
Research Article
Austin Chromatogr.2014;1(5): 1022.
HPLC-DAD-ESIMS Analyses of Hyoscyamus niger and H. reticulatus for their Antioxidant Constituents
Amir Reza Jassbi¹*, Ramin Miri¹, Mehdi Masroorbabanari¹, Mojtaba Asadollahi¹, Mahshid Attarroshan¹ and Ian T Baldwin²
Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences, Iran
Department of Molecular Ecology, Max Planck Institute for Chemical Ecology, Germany
*Corresponding author: Amir Reza Jassbi, Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences, PO Box 71345-3388, Shiraz, Iran
Received: November 17, 2014; Accepted: December 29, 2014; Published: December 31, 2014
Abstract
Different extracts from leaves, epicalyxes and seeds of the plants Hyoscyamus niger and H. reticulatus were subjected to total phenolic measurements using Folin-Ciocalteu reagent and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assays and high performance liquid chromatography (HPLC-UV) analyses. Among the tested plant’s extracts, the 80% methanol extracts of H. reticulatus leaves (173.1±0.0), H. reticulatus epicalyxes (183.3±10.1), H. niger leaves (221.3±26.6) and H. niger epicalyxes (362.3±11.6) had lower IC50s (μg plant extracted/1 mL 10-4 M DPPH) which were in agreement with the increasing order of their total phenolic contents. The constituents of the most active 80% methanol extracts of H. niger and H.reticulatus were determined by HPLC-Diode Array Detector-Electrospray Ionization Mass Spectroscopy (LC-DAD-ESIMS). Three major phenolic constituents were identified and quantified: chlorogenic acid, rutin and Quercetin-3O-Glucoside-Rhamnoside-Rhamnoside (QGRR). In this paper, we correlate the antioxidant activity of the plants extracts with the concentrations of their phenolics.
Keywords: Hyoscyamus niger; Hyoscyamus reticulatus; Solanaceae; Quercetin glycosides; Antioxidants
Introduction
The genus Hyoscyamus is represented by 18 species in Iran, half of which are reported as native plants [1,2]. H. niger and H. reticulatus grow wild in different parts of the country and are rich in tropane alkaloids, including: hyoscyamine, scopolamine and atropine with diverse biological activities such as antispasmodic, anticholinergic, analgesic and sedative effects [3-5]. Most of these bioactive compounds have been purified from the seeds of H. niger, but a few papers report the chemical composition of its leaves [6] and non-alkaloidal secondary metabolites, including biologically active flavonoids, lignans [7-11], steroidal saponins [12] and coumarinolignans [13]. Recently, the aerial parts of H. reticulatus collected from Turkey were assessed for their antioxidant activity and fatty acids composition [4] but the plant’s antioxidant constituents were not characterized.
As a part of our program for phytochemical screening of solanaceous plants, we have subjected different extracts of the above mentioned plants to the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging test and quantification of their total phenoliccontents. The active extracts were then analyzed by high performance Liquid Chromatography-Ultraviolet Spectroscopy-Electrospray Ionization-Mass Spectrometry (LC-UV-ESIMS) and HPLC-UV for qualitative and quantitative analyses.
Material and Methods
Reagents
Quercetin and rutin were obtained from Acros Organics (Geel, Belgium). Folin-Ciocalteu reagent, methanol, acetonitrile, phosphoric acid, gallic acid, and sodium carbonate were purchased from Merck (Darmstadt, Germany). The solvents were HPLC grades. Caffeic acid and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Plant material
Hyoscyamus niger L. was collected in June 2011 from Shiraz to Kharameh, Fars Province at 29º 36´ 50.6˝ N and 52º 53´ 05.9˝ E and altitude of 1593 m while Hyoscyamus reticulatus L. was collected from Dasht-e-Arjan, Fars Province at location coordinates of 29º, 35´ N and 51º, 56´ E and altitude of 1700 m. The plants were identified [1] at Medicinal and Natural Products Chemistry Research Center (MNCRC), Shiraz, Iran by Mojtaba Asadollahi. The voucher specimens for H. niger and H. reticulatus with herbarium numbers PC- 90-1 and PC-90-2 were deposited at MNCRC herbarium respectively. Aerial parts of the plants were air-dried at room temperature in the shade and were used for solvent extraction.
