
Review Article
Austin Chromatogr. 2014;1(5): 1021.
Analysis of Phytosterols in Plants and Derived Products by Gas Chromatography – A Short Critical Review
Marco Aurelio Ziemann dos Santos1, Miguel Roehrs2, Claudio Martins Pereira de Pereira1, Rogério Antonio Freitag1 and Andre Valle de Bairros1*
1Center of Chemical, Pharmaceutical and Food Sciences, Federal University of Pelotas, Brazil
2Center of Rural Sciences, Federal University of Santa Maria, Brazil
*Corresponding author: Andre Valle de Bairros, Center of Chemical, Pharmaceutical and Food Sciences, Federal University of Pelotas, Campus Universitário Capão do Leão, s/n°, Prédio 32, Laboratório 412, CEP: 96010-900, Pelotas, RS, Brazil
Received: November 24,2014; Accepted: December 05, 2014; Published: December 08, 2014
Abstract
Phytosterols are sterols present in plant cells and studies of these molecules suggested positive biological effects. Government agencies proposed to includefree phytosterols in foods as well as its determination in many products to avoid adulterations by chromatography such as Gas Chromatography (GC). Limitations and suggested alternatives for Phytosterols analysis by GC are cited in this review article as information source for future studies.
Keywords: Phytosterol; Gas chromatography; Limitations; Alternatives
Introduction
Phytosterols are an important family of lipids present in plant cells and can be classified either as 4-desmethylsterols, 4-methylsterols, or 4,4’-dimethylsterols. Sitosterol, campesterol, and stigmasterol (4-desmethylsterols group) are found in abundance in most of the plants. However, there are over 200 different sterol structures that have been discovered in various plant species [1]. Phytosterols can exist in vegetables in their free form, as esters with fatty acids, ferulic acid, or p-coumaric acid, or as glycosides and acylatedsteryl glycosides [2-4].
Studies have been suggested that phytosterols have antiinflammatory, antibacterial, antifungal, antiulcerative and antitumoral activities [5,6]. Moreover, these molecules show ability to lower blood cholesterol [7,8]. Because of its positive biological effects, Food and Drug Administration (FDA) and European Union (EU) proposed to include free phytosterols in conventional foods and established labeling guidelines. Therefore, the determination of sterol profile in medicinal products and vegetable oils on the basis of phytosterols is important to avoid adulterations [9-11]. So, there is an increase interest in the analysis of these molecules [12,13].
In order to promote the analysis of phytosterols, chromatographic methods are the most widely used equipment for the determination of these molecules, mainly Gas Chromatography (GC) [10,11,14-16]. However, there are some limitations when GC is used to determinate these types of lipids.
The goal is to present a short critical review of the limitations and proposed alternatives for the determination of phytosterols in plants and derived products by GC.
Phytosterols analysis by gas chromatography (GC)
Crude extract of plant and its derived products are considered complex matrices. Saponification is a sterol transformation method in its free form and extractive techniques based on principles of Liquid-Liquid Extraction (LLE) and Solid Phase Extraction (SPE) are performed to promote the extraction [17,18]. Moreover, SPE allows purification and higher concentrations of these molecules compared to LLE [18]. Regardless of the extraction technique, both procedures are required for phytosterols analysis by GC. GC is a physical separation method which allows high efficiency analysis of different compounds based on volatilization of the analytes and interaction between analyte and stationary phase from capillary column, since mobile phase (carrier gas) is inert [19-21]. This equipment is an important tool in the process of detection, identification and quantification of Phytosterols [19,20] showing a wide range of columns for efficient and reliable analysis [21], with standard Gas Chromatographic-Flame Ionization Detection (GCFID) and Gas Chromatographic-Mass Spectrometry (GC-MS).
