
Special Article - RP-HPLC
Austin Chromatogr. 2014;1(5): 1023.
Development and Validation of Stability Indicating HPTLC Method for Simultaneous Estimation of Fluocinolone Acetonide and Miconazole Nitrate in Ointment
Archita J Patel*
K.B. Institute of Pharmaceutical Education and Research, India
*Corresponding author: Archita J Patel, K.B. Institute of Pharmaceutical Education and Research, India
Received: December 10,2014; Accepted: January 05, 2014; Published: January 07, 2014
Abstract
Fluocinolone acetonide is a steroidal drug and Miconazole nitrate is an antifungal azole. The combination of these drugs has a highly beneficial effect on dermatological inflammatory disorders associated with fungal infections. A simple, specific, accurate, precise, robust and stability indicating HPTLC method has been developed and validated for the simultaneous estimation of Fluocinolone acetonide and Miconazole nitrate in bulk drug and ointment dosage form. Chromatography was performed using pre-coated silica gel aluminium plate 60F254, (10 ×10 cm) as stationary phase and n-Hexane: Ethyl acetate (1:9, v/v) as mobile phase. Detection was carried out at 254 nm. The Rf value for Miconazole nitrate and Fluocinolone acetonide was found to be 0.46 and 0.64 respectively. The optimized conditions develop showed a linear response from 200-700 ng/spot (r2 =0.983) for Fluocinolone acetonide and 40000-140000 ng/spot (r2 =0.990) for Miconazole nitrate. The study involved observation on degradation products formed under different stress condition. The developed method successfully separated drug substances from degradation products formed under various stress conditions.
Keywords: Miconazole nitrate; Fluocinolone acetonide; Stability indicating;HPTLC
Introduction
Fluocinolone Acetonide (FA), 6α, 9α-diFAoro-11β, 21-dihydroxy- 16α, 17α-isopropylidenedioxy-1, 4-diene, 3, 20-Dione (Figure 1) is a corticosteroid used topically for treatment of a variety of skin disorders and inflammatory eye, ear, and nose diseases [1]. It has high antiinflammatory activity and is usually used formulated as a cream, gel, lotion, or ointment [2,3]. Miconazole nitrate (MCZ), 1H-imidazole, 1-[2-(2, 4-dichlorophenyl)-2-[(2, 4- icholophenyl) methoxy] ethyl] - (±) nitrate (Figure 1) is an antimycotic imidazole derivative [1]. With a wide activity spectrum, it is endowed with a powerful activity against dermatophytes and Candida albicans, as well as against several Gram positive germens [3,4]. The generally accepted mode of action of azoles antifungal is the inhibition of 14-alpha-lanosterol demethylase, a key enzyme in ergosterol biosynthesis, resulting in depletion of ergo sterol and accumulation of toxic 14-alpha-methylated sterols in the membrane of susceptible yeast species [5]. MCZ has been extensively applied in the management of dermal, oral, and vaginal mycosis. Currently, it is used in a variety of pharmaceutical formulations such as injections, tablets, oral gels, creams, ointments, topical powders and vaginal suppositories. The most usual application forms include creams, ointments or gels at 2.0% concentration level, alone or associated with anti-inflammatory steroids, or other antimicrobials such as gentamycin for the treatment of dermatitis [6,7].
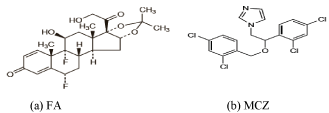
Figure 1: Chemical structure of FA (a) and MCZ (b).
Literature review reveals that detection and quantitative determination of FA and MCZ in routine analysis of individual drug, with combination of other drugs as well as in biological matrices, including human plasma, serum, and urine, is accomplished by variety of methods, including potentiometry [1,8], spectrophotometry [9,10], High Performance Liquid Chromatography (HPLC) [10-21], HPLC with spectrophotometry [14,18], HPLC with Mass Spectroscopy (LC-MS) [22-24], Gas Chromatography (GC) [8,25], Thin Layer Chromatography (TLC) [26], High Performance Thin Layer Chromatography (HPTLC) [27], etc in various dosage forms like cream, gel, tablets, ovule, ointment. However best of our knowledge, no method has been reported yet for simultaneous estimation of above mentioned drugs.