Secondary metabolites HPLC analysis
The extraction and HPLC methods for analyses of rutin, chlorogenic acid, quercetin derivatives were the same as we described earlier [14]. The dried powdered leaves (50 mg) were extracted using 1.5 mL of 80% methanol, initially 45 min in an ultrasound bath and then kept for 24 h at room temperature. Reversed-phase (RP-18) HPLC analyses were performed using a Knauer analytical HPLC with a K-1001 pump and a four channel K-2600 UV detector set at λ 210, 254, 320 and 365 nm in a wavelength range of 190-740 nm [14]. The HPLC column (Eurospher-100 C18, 250 × 4.6 mm, Knauer, Germany) was eluted with acetonitrile (solvent B) and 0.25 % H3PO4 in ultrapure water (solvent A) as follows: 0-6 min, 0-12% of B; 6-10 min, 12-18% of B; 10-30 min, 18-58% of B; 30-35 min, 58-80% of B; 35-45 min, 100% of B and 45-50 min, 100-0% of B. The flow rate of the mobile phase was set at 1 mL/ min. Rutin and caffeic acid were used as external standards for quantification of QGs (quercetin glycosides) and chlorogenic acid, measured at λ 365 and 320 nm respectively.
LC-DAD-ESIMS analysis
For the LC-DAD-ESIMS analyses we used a Shimadzu LCMS- 2010EV, with an ESI mass and a SPD-M20A diode array detector. For the HPLC coupled to mass spectrometer, the column was Shim Pack XR-ODS 2 mm × 50 mm and LC solvent program and pump (LC 20AD) were; flow rate: 0.2 mL/min; solvent B: acetonitrile; solvent A: 0.25% formic acid in water. The injection volume was 5 μL of the 10 times-diluted of the sample solutions that were used for the HPLC analyses. The column was eluted with 0% B at the beginning, at 3 min 12% B, at 5 min 18% B, at 15 min 58% B, at 20 min 80% B, at 25 min 80% B and at 30 min 0% B. ESI was used as the ionization source for the MS in positive and negative modes. The MS parameters were as follows: MS detector voltage: ±1.5 kV, interface: ±4.5 kV, CDL: ±10 V, and Q-array (Rf: ±150 V) voltages uploaded from the tuning file. The scanned mass range was 100-1000 mass unit, nebulizing gas was N2 with flow rate of 1.5 L/min and heat block and CDL temperatures were set at 230 and 275 °C respectively. The UV spectra were recorded on the DAD detector at λ190-600 nm and the cell and column temperature was set at 40 °C.
Free radical scavenging activity
The free radical scavenging activity of the plant extracts was measured by the method of Blois [15] with some modifications [16] and compared to that for quercetin as a standard radical scavenger. The extraction procedure was similar to that reported for HPLC analysis except for the amount of the plant material (25 mg) and the duration of extraction (48 hours). Twenty-five μL of this and a series of its two-fold diluted extracts were added to 4 mL 10-4 M DPPH methanol solutions. After 30 min shaking of the solutions in the dark, the absorptions of the DPPH solutions were measured at 517 nm on a Perkin Elmer Lambda 1 UV/VIS spectrophotometer. The percentage of the reduced DPPH was calculated by the following equation:
Percentage of DPPH reduction = ((A0 – A1) / A0) x 100), where A0 is the absorbance of the control, and A1 is the absorbance in the presence of sample. The IC50s were calculated by linear regression equations of the DPPH inhibition percentage from different concentrations of the extracts and the standard antioxidants, using Microsoft Excel and Curve Expert statistical programs and expressed as μg plant material extracted with the solvent/1 mL 10-4 M DPPH (μg PM /ml DPPH).
Total phenol contents
The total phenol contents of the plant extracts were determined by the Folin-Ciocalteu method as described previously with some modifications [17]. Briefly, 3.16 mL water and 200 μL Folin-Ciocalteu reagent were added to a 40 μL solution of the plant extract (80% methanol or methanol), and the mixture was shacked well. 600 μl of a 0.25% sodium carbonate was added to this solution after 8.5 min incubation at room temperature. The above solution was further incubated at RT for 2 h and its absorbance was measured at 765 nm against the blank. The concentrations of the total phenolics were measured against a series of gallic acid standard solutions and expressed as mg equivalent of gallic acid in 1g dry plant material (mg EG/g PM) [17].