Gas Chromatographic-Flame Ionization Detection (GC-FID) is widely used in the analysis of phytosterols because of easy handling, low cost and good sensitivity [15,22,23]. However, the difficulty of the analysis of phytosterols in foods and plants requires complex processes extractives, purifications and efficient derivatization (if necessary) [19,20,23,24]. Furthermore, chromatographic determination of these molecules shows some limitations such as co elution of some compounds. This behavior can happen when a fully nonpolar capillary column with methyl groups attached is used as stationary phase, such as dimethylpolysiloxane (100%) or phenyldimethylpolysiloxane (5%-95%). According to Laakso [23], delta- 5-avenasterol/sitostanol, campesterol/campestenol and sitosterol/ sitostanol demonstrated problematic separation with these columns.
A chromatogram developed in our laboratory used reference standards of cholesterol, brassicasterol, ergo sterol, campesterol, stigma sterol, beta-sitosterol and fucosterol (Sigma-Aldrich®, MO, USA) with intention to demonstrate the coelution in these analytes by GC-FID using capillary column Elite-5 (crossbond 5% diphenyl - 95% dimethyl polysiloxane) with 30 m x 0.25 mm x 0.25 μm film thickness. The result is demonstrated in the Figure 1, showing coelution between beta-sitosterol and fucosterol with this stationary phase. Different temperature programs were evaluated and the retention time of betasitosterol and fucosterol were quite close.
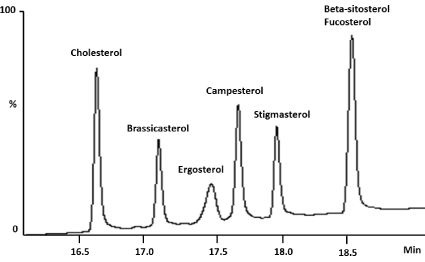
Figure 1: GC-FID 2010 model (Shimadzu®) in the following conditions:
Programmed Temperature Vaporizer (PTV) in direct inject mode at 250°C
using hydrogen as carrier gas and column flow in 2.04 mL/min at a constant
pressure mode. The column oven temperature program started 150°C (hold
1 min), then programmed at 10°C/min to 320 °C (hold 4 min). The total
analytical time was 22 min. Detector temperature was 320°C.
One alternative is the derivatization step to promote chromatographic separation, since most phytosterols having hydroxyl group (-OH group) can easily bederivatized by silylatingagents as N,Obis (trimethylsilyl) trifluoroacetamide with 1% trimethylchlorosilane (BSTFA) or N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide with 1% tert-butyldimethylchlorosilane (MTBSTFA) [25-28]. Indeed, an increase of sensitivity after this chemical reaction occurs due a better volatilization of these compounds because phytosterols requires high temperatures to perform the volatilization and consequently, its determination by GC. However, separation of the analytes cannot be modified because elution orders of the phytosterolsremains the same manner after derivatization. This particular behavior happened between beta-sitosterol and fucosterol.
To solve this problem, columns with different chemical characteristics are required. Columns with stationary phase with 14%cyanopropylphenil and 86% methyl polysiloxanecan are used to promote the separation of these compounds [23]. Similarly, it is possible to use columns of medium polarity based in 50% phenyl and 50% methylpolysiloxane [20] or stationary phase with 95% dimethyl-5% diphenilpolysiloxane [29]. Nevertheless, chromatographic separation with a type of capillary column for determinated phytosterols (ex.: beta-sitosterol and fucosterol) can be promote coelution among other sterols, mainly complex matrices with a mixture of these molecules.
Because of the phytosterols and matrices complexity, Multidimensional Gas Chromatography (MDGC) and comprehensive two-dimensional Gas Chromatography (GCxGC) would be interesting alternatives for chromatographic separations [30-33]. MDGC uses two columns of different polarities interconnected with a modulator transfer, where selected bands of overlapping compounds from conventional nonpolar column (first dimension) are passed to a short polar column (second dimension) [34]. For GCxGC, two columns (first dimension is a conventional column and second dimension is a short fast type) are connected sequentially with a modulator between them which is powered by liquid nitrogen. The modulator functions are continuously collect small fractions from first dimension, focus or refocus of a narrow band and quickly transfer of fraction collected to second dimension [31]. Both techniques have been poorly explored for determination of phytosterols. MDGC was used to analyze of plant sterol profile from unsaponifiable lipid fraction [30] and GCxGC was applied to evaluate phytosterols in vegetable oil [32]. GCxGC, MDGC and traditional GC can be easily coupled to MS, ensuring other important parameter, mass spectrum, which allows the determination of these analytes.