The International Conference on Harmonization (ICH) guideline entitled Stability Testing of New Drug Substances and Products requires the stress testing to be carried out to elucidate the inherent stability characteristics of the active substance [28]. Susceptibility to oxidation is one of the required tests. Also the hydrolytic and the photolytic stability are required. An ideal stability-indicating method is one that quantifies the standard drug alone and also resolves its degradation products. Ferenczi-Fodor and colleagues [29] explained basic acceptance criteria for evaluation of validation experiments based on practical experience for planar chromatographic procedures, which may be used at different levels either in qualitative identity testing, assays, semi-quantitative limit tests or quantitative determination of impurities. The parameters for robustness testing of the given procedures and quality assurance of quantitative planar chromatographic testing have been described as per ICH guidelines.
Nowadays HPTLC is becoming a routine analytical technique due to its advantages of low operating cost, high sample throughput and need for minimum sample clean up. The major advantage of HPTLC is that several samples can be run simultaneously using a small quantity of mobile phase unlike HPLC, thus lowering the analysis time and cost per analysis.
The aim of the present work was to develop and validate a new simple, rapid, selective, cost effective and stability indicating HPTLC method for simultaneous determination of FA and MCZ in ointment formulation in the presence of its degradation products. The proposed method was validated as per ICH guidelines [30,31].
Experimental
Materials
Pharmaceutical grade of MCZ (Luxica Pharma Inn., Ankleshwer, Gujarat, India) and FA (Dermocare Labs Ltd., Ahmedabad, Gujarat, India) were kindly supplied as a gift sample. All chemicals and reagents used were of analytical grade and were purchased from Merck Chemicals, India.
HPTLC instrumentation
The samples were spotted in the form of bands of width 6mm with a Camag 100 μL syringe on pre-coated silica gelaluminium Plate 60 F–254 (20 × 10 cm with 250 μm thickness; E. Merck, Darmstadt, Germany, supplied by An chrome Technologists, Mumbai, India) using a Camag Linomat V(Muttenz, Switzerland). The plates were prewashed by methanol and activated at 60 °C for 5 min prior to chromatography. A constant application rate of 0.1 μl/s was employed and space between two bands was 5 mm. The slit dimension was kept at 5 × 0.45 mm and scanning speed was employed 10 mm/s. The monochromatic bandwidth was set at 20 nm, each track was scanned thrice and baseline correction was used. The mobile phase consisted of n-Hexane/Ethyl acetate (1:9 v/v) and 10 ml of mobile phase was used as per chromatography. Linear ascending development was carried out in 10 × 10 cm twin trough glass chamber (Camag, Muttenz, Switzerland) saturated with the mobile phase. The optimized chamber saturation time for mobile phase was 30 min at room temperature (25 °C ± 2) at relative humidity of 60 ±± 5%. The length of chromatogram run was 8 cm. Subsequent to the development; TLC plates were dried in a current of air with the help of an air dryer. Densitometry scanning was performed on Camag TLC scanner III in there flectance–absorbance mode at 260 nm and was operated by CATS software (V 3.15, Camag). The source of radiation utilized was deuterium lamp emitting a continuous UV spectrum between 190 and 400 nm. Concentrations of the compound chromatographer were determined from the intensity of diffusely reflected light. Evaluation was via peak areas with linear regression.
Preparation of standard solutions
Accurately weighed portion of MCZ (250 mg) and FA (25 mg) was transferred to a separate 25 ml volumetric flask and dissolved and diluted to the mark with methanol to obtain standard solution having concentrations of MCZ (10000μg/ml) and FA (1000μg/ml). Further dilute FA 10 times to obtain standard stock solution having concentration 100 μg/ml.
