia
methanol
6.72±0.26 b
1.59±0.22 d
9.73±1.58 f
86.0: 14.0
37.2: 62.8
S. eremophila
80% methanol
15.46±0.69 c
17.64±1.05 e
39.05±0.64 g
68.9: 31.1
21.4: 78.6
S. eremophila
methanol
6.96±036 b
4.27±0.4 d
20.16±0.74 h
82.5: 14.5
22.2: 77.8
Table 1: Reversed phase HPLC analyses of different plant extracts of S. eremophila and S. santolinifolia.
Phenolic compounds are appropriate chemical markers in chemotaxonomic research and are used for classifying Salvia [20]. In Iran, this genus is divided taxonomically into five groups among which S. eremophila Boiss and S. santolinifolia Boiss grow in deserts and are similar in morphology and essential oil’s composition and are classified in the same group [4,5]. However, they can be identified easily using micro-morphological characters of floral and leaves segments (Figure 1) [6].
We classified different sage plants of Iran based on their essential oil’s composition into four main chemical groups [5]. Both S. eremophila and S. santolinifolia contained monoterpenes and fall into the first chemical group [5]. The present study shows that these two species are very similar in their non-volatile chemical compositions which support their previously reported botanical and chemical similarities.
Apart from identification of the non-volatile chemical markers of the plants, searching the literature showed that these compounds (1-3) are the main reason for the high biological activity of the above plants. The antibacterial potential of S. eremophila was higher than those reported for S. santolinifolia which was compatible with higher concentrations of the active compounds; 2 and 3 in its extracts [15]. However, the antioxidant, total phenol contents and cytotoxic potential of the two plant extracts were statistically similar and highest among the eleven sage plants [15]. While the neuroprotective, ROS and apoptosis inhibition potential of S. santolinifolia was higher compared to those reported for S. eremophila [16]. The above observations may be due to the presence of other undetectable biological active substances or synergetic/ antagonistic effects of the major compounds.
All of the three compounds, 1-3 were found to be active radical scavengers, cytotoxic and antibacterial. Carnosol and carnosic acid are two antibacterial constituents that were reported from S. officinalis and have shown synergetic effect when combined with amynoglycoside antimicrobial compounds [21]. The two diterpenes2 and 3 are lipophilic antioxidants which may act as protective components against oxidative stress in rosemary plants in which 3 is the main constituents [22]. The antioxidant potential of rosmarinic acid and carnosol were compared to those of the crude extract of rosemary [23] and found to be more active than standard antioxidative agents: butylated hydroxytulene and butylated hydroxyanisol, but less than tertiary butyl hydroquinone [24]. Different extracts of rosemary and its constituents 1, 3 were tested on the growth of several cancer cell lines, carnosol showed cytotoxic effects but rosmarinic acid had proliferative activity on all cell lines and when combined with TNF-a, exhibited induced apoptosis [25]. Rosmarinic acid the most abundant and active antioxidant constituents of S. virgata [26] were found as the major constituents in several members of the genus Salvia [17,27- 30].
In the present report, we confirmed that S. eremophila and S. santolinifolia in addition to their similarity in their morphology are very similar in their chemical constituents and we may suggest them chemically identical. Considering the high levels of rosmarinic acid, carnosol and carnosic acid in the two sage plants (Table 1), which is comparable to those reported in rosemary [31], we may introduce S. eremophila and S. santolinifolia as alternative plants to be used in food industry such as natural preservatives.
Acknowledgment
We are grateful to the vice-chancellor for research, Shiraz University of Medical Sciences, Iran (Grants: 4924-88, 88-4923 and 88-4811) and Alexander von Humboldt foundation under the Research Group Linkage Project (3.4 - IRN/1101775-2012- 2013) for financial support of this project.
References
- Ulubelen A, Topcu G, Johansson CB. Norditerpenoids and diterpenoids from Salvia multicaulis with antituberculous activity. J Nat Prod. 1997; 60: 1275-1280.
- Aggarwal KK, Khanuja SPS, Ahmad A, Santha Kumar TR, Gupta VK, Kumar S. Enantiomeric distribution of some monoterpenes in the essential oils of some Salvia species. Flavour Frag J. 2002; 17: 54-58.
- Jamzad Z. Lamiaceae. In Flora of Iran. Assadi M, Maassoumi A, Mozaffarian V, editors. Research Institute of Forests & Rangelands: Tehran. 2012; 76: 811-816.
- Hedge IC, Flora Iranica. Akademische Druck und Verlagsanstalt: Graz, Austria. 1982; 150: 403-476.
- Jassbi AR, Asadollahi M, Masroor M, Schuman MC, Mehdizadeh Z, Soleimani M, et al. Chemical classification of the essential oils of the Iranian Salvia species in comparison with their botanical taxonomy. Chem Biodivers. 2012; 9: 1254-1271.