In this regard, GC-MS is still considered the gold standard equipment for phytosterols determination [10,11,28,33]. Chromatographic separation problems remains if different types of columns are not used or the equipment is not a MDGC or GCxGC. However, mass fragmentation of each phytosterol allows selectivity of the compounds, including bands of overlapping compounds. By selection of the ions (m/z), chromatographic peak becomes evident and consequently, the identification of the compounds [24]. Figure 2 is a typical chromatogram produced by GC-MS in our laboratory showing coelution between beta-sitosterol and fucosterol and the identification of these analytes was performed based in ions of interest.
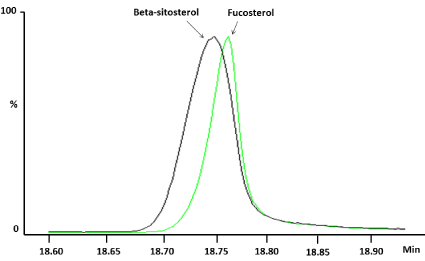
Figure 2: GC-MS QP2010 model (Shimadzu®) using capillary column RTx-
5MS (cross bond 5% diphenyl – 95% dimethylpolysiloxane) with 30m x
0.25mm x 0.25 Ám film thickness in the following conditions: Split less inject
mode at 250°C using helium as carrier gas. Column flow in 1.11 mL/min
at a constant linear velocity mode. The column oven temperature program
started 150°C (hold 1 min), then programed at 10°C/min to 320 °C (hold
4 min). The total analytical time was 22 min. Transfer line was 320°C and MS
was operated by electron ionization (70 eV) in Selected Ion Monitoring (SIM)
mode. Ions were chosen (m/z): 414, 396 and 354 for beta-sitosterol; 314, 299
and 281 for fucosterol. Base peak are highlighted and underlined.
Nevertheless, determination of phytosterols is discouraged when these analytes shows very close retention time and similar mass fragmentation. In this case, it is possible to identify these analytes using other types of advanced MS (triple quadrupole, time-offlight and hybrids MS), avoiding different strategies with respect to chromatographic separation [35-37].
For quantification of compounds, the most of the analysis requires Internal Standard (IS) [21,24]. Cholesterol has been used as Internal Standard (IS) in GC-FID for analysis of unsaponifiables compounds in vegetable distillate oils [38]. However, cholesterol is present in algae sin significative levels [14] and small concentrations (less than 1%) of crude extra-virgin olive oil [39]. Cholestane (5a-cholestane), dihydrocholesterol (5a-cholestan-3β-ol), copostranol (5β -cholestan -3β-ol) and epicoprostranol (5 β-cholestan- 3a-ol) have been used with success as IS for GC-FID and GC-MS [23,28,40-44]. Indeed, deuteratedanalytes are the best option as IS for phytosterols determination when GC-MS is available.
Conclusion
This critical review article showed limitations of GC for determination of phytosterols, demonstrating alternatives to solve chromatographic problems for future studies with these analytes in plants and derived products.
References
- Moreau RA, Whitaker BD, Hicks KB. Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res. 2002; 41: 457-500.
- Seitz LM. Stanol and sterol esters of ferulic acid and p-coumaric acids in wheat, corn, rye and triticale. J Agric Food Chem. 1989; 37: 662-667.
- Piironen V, Toivo J, Lampi A-M. Plant sterols in cereals and cereal products. Cereal Chem. 2002; 79: 148-154.
- Jiang Y, Wang T. Phytosterols in cereal by-products. J AOCS. 2005; 82: 439-444.
- Awad AB, Downie A, Fink CS, Kim U. Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer Res. 2000; 20: 821-824.
- Berger A, Jones PJ, Abumweis SS. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004; 3: 5.
- Jones PJ. Cholesterol-lowering action of plant sterols. Curr Atheroscler Rep. 1999; 1: 230-235.