Statistical analysis
The values shown in tables 2 and 3 are the average of 3-5 measurements ± SE. The IC50s were calculated using Curve Expert statistical program. One-way analyses of variance (ANOVA) and post hoc multiple comparison (Tukey) tests were used for determination of signification between different measurements using SPSS software and expressed as probability factor; p value. p≤0.05 was considered to be significant.
Results and Discussion
We identified chlorogenic acid; rutin and quercetin-3Oglucoside- rhamnoside–rhamnoside (QGRR) (Figure 1) in an 80% methanol extract of leaves and epicalyxes of H. niger and H. reticulatus (Figure 2, Table 1). The identification of the compounds was deduced according to the Electrospray Ionization (ESI) mass and UV spectral data (Table 1) and the quantification was performed using reversed-phase HPLC-UV analyses of the extracts (Figure 2, Table 2) using rutin and caffeic acid as external standards. Rutin was previously identified in the seeds of H. niger [11] but not in our sample seeds, this may be due to different origin of the plant material or the extraction procedure. To the best of our knowledge, this is the first report of QGRR from these species. We propose the structure based on the positive ESI mass spectral data, which revealed protonated precursor ion at m/z 757 [M+H]+, and fragment ions at m/z 611 [M-146]+, 465 [M-2×146]+, 303 [M-2×146-162]+, indicating the loss of two rhamnose (deoxyhexose) and one glucose (hexose) from the molecular ion respectively [18-20].
Compound
+ ESI MS
m/z
- ESI MS
m/z
UV abs. nm
RT
min
FW
MF
Chlorogenic acid
-
353 [M-H]-, 191
239, 326
7.7
354
C16H18O9
Rutin
611 [M+1]+, 473, 303
609 [M-H]-, 302
205, 264, 352
11.8
610
C27H30O16
QGRRa
757 [M+H]+, 611, 465, 460, 303
755 [M-H]-, 463, 301, 285
205, 263, 352
13.9
756
C33H40O20
a) The quercetin glycoside is determined as: quercetin-3O-glucoside-rhamnoside-rhamnoside (QGRR)
Table 1: The spectral data recorded for the phenolic constituents in the H. niger and H. reticulatus.
Plant name
Rutin
(RT 15.9)
QGRRa
(RT 17.3)
Chlorogenic acid
(RT 18.6)
H. niger (leaves)
9.2±0.5 *
2.2±0.1
0.4±0.0
H. niger (epicalyxes)
3.5±0.4
-
1.1±0.1
H. reticulatus (leaves)
8.9±0.3 *
19.9±0.1*
3.4±0.1 *
H. reticulatus (epicalyxes)
0.1±0.0
2.2±0.1
1.8±0.1
Values are presented as mean ± SE of 5 experiments. a) The quercetin glycoside is determined as: quercetin-3O-glucoside-rhamnoside-rhamnoside (QGRR)
* significantly p< 0.0001 higher than other values in the columns
Table 2: Reversed phase-HPLC analyses of 80% methanol extracts of H. niger and H. reticulatus (mg/g dry plant material).
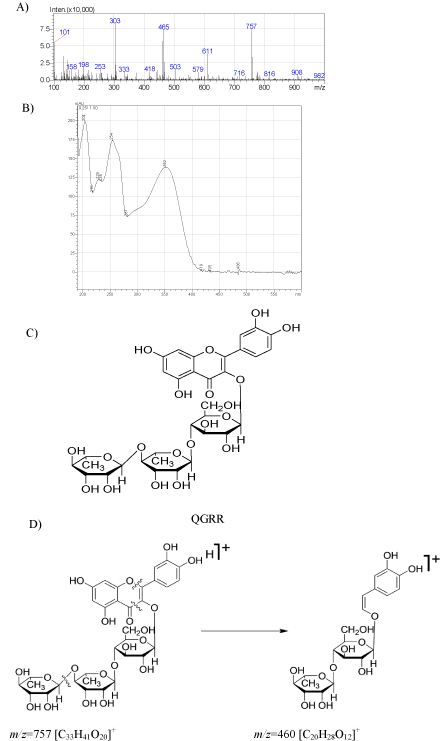
Figure 1: ESI-MS (A) and UV (B) spectra of the proposed structure (C) for
the quercetin-3O-glucoside-rhamnoside-rhamnoside (QGRR) detected in
the Hyoscyamus species and the fragment (D) observed for the tentative
structure at m/z=460 in the mass spectrum.