- Jafari A, Nikian M. Micromorphological, anatomical and pollen ornamentation study on four desert species of Salvia in Center of Iran. Asian J Plant Sci. 2008; 7: 736-741.
- Farimani MM, Moghaddam FM, Esmaeili MA, Amin G. A lupane triterpenoid and other constituents of Salvia eremophila. Nat Prod Res. 2012; 26: 2045-2049.
- Moghaddam FM, Farimani MM, Amin G. Carnosol from Salvia eremophila Boiss Daru. 2000; 8: 45-46.
- Ahmad Z, Fatima I, Mehmood S, Ifzal R, Malik A, Afza N. New epoxydammarane triterpenes from Salvia santolinifolia. Helv ChimActa. 2008; 91: 73-78.
- Ahmad Z, Mehmood S, Ifzal R, Malik A, Afza N, Ashraf M, et al. A new ursane-type triterpenoid from Salvia santolinifolia. Turk J Chem. 2007; 31: 495-501.
- Mehmood S, Riaz N, Nawaz SA, Afza N, Malik A, Choudhary MI. New butyrylcholinesterase inhibitory triterpenes from Salvia santolinifolia. Arch Pharm Res. 2006; 29: 195-198.
- Mehmood S, Ahmad Z, Malik A, Afza N. Phytochemical studies on Salvia santolinifolia. J Chem Soc Pak. 2008; 30: 311-314.
- Mehmood S, Fatima I, Malik A. Salvicins A and B, new lignans from Salvia santolinifolia. J Asian Nat Prod Res. 2011; 13: 588-591.
- Mehmood S, Riaz N, Ahmad Z, Afza N, Malik A. Lipoxygenase inhibitory lignans from Salvia santolinifolia. Pol J Chem. 2008; 82: 571-575.
- Firuzi O, Miri R, Asadollahi M, Eslami S, Jassbi AR. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven salvia species from iran. Iran J Pharm Res. 2013; 12: 801-810.
- Tavakkoli M, Miri R, Jassbi AR, Erfani N, Asadollahi M, Ghasemi M, et al. Carthamus, Salvia and Stachys species protect neuronal cells against oxidative stress-induced apoptosis. Pharm Biol. 2014.
- Lamien-Meda A, Nell M, Lohwasser U, Borner A, Franz C, Novak J. Investigation of antioxidant and rosmarinic acid variation in the sage collection of the genebank in Gatersleben. J Agric Food Chem. 2010; 58: 3813-3819.
- Hu P, Liang QL, Luo GA, Zhao ZZ, Jiang ZH. Multi-component HPLC fingerprinting of Radix Salviae Miltiorrhizae and its LC-MS-MS identification. Chem Pharm Bull (Tokyo). 2005; 53: 677-683.
- Mulinacci N, Innocenti M, Bellumori M, Giaccherini C, Martini V, Michelozzi M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta. 2011; 85: 167-176.
- Nakiboglu M. The classification of the Salvia L. (Labiatae) species distributed in West Anatolia according to phenolic compounds. Turk J Bot. 2002; 26: 103-108.
- Horiuchi K, Shiota S, Kuroda T, Hatano T, Yoshida T, Tsuchiya T. Potentiation of antimicrobial activity of aminoglycosides by carnosol from Salvia officinalis. Biol Pharm Bull. 2007; 30: 287-290.
- Munne-Bosch S, Alegre L. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiol. 2001; 125: 1094-1102.
- Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008; 110: 76-82.
- Richheimer SL, Bernart MW, King GA, Kent MC, Bailey DT. Antioxidant activity of lipid-soluble phenolic diterpenes from rosemary. JAOCS, J AmOil Chem Soc. 1996; 73: 507-514.
- Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr. 2010; 65: 158-163.
- Kosar M, Goger F, Can Baser KH. In vitro antioxidant properties and phenolic composition of Salvia virgata Jacq. from Turkey. J Agric Food Chem. 2008; 56: 2369-2374.
- Akkol EK, Goger F, Kosar M, Baser KHC. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 2008; 108: 942-949.
- Gohari AR, Saeidnia S, Malmir M, Hadjiakhoondi A, Ajani Y. Flavones and rosmarinic acid from Salvia limbata. Nat Prod Res. 2010; 24: 1902-1906.
- Skoula M, Abbes JE, Johnson CB. Genetic variation of volatiles and rosmarinic acid in populations of Salvia fruticosa mill growing in Crete. Biochem Syst Ecol. 2000; 28: 551-561.
- Tepe B. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia virgata (Jacq), Salvia staminea (Montbret & Aucher ex Bentham) and Salvia verbenaca (L.) from Turkey. Bioresour Technol. 2008; 99: 1584-1588.
- Jordán MJ, Lax V, Rota MC, Loran S, Sotomayor JA. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) methanolic extracts. J Agric Food Chem. 2012; 60: 9603-9608.