- Garoufi A, Vorre S, Soldatou A, Tsentidis C, Kossiva L, Drakatos A, et al. Plant sterols-enriched diet decreases small, dense LDL-cholesterol levels in children with hypercholesterolemia: a prospective study. Ital J Pediatr. 2014; 40: 42.
- Blas OJ, Gonzáles AV. Determination of sterols by capillary column gas chromatography. Differentiation among different types of olive oil: Virgin, refined, and solvent-extracted. J AOCS. 1996; 73: 1685-1689.
- Azadmard-Damirchi S. Review of the use of phytosterols as a detection tool for adulteration of olive oil with hazelnut oil. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010; 27: 1-10.
- Inchingolo R, Cardenia V, Rodriguez-Estrada MT. Analysis of phytosterols and phytostanols in enriched dairy products by Fast gas chromatography with mass spectrometry. J Sep Sci. 2014; 37: 2911-2919.
- Food and Drug Administration (FDA). Department of Health and Human Services, Food labeling; health claim; phytosterols and risk of coronary heart disease. Federal Register. 2010; 75: 76536-76571.
- European Commission regulation (EC). 608/2004 of 31st March, 2004 concerning labeling of foods and food ingredients with added phytosterols, phytosteryl esters, phytostanols, and/or phytostanyl esters. Off J Eur Comm. 2004; 97: 44-45.
- Govindan M, Hodge JD, Brown KA, Nuñez-Smith M. Distribution of cholesterol in Caribbean marine algae. Steroids. 1993; 58: 178-180.
- Heupel RC. Isolation and primary characterization of of sterols. In: Analysis of Sterols and Other Biologically Significant Steroids. W.D. Nes and E.J. Parish (eds), Academic Press, Inc, San Diego, CA. 1989; 1-32.
- Abidi SL. Chromatographic analysis of plant sterols in foods and vegetable oils. J Chromatogr A. 2001; 935: 173-201.
- Bligh eg, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959; 37: 911-917.
- Mitei YC, Ngila JC, Yeboah SO, Wessjohann L, Schmidt J. Profiling of phytosterols, tocopherols and tocotrienols in Selected Seed Oils from Botswana by GC–MS and HPLC. J Am Oil Chem Soc. 2009; 86: 617–625.
- Kongduang D, Wungsintaweekul J, De-Eknamkul W. Established GC-FID for simultaneous determination of diterpenes and phytosterols in Plaunoi (Croton stellatopilosusOhba). Songklanakarin J. Sci. Technol. 2012; 34: 623-628.
- Bezerra KS, Antoniosi Filho NR. Characterization and quantification by gas chromatography of free steroids in unsaponifiable matter of vegetable oils. J Braz Chem Soc. 2014; 25: 238-245.
- Holler FJ, Skoog DA. Princípios de análiseinstrumental. 6th edn. Porto Alegre: Bookman. 2009.
- Clement LM, Hansen SL, Costin CD, Perri GL. Quantitation of sterols and steryl esters in fortified foods and beverages by GC/FID. J AOCS. 2010; 87: 973–980.
- Laakso PH. Determination of plant stanols and plant sterols in phytosterol enriched foods with a gas chromatographic-flame ionization detection method: NMKL collaborative study. J AOAC Int. 2014; 97: 1097-1108.
- Hübschmann HJ. Handbook of GC/MS: fundamentals and applications. 2ndedn.Weihneim. Willey-VCH. 2008.
- Domeno C, Ruiz B, Nerin C. Determination of sterols in biological samples by SPME with on-fiber derivatization and GC/FID. Anal Bioanal Chem. 2005; 381: 1576-1583.
- Ahmida HS, Bertucci P, Franzo L, Massoud R, Cortese C, Lala A, et al. Simultaneous determination of plasmatic phytosterols and cholesterol precursors using gas chromatography-mass spectrometry (GC-MS) with selective ion monitoring (SIM). J Chromatogr B Analyt Technol Biomed Life Sci. 2006; 842: 43-47.