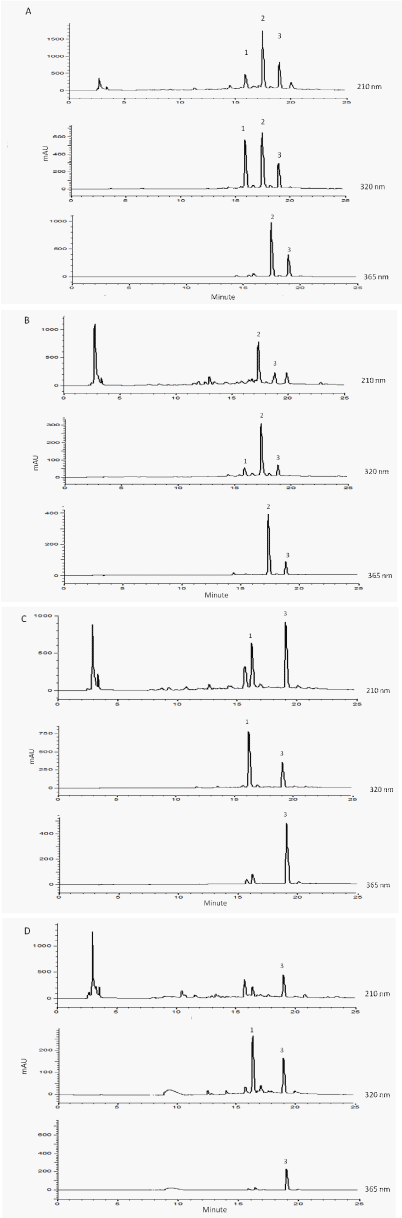
Figure 2: HPLC chromatograms of the 80% methanol extract of A) H.
reticulatus (leaves), B) H. reticulatus (epicalyxes), C) H. niger (leaves),
D) H. niger (epicalyxes). 1: chlorogenic acid, 2: quercetin-3O-glucosiderhamnoside-
rhamnoside (QGRR) and 3: rutin, measured at λ 210, 320 and
365 nm.
The UV absorption maxima at λ 205, 263 and 352 nm for rutin and the quercetin glycoside suggests a similar oxidation and glycosylation pattern for both compounds. Therfore we propose that both compounds are glycosides of quercetin at the C-3(OH) position. Simillar quercetin glycosides have been reported from Arabidopsis thaliana particularly, two isomers Q1 and Q4 (quercetin-glucosiderhamnoside- rhamnoside) [20]. The only difference between the mass spectra of QGRR and those reported in A. thaliana was that the peak observed at m/z 449 [M-146-162]+ for Q1 and Q2 indicated the loss of a rhamnose followed by a glucose (m/z 162) lost from the ions at m/z 611 which in turn suggests an extra rhamnose on any of the free hydroxyls of either A or B rings of the aglycone [20], while in the mass spectrum of QGRR an ion at m/z 465 [M-146-146]+ suggested the loss of an additional rhamnose rather than a glucose from the molecule. In addition to the above mentioned fragments, a strong peak at m/z 460 (C20H28O12) may be resulted from elemination of the A ring together with O-1 and the C-3 carbonyl function and one rhamnosyl from the QGRR molecule [18]. The above mentioned fragments may suggest a furhter glycosylation of rhamnose on QGRR similar to that of rutin on one of the other free hydroxyl groups of the sugar moiety at C-3 (Figure 1).