Preparation of sample solutions
Weigh an accurately about 2 grams of ointment containing 2% of MCZ and 0.01% of FA in 10 ml volumetric flask. Add 2/3rd quantity of methanol into flask and sonicate the solution for 10 min to get clear solution. Add methanol up to mark to make the volume 10 ml. get the test solution contains 4000μg/ml MCZ and 20 μg/ml FA.
Preparation of calibration curve
To set up the linearity range 40000-140000 ng/spot for MCZ and 200-700 ng/spot FA. From the combined working standard solution (20 μg/ml of FA and 4000 μg/ml of MCZ) aliquots of 10, 15, 20, 25, 30 and 35 μl were spotted on the TLC plate and developed and analysed. The above working standard of MCZ and FA were spotted in band width 6 mm using Hamilton 100 μL syringe on pre-coated silica gel aluminium plate 60 F254using automatic application device. Linear ascending development was carried out in 10 × 10 cm twin trough glass chamber saturated with the mobile phase for 30 min. The plate was removed from the chamber, subsequently dried in a current of air and densitometry scanning was performed on Camag TLC scanner III in the reflectance-absorption mode at 254 nm and operated by win CATS software. Peak areas were recorded for all the peaks. The calibration curve for MCZ and FA were constructed by plotting peak area versus concentration (ng/spot) corresponding to each spot.
Method validation
Precision
a. Repeatability
System precision experiment was performed by application of 15μL of combined working standard solution (20 μg/mL of FA and 4000 μg/mL of MCZ) for six times on same TLC plate. Plate was developed and analyzed as described in section 4.2.1. The areas of six replicate spots were measured and % RSD was calculated.
b. Intermediate Precision (Reproducibility)
The intra-day and inter-day precision of the proposed method was determined by analyzing mixed standard solution of FA and MCZ at 3 different concentrations (200, 400, 600 ng/spot for FA and 40000, 80000, 120000 ng/spot for MCZ) on the same day and on different days. The results are reported in terms of relative standard deviation (%RSD).
Robustness of the method: By introducing small changes in the mobile phase composition, the effects on the results were examined. Mobile phases having different composition like n- Hexane/ Ethyl acetate (0.9:9.1 v/v) and (1.1:8.9, v/v) were tried and chromatograms were run. The amount of mobile phase, temperature and relative humidity was varied in the range of ± 5%. The plates were prewashed by methanol and activated at 60 ± 5°C for 2, 5 and 7min. prior to chromatography. Time from spotting to chromatography and from chromatography to scanning varied from 0, 25 and 35 min. Robustness of the method was done at 4000 ng/spot of MCZ and 20 ng/spot FA.
Limit of detection and limit of quantization: The limits of detection and quantification of the developed method were calculated from the standard deviation of the intercepts and mean slope of the calibration curves of FA and MCZ using the formulae as given below.
LOD = 3.3 ?σ/S
LOQ = 10 ?σ/S
Where, σ = the standard deviation of the response
S = slope of the calibration curve.
Specificity: The specificity of the method was as certained by analysing standard drug and sample. The spot for MCZ and FA in sample was confirmed by comparing the Rf and spectra of the spot with that of standard. The peak purity of MCZ and FA were assessed by comparing the spectra at three different levels; i.e. peak start (S), peak apex (M) and peak end (E) positions of the spot.
Accuracy: The samples were spiked with extra 80%, 100%and 120% of the standard MCZ and FA solutions, and the mixtures were reanalysed by the proposed method. The experiment was conducted in triplicate. This was done to check for the recovery of the drug at different levels in the formulations.
Analysis of the marketed formulation
20 μl of sample solution was applied on the TLC plate followed by development and scanning as described in Section 2.2. The analysis was repeated in triplicate. The amount MCZ and FA present in the sample solutions were determined by fitting the response into the respective regression line equation for MCZ and FA.
Forced degradation
Forced degradation of FA and MCZ was carried out under acidic and alkaline hydrolysis, oxidative and photolytic stress conditions.
Acidic hydrolysis: Accurately weighed 2 mg of FA and 400 mg of MCZ was transferred into 10 ml volumetric flask, dissolved in 5 ml methanol and diluted up to the mark with 2N HCl. The solution was refluxed for 1 hour at room temperature. Aliquot of 1 ml was transferred to 10 ml volumetric flask and neutralized by 2N NaOH. Volume was made up to mark with methanol. The resulting solution (15 μl) was applied to TLC plate and the chromatogram was developed.
Alkaline hydrolysis: Accurately weighed 2 mg of FA and 400 mg of MCZ was transferred into 10 ml volumetric flask, dissolved in 5 ml methanol and diluted up to the mark with 2N NaOH. The solution was refluxed for 1 hour at room temperature. Aliquot of 1 ml was transferred to 10 ml volumetric flask and neutralized by 2N HCl. Volume was made up to mark with methanol. The resulting solution (15 μl) was applied to TLC plate and the chromatogram was developed.
Oxidative degradation: Accurately weighed 2 mg of FA and 400 mg of MCZ was transferred into 10 ml volumetric flask, dissolved in 5 ml methanol and diluted up to mark with 6% hydrogen peroxide (H2O2). The solution was kept at room temperature (25± 20C) for 1 hour. From sample solution 1 ml aliquot was transferred in to 10 ml volumetric flask and volume made up to mark with methanol. The resulting solution (15 μl) was applied to TLC plate and the chromatogram was developed.
Photolytic degradation: For photolytic degradation studies, working standard solution of FA (20μg/ml) and MCZ (4000μg/ml) was exposed to direct sunlight (afternoon, 32±0.5°C) for 3 hr on a wooden plank kept on terrace. 15 μl of the solution was applied on TLC plate and the chromatogram was developed.
Results and Discussion
Mobile phase optimization
The HPTLC procedure was optimized with a view to develop stability indicating assay method. Both, MCZ and FA were spotted on TLC plates and run in different mobile phase systems. The mixture of n-Hexane: Ethyl acetate (1:9, v /v) was proven to be better than the other mixtures in terms of resolution and peak shape.
Validation of the proposed method
Specificity: The peak purity of FA and MCZ were assessed by comparing its HPTLC chromatogram with standard at peak start, apex, and peak end positions of the spot.
Good correlation (r= 0.9990) was obtained between standard and sample spectra of FA and MCZ respectively.
Peak purity spectra of test FA and MCZ are shown in Figures 2 and 3 respectively. Data of peak purity are shown in Table 1.
Substance
r(s, m)
r(m, e)
Fluocinolone acetonide
0.9996
0.9995
Miconazole nitrate
0.9999
1.000
Table 1: Data of peak purity of sample FA and MCZ.
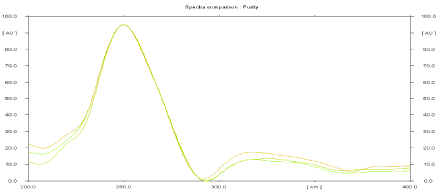
Figure 2: Peak purity spectra of FA in sample.
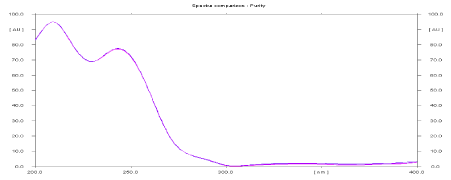
Figure 3: Peak purity spectra of MCZ in sample.
Linearity: Linear responses were observed in the concentration range of 200-700 ng/spot for FA and 40000-140000 ng/spot for MCZ. Correlation co-efficient for calibration curve of MCZ and FA were found to be 0.990 and 0.983 respectively. 3D chromatogram of standard MCZ and FA in linearity range is depicted in Figure 4.
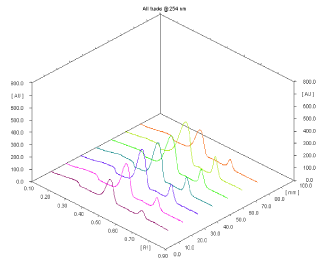
Figure 4: 3D chromatogram of MCZ (40000-140000 ng/spot) and FA (200-
700 ng/spot).
The data for linearity range of MCZ and FA is depicted in Table 2 and 3 respectively. Calibration curves of MCZ and FA are shown in Figure 5 and Figure 6 respectively.
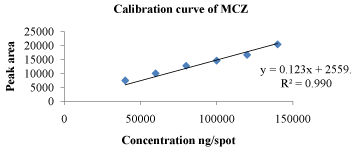
Figure 5: Calibration curve of MCZ at 254 nm.
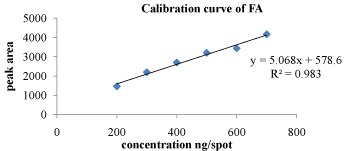
Figure 6: Calibration curve of FA at 254 nm.
Sr.
No.
Concentration
(ng/spot)
Peak Area ± S.D.
(n= 6)
%RSD
1
40000
7485.3±93.2
1.24
2
60000
10019.4±91.2
0.91
3
80000
12737.1±94.9
0.74
4
100000
14562±271.07
1.86
5
120000
16625.3±266.86
1.60
6
140000
20387±259.83
1.27
Table 2: Calibration data of MCZ.
Sr.
No.
Concentration
(ng/spot)
Peak Area ± S.D.
(n= 6)
%RSD
1
200
1466.9±26.55
1.81
2
300
2191.6±10.95
0.49
3
400
2701.6±21.54
0.79
4
500
3202.3±32.50
1.01
5
600
3441.4±32.27
0.93
6
700
4161.7±22.30
0.53
Table 3: Calibration data of FA.
The regression line equation for MCZ and FA are as following
y = 0.123x + 2559 for MCZ
y = 5.068x + 578.6 for FA
Where, y= Peak area
x= Concentration in ng/spot
Precision
System and method precision: %RSD of system precision of FA and MCZ were found to be 1.37 and 1.59 respectively. %RSD of method precision of FA and MCZ were found to be 1.75 and 1.63 respectively. The data for system and method precision of FA and MCZ are depicted in Table 4 and 5 respectively.
Sr.No.
Peak Area
MCZ(60000 ng/spot)
FA(300 ng/spot)
1
9828.4
2114.7
2
10069.7
2126.7
3
10019.8
2123.1
4
9904.9
2107.5
5
10184.6
2102.4
6
10143.2
2195
Mean
10025.19
2128.23
S.D.
137.51
33.96
% RSD
1.37
1.59
Table 4: Repeatability data for FA and MCZ.
Concentration of MCZ
(ng/spot)
Intra-day precision
Inter-day precision
Area
Mean ± SD(n= 3)
%RSD
Area
Mean ± SD(n= 3)
%RSD
60000
10019.4 ± 91.20
0.9102
10453.67 ± 146.79
1.40
100000
14562.267 ± 271.07
1.86
14453.67 ± 146.791
1.0155
140000
20387 ± 270.687
1.31
20953.67 ± 322.108
1.548
Mean RSD
1.36
Mean RSD
1.32
Table 5: Intermediate precision data for MCZ.
Intra-day and Inter-day precision: Mean RSD for intra-day and inter-day precision of MCZ was found to be 1.36 and 1.32 respectively. The Mean RSD for intraday and inter day precision of FA was found to be 0.6818 and 1.36 respectively. The data for intra-day and interday precision for MCZ is depicted in Table 6 and for FA in Table 7.
Concentration of FA (ng/spot)
Intra-day precision
Inter-day precision
Area
Mean ± SD (n= 3)
%RSD
Area
Mean ± SD
(n= 3)
%RSD
300
2191.6 ± 10.95
0.50
2191.6 ± 10.95
0.85
500
3202.3 ± 32.50
1.01
3202.3 ± 32.50
1.60
700
4161.7 ± 22.3
0.53
4161.7 ± 22.3
1.65
Mean RSD
0.68%
Mean RSD
1.36%
Table 6: Intermediate precision data for FA.
Sr. No.
Amount taken
(ng/spot)
Amount added
(ng/spot)
Total
(ng/spot)
Area
Amount Recover
(ng/spot)
% Recovery
Mean % Recovery
1
300
240
540
3320.6
241.04
100.43
100.63
240
540
3293.6
235.71
98.21
240
540
3354.9
247.81
103.25
2
300
300
600
3621.4
300.39
100.13
99.44
300
600
3615.7
299.27
99.75
300
600
3595.6
295.30
98.43
3
300
360
660
3933.4
361.95
100.54
100.41
360
660
3914.6
358.24
99.51
360
660
3945.2
364.28
101.19
Mean Recovery = 100.16%
Standard Deviation = 0.63
% Relative Standard Deviation = 0.63%
Table 7: Recovery data of FA from formulation.
These results indicate that method is precise.
Accuracy: Accuracy of the method was confirmed by recovery study from marketed formulation at three level of standard addition. The % recovery of FA and MCZ was found to be 100.163 and 98.73 respectively. The data for accuracy of FA and MCZ are depicted in Table 7 and Table 8 respectively. These results indicate that method is accurate.
Sr No.
Amount taken
(ng/spot)
Amount added
(ng/spot)
Total
(ng/spot)
Area
Amount Recover
(ng/spot)
% Recovery
Mean % Recovery
1
60000
48000
108000
15956.4
48918.71
101.91
99.59
48000
108000
15865.4
48182.13
100.38
48000
108000
15635.7
46314.65
96.49
2
60000
60000
120000
17213.6
59143.14
98.57
98.73
60000
120000
17358.4
60320.36
100.53
60000
120000
17105.9
58267.51
97.11
3
60000
72000
132000
18667.4
70962.65
98.55
97.88
72000
132000
18420.9
68958.53
95.77
72000
132000
18735.1
71513.11
99.32
Mean Recovery = 98.73%
Standard Deviation = 0.85
% Relative Standard Deviation = 0.86%
Table 8: Recovery data of MCZ from formulation.
Limit of Detection (LOD) and Limit of Quantification (LOQ): The LOD for FA and MCZ were found to be 11.71 ng/spot and 2887.56 ng/spot respectively.
The LOQ for FA and MCZ were found to be 34.9769 ng/spot and 8750.20 ng/spot respectively. The data for LOD and LOQ of FA and MCZ are depicted in Table 9.
Parameters
FA
MCZ
Standard deviation of the Y- intercepts of the three calibration curves.
17.71
107.33
Mean slope of the three calibration curves.
5.06
0.12
LOD = 3.3 × (SD/Slope) (ng/spot)
11.54
2887.56
LOQ = 10 ×× (SD/Slope) (ng/spot)
34.97
8750.20
Table 9: LOD and LOQ data for FA and MCZ nitrate.
Robustness: Robustness data clearly shows that the proposed method is robust at small but deliberate change. Robustness data are depicted in Table 10 and 11.
Sr. No.
FA
MCZ
Change in saturation time
25 min
35 min
25 min
1
2114.7
2123.1
1
2114.7
2
2126.7
2107.5
2
2126.7
Mean
2118
9955.7
S.D
8.61
109.38
%RSD
0.40
1.09
Chang in Mobile phase ratio: n- Hexane: Ethyl acetate
0.9:9.1, v/v
1.1:8.9, v/v
0.9:9.1, v/v
1
2058.28
2060.63
1
2058.28
2
2112.51
2131.08
2
2112.51
Mean
2102.6
9962.63
S.D
26.06
89.92
% RSD
1.23
0.90
Change in sonication time for sample preparation:
5 min
15 min
5 min
1
2102.7
2113.1
1
2102.7
2
2085.4
2107.5
2
2085.4
Mean
2102.175
10090.62
S.D
11.96
120.17
% RSD
0.56
1.19
Table 10: Robustness parameters for FA and MCZ.
Drug
Label claim
Amount found
% Content
FA
0.01% w/w
0.009882% w/w
98.82%
MCZ
2.0% w/w
1.9994% w/w
99.97%
Table 11: Estimation of FA and MCZ in pharmaceutical formulation (Zole-F ointment).
Summary of all validation parameters are depicted in Table 12.
Parameters
Value
Video integration
no
Baseline correction
yes
Peak thresh. height
10 AU
Peak thresh. area
300
Peak thresh. slope
3
Filter factor
3
Data selection factor
1
Track start position
85 mm
Track end position
135 mm
Table 12: Summary of validation parameters.
Analysis of marketed formulations (ointment) by proposed method
The assay results obtained by using the proposed method for the analysis of a marketed ointment formulation containing FA (0.01%, w/w) and MCZ (2%, w/w) were in good agreement with the labeled amounts of FA and MCZ. The average contents of FA and MCZ were 0.009882g/100g ointment (0.009882% w/w) and 1.9994g/ 100g ointment (1.9994 %, w/w), respectively. So the % drug obtained was 98.82% of FA and 99.97% of MCZ. No interference of the excipients with the peaks of interest appeared; hence the proposed method is applicable for the routine estimation of FA and MCZ in pharmaceutical dosage forms. Results obtained are shown in following Table 13. Densitogram of test solution containing MCZ (60000 ng/spot) and FA (300 ng/spot) is depicted in Figure 7.
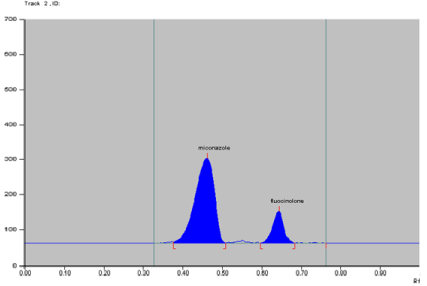
Figure : Densitogram of test peak containing 60000 ng/spot MCZ and 300
ng/spot FA.
Sr.
No.
Parameters
FA
MCZ
1
Linearity Range
200-700 ng/spot
4000-140000 ng/spot
2
Correlation Co-efficient (R2)
0.990
0.983
3
Precision (% RSD)
Repeatability (n=6)
Intraday precision (n=3)
Inter day precision (n=3)
1.54
0.681
1.366
1.76
1.36
1.32
4
% Recovery
100.163%
98.79%
5
Limit of Detection (LOD)
11.5424
2887.56
6
Limit of Quantification (LOQ)
34.9769
8750.20
Table 13: Integration parameters for HPTLC method.
Stability indicating property
Acid hydrolysis: The rate of degradation in acid was found to be slower as compared to that of alkali. After applying 1 N HCl exposure for 1 hour, about 30.7% degradation was observed. Three additional peak of degradation product was observed at Rf 0.12, 0.32 and 0.87 (Figure 8). Data of acid hydrolysis is given in Table 14.
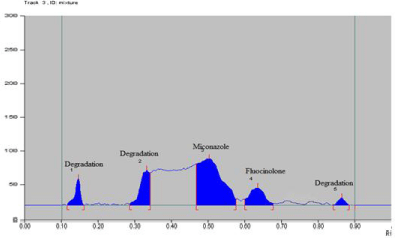
Figure 8: Densitogram of acid hydrolysis.
Peak No.
Rf value
% Area
Substance
1
0.14
10.65
Degradation
2
0.32
14.79
Degradation
3
0.50
49.47
MCZ
4
0.64
19.53
FA
5
0.87
5.26
Degradation
Table 14: Peak table of acid degradation study.