- Saraiva D, Semedo R, Castilho Mda C, Silva JM, Ramos F. Selection of the derivatization reagent--the case of human blood cholesterol, its precursors and phytosterols GC-MS analyses. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879: 3806-3811.
- Saraiva D, Castilho MC, Martins MR, Silveira MIN, Ramos F. Evaluation of phytosterols in milk and yogurts used as functional foods in Portugal. Food Anal Methods. 2011; 4: 28–34.
- Herchi W, Harrabi S, Sebei K, Rochut S, Boukhchina S, Pepe C, et al. Phytosterols accumulation in the seeds of Linum usitatissimum L. Plant Physiol Biochem. 2009; 47: 880-885.
- Breinhölder P, Mosca L, Lindner W. Concept of sequential analysis of free and conjugated phytosterols in different plant matrices. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 777: 67-82.
- Mondello L, Tranchida PQ, Dugo P, Dugo G. Comprehensive two-dimensional gas chromatography-mass spectrometry: a review. Mass Spectrom Rev. 2008; 27: 101-124.
- Tranchida PQ, Salivo S, Franchina FA, Bonaccorsi I, Dugo P, Mondello L. Qualitative and quantitative analysis of the unsaponifiable fraction of vegetable oils by using comprehensive 2D GC with dual MS/FID detection. Anal Bioanal Chem. 2013; 405: 4655-4663.
- Sakouhi F, Absalon C, Sebei K, Fouquet E, Boukhchina S, Kallel H. Gas chromatographic–mass spectrometric characterisation of triterpene alcohols and monomethylsterols in developing Oleaeuropaea L. fruits. Food Chemistry. 2009; 116: 345–350.
- Cherif AO, Messaouda MB, Kaabi B, Pellerin I, Boukhchina S, Kallel H, et al. Characteristics and pathways of bioactive 4-desmethylsterols, triterpene alcohols and 4a-monomethylsterols, from developing Tunisian cultivars and wild peanut (Arachishypogaea L.). Plant PhysiolBiochem. 2011; 49: 774-781.
- Ai J. Rapid Measurement of Free Phytosterols in Tobacco by Short-Column GC/MS/MS. J Agri Food Chem. 1997; 45: 3932-3935.
- Wewer V, Dombrink I, vom Dorp K, Dörmann P. Quantification of sterol lipids in plants by quadrupole time-of-flight mass spectrometry. J Lipid Res. 2011; 52: 1039-1054.
- Matysik S, Klünemann HH, Schmitz G. Gas chromatography-tandem mass spectrometry method for the simultaneous determination of oxysterols, plant sterols, and cholesterol precursors. Clin Chem. 2012; 58: 1557-1564.
- Naz S, Sherazi STH, Talpur FN, Talpur MY, Kara H. Determination of unsaponifiable constituents of deodorizer distillates by GC–MS. J AOCS. 2012; 89: 973–977.
- Rocco A, Fanali S. Analysis of phytosterols in extra-virgin olive oil by nano-liquid chromatography. J Chromatogr A. 2009; 1216: 7173-7178.
- Esche R, Scholz B, Engel KH. Analysis of free phytosterols/stanols and their intact fatty acid and phenolic acid esters in various corn cultivars. J Cereal Sci. 2013; 58: 333-340.
- Rumiyati, Jayasena V, James AP. Total phenolic and phytosterol compounds and the radical scavenging activity of germinated Australian sweet lupin flour. Plant Foods Hum Nutr. 2013; 68: 352-357.
- Nurmi T, Lampi AM, Nyström L, Hemery Y, Rouau X, Piironen V. Distribution and composition of phytosterols and sterylferulates in wheat grain and bran fractions. J Cereal Sci. 2012; 56: 379-388.
- Mo S, Dong L, Hurst WJ, van Breemen RB. Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography-tandem mass spectrometry. Lipids. 2013; 48: 949-956.
- Santos MAZ, Alicieo TVR, Pereira CMP, Ramis-Ramos G, Mendonça CRB. Profile of bioactive compounds in avocado pulp oil: influence of the drying processes and extraction methods. J AOCS. 2014; 91: 19-27.