Since the above phenolic constituents are known to have antioxidant properties, [21,22] seeds, leaves and epicalyxes were subjected to DPPH radical scavenging test [15,16] and measures of total phenolic-contents using the Folin-Ciocalteu reagent [23]. In all of 80% methanol extracts of the tested plant material, the total phenol contents of the plant’s extracts were higher and the respective DPPH IC50s lower than those measured in the methanol extracts (Table 3). Therefore, the chemical constituents of the 80% methanol extracts were quantified using Reversed Phase (RP) HPLC (Figure 2, Table 2). The leaves of both plants had higher levels of the phenolics compared to their epicalyxes, and none of the above phenolics could be detected in the seeds. The levels of phenolics were lower and therefore the DPPH IC50s was significantly higher in the seeds of H. niger but the above values were not measurable in the seeds of H. reticulatus. The leaves of H. reticulatus contained QGRR (19.9±0.1 mg/ g PM), rutin (8.9±0.3 mg/ g PM) and chlorogenic acid (3.4±0.1 mg/ g PM) as their major phenolic constituents, while rutin (9.2±0.5 mg/ g PM) was the most abundant component in the leaves of H. niger (Table 2).
Plant name
IC50DPPHa
(MeOH)
IC50 DPPH (80%MeOH)
Total Phenolb
(MeOH)
Total Phenol
(80%MeOH)
H. niger (leaves)
270.2±8.3cfh
221.3±26.6hcfkl
7.5±0.0mpqrsv
8.5±0.2rmpqv
H. niger (epicalyxes)
451.3±2.2d
362.3±11.6ig
2.7±0.3nsoqt
4.7±0.3smntq
H. niger (seeds)
1485±34.9e
680.2±10.4j
2.1±0.4ont
3.3±0.1tnos
H. reticulatus (leaves)
224.2±14.0fch
173.1±0.0kl
9.4±1.0pmrv
14.3±0.7u
H. reticulatus (epicalyxes)
339.2±10.0gi
183.3±10.1lk
5.3±0.1qmrnst
9.1±1.5vrp
H. reticulatus (seeds)
NMw
NM
NM
NM
Quercetin
1.79±0.046
-
-
-
alues are presented as mean ± SE of 3-5 experiments.
a) DPPH IC50 (µg plant extracted or µg quercetin/1 mL 10-4 M DPPH)
b) Total phenol (mg eq. gallic acid in 1g dried plant)
c-v) Values with different letters, have p values which are significantly different, (p<0.05) in the columns and the rows
w) NM: not measurable
Table 3: Total phenolic content and DPPH radical scavenging potential of the methanol and 80% methanol extracts of plants.
Conclusion
The phenolics in the diets of humans particularly, quercetin and its glycosides, play important roles as potential protectors against diseases such as coronary heart disease, cancer and inflammatory disease [21,24,25]. The QGs are even more potent antioxidants than their aglycones [21,22]. Kim and coworkers have reported that, the glycoside part of rutin prevents the compound from being adsorbed in the small intestine. This allows rutin to reach the colon and be cleaved there, so that the aglycone (quercetin) can have antiinflammatory activity against Inflammatory Bowel Diseases (IBD) in test rats [24]. The action of the compound is comparable to that observed for sulfasalazine, a pro-drug of 5-amino salicylic acid used for treatment of IBD [24]. The high concentrations of both rutin and its rhamnoside derivative suggest H. reticulatus could be used as a source and the larger sugar moiety of the QG may affect its absorption in the small intestines [26] and thereby be another candidate for treatment of IBD.
Acknowledgment
We are grateful to the Alexander von Humboldt foundation, Max Planck Institute for Chemical Ecology in Jena, Germany and the research council of Shiraz University of Medical Sciences for financial support under the Research Group Linkage Project (3.4 - IRN/1101775-2012- 2013).
References
- Schonbeck-Temesy E. Hyoscyamus. In: Rechinger KH, editor. Flora Iranica: Volume 100, Akademische Druck and Verlagsanstalt, Graz. 1972; 49-79.
- Mozaffarian V. A dictionary of Iranian plant names. Farhang Mo’aser, Tehran. 2003.
- Bahmanzadegan A, Sefidkon F, Sonboli A. Determination of hyoscyamine and scopolamine in four Hyoscyamus species from Iran. Iran J Pharm. Res. 2009; 8: 65-70.
- Guler GO. Studies on antioxidant properties of the different solvent extracts and fatty acid composition of Hyoscyamus reticulatus L. J Food Biochem. 2012; 36: 532-538.
- Mohammad MK, Almasri IM, Tawaha K, Issa A, Al-Nadaf A, Hudaib M, et al. Antioxidant, antihyperuricemic and xanthine oxidase inhibitory activities of Hyoscyamus reticulatus. Pharm Biol. 2010; 48: 1376-1383.