Alkaline hydrolysis: The drug was found o be highly labile to alkaline hydrolysis. After applying 1 N NaOH exposure for 1 hour, about 40% degradation was observed. Additional peak of degradation product were observed at Rf 0.36, 0.42 and 0.59 (Figure 9). Data of alkaline hydrolysis is given in Table 15.
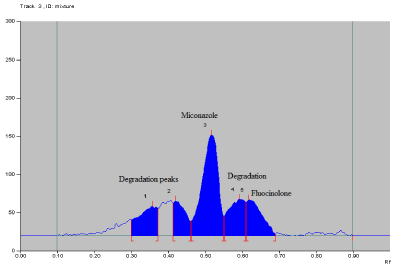
Figure 9: Densitogram of alkaline hydrolysis
Table 15: Peak table of alkali degradation.
Peak No.
Rf value
% Area
Substance
1
0.36
12.71
Degradation
2
0.42
10.65
Degradation
3
0.51
44.62
MCZ
4
0.59
16.53
Degradation
5
0.64
15.49
FA
Table 15: Peak table of alkali degradation.
Oxidative degradation: The drugs were found to be slightly labile to oxidative degradation. After applying 3% hydrogen peroxide (H2O2) at room temperature (25 ± 20C) for one hour, about 16.17% degradation was observed. Additional two degradation peaks are observed at Rf 0.21 and 0.29 (Figure 10). Data of oxidative degradation is given in Table 16.
Figure 10: Densitogram of oxidation induced degradation study.
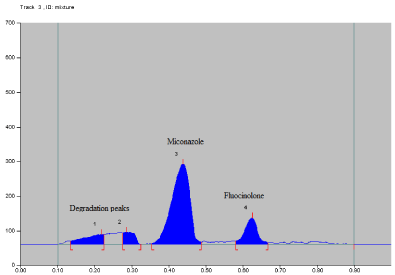
Figure 10: Densitogram of oxidation induced degradation study.
Table 16: Peak table of oxidative degradation.
Peak No.
Rf value
% Area
Substance
1
0.21
5.43
Degradation
2
0.29
10.74
Degradation
3
0.45
62.57
MCZ
4
0.64
20.26
FA
Table 16: Peak table of oxidative degradation.
Photo degradation: When drug solution was exposed to direct sunlight for 3 hr, 35.63 % degradation was observed. Three additional peaks were observed at Rf 0.23, 0.39 and 0.54 (Figure 11). Data of photo degradation is given in Table 17 and 18.
Figure 11: Densitogram of photodegradation.
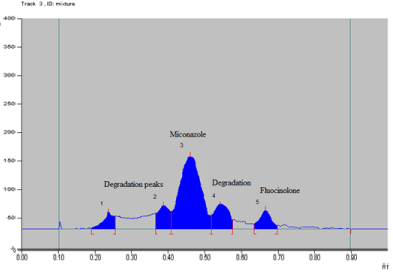
Figure 11: Densitogram of photodegradation.
Table 17: Peak table of Photo degradation.
Peak No.
Rf value
% Area
Substance
1
0.21
5.43
Degradation
2
0.29
10.74
Degradation
3
0.45
62.57
MCZ
4
0.64
20.26
FA
Table 17: Peak table of Photo degradation.
Table 18: Summary of forced degradation study of FA and MCZ.
Sr. No.
Stress type*
Stress condition
Peak area of FA
Peak area of MCZ
% Degradation
1
Acid hydrolysis
1 N HCl at
Room temperature, for 1 hour
973.1
4153.8
30.7%
2
Alkali hydrolysis
1 N NaOH at
Room temperature, for 1 hour
1837.1
5374
40%
3
Oxidation
3% W/V H2O2 at
Room temperature, for 1 hour
2430
10514
16.17%
4
Photolytic degradation
Direct sunlight for 3 hour
976.8
12018.9
35.63%
*Concentration of FA: 300 ng/spot and for MCZ: 60000 ng/spot.
Table 18: Summary of forced degradation study of FA and MCZ