- Chalabian F, Majd A. Research of change of tropane alkaloids quantities in different stages of growth of Hyoscyamus reticulatus L. in natural condition and assessment of sugar and elements changes on biosynthesis of these alkaloids in In vitro. J Med Plants. 2004; 3: 39-46.
- Begum AS. Bioactive non-alkaloidal secondary metabolites of Hyoscyamus niger Linn. Seeds: A review. Res J Seed Sci. 2010; 3: 210-217.
- Begum AS, Verma S, Sahai M, Asai T, Hara Nand Fujimoto Y. Hyosmin, a new lignan from Hyoscyamus niger L. J Chem Res. 2006; 10: 675-677.
- Begum AS, Verma S, Sahai M, Schneider K, Sussmuth R. Hyoscyamal, a new tetrahydrofurano lignan from Hyoscyamus niger Linn. Nat Prod Res. 2009; 23: 595-600.
- Begum S, Saxena B, Goyal M, Ranjan R, Joshi VB, Rao CV, et al. Study of anti-inflammatory, analgesic and antipyretic activities of seeds of Hyoscyamus niger and isolation of a new coumarinolignan. Fitoterapia. 2010; 81: 178-184.
- Ma CY, Liu WK, Che CT. Lignanamides and nonalkaloidal components of Hyoscyamus niger seeds. J Nat Prod. 2002; 65: 206-209.
- Lunga I, Bassarello C, Kintia P, Shvets S, Piacente S, Pizza C. Steroidal glycosides from the seeds of Hyoscyamus niger L. Nat Prod Com. 2008; 3: 731-734.
- Sajeli B, Sahai M, Suessmuth R, Asai T, Hara N, Fujimoto Y. Hyosgerin, a new optically active coumarinolignan, from the seeds of Hyoscyamus niger. Chem Pharm Bull (Tokyo). 2006; 54: 538-541.
- Jassbi AR, Miri R., Alizadeh M, Masroorbabanari M, Asadollahi M, Baldwin IT. Quantification of phenolic diterpenoids and rosmarinic acid in Salvia eremophila and S. santolinifolia using LC-DAD-MS. Austin Chromatogr. 2014; 1: 5.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1985; 181: 1199-1200.
- Firuzi O, Miri R, Asadollahi M, Eslami S, Jassbi AR. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven salvia species from iran. Iran J Pharm Res. 2013; 12: 801-810.
- Waterhouse. Determination of total phenolics. In: Wrolstad, editor. Current protocols in food analytical chemistry, Wiley, New York. 2002; I1.1.1–I1.1.8.
- Domon B, Hostettmann K. Mass spectrometric studies of underivatized polyphenolic glycosides. Phytochemistry. 1985; 24: 575-580.
- Jones P, Messner B, Nakajima J, Schaffner AR, Saito K. UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J Biol Chem. 2003; 278: 43910-43918.
- Le Gall G, Metzdorff SB, Pedersen J, Bennett RN, Colquhoun I. Metabolite profiling of Arabidopsis thaliana (L.) plants transformed with an antisense chalcone synthase gene. J Colquhoun Metabolomics. 2005; 1: 181-198.
- Choi SJ, Tai BH, Cuong NM, Kim YH, Jang HD. Antioxidative and anti-inflammatory effect of quercetin and its glycosides isolated from mampat (Cratoxylum formosum). Food Sci Biotech. 2012; 21: 587-595.
- Kim MR, Lee JY, Lee HH, Aryal DK, Kim YG, Kim SK, et al. Antioxidative effects of quercetin-glycosides isolated from the flower buds of Tussilago farfara L. Food Chem Toxicol. 2006; 44: 1299-1307.
- Singleton VL, Rossi Jr JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965; 16: 144-158.
- Kim H, Kong H, Choi B, Yang Y, Kim Y, Lim MJ, et al. Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm Res. 2005; 22: 1499-1509.
- Shen SC, Chen YC, Hsu FL, Lee WR. Differential apoptosis-inducing effect of quercetin and its glycosides in human promyeloleukemic HL-60 cells by alternative activation of the caspase 3 cascade. J Cell Biochem. 2003; 89: 1044-1055.
- Arts ICW, Sesink ALA, Faassen-Peters M, Hollman PCH. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Brit J Nutr. 2004; 91: 841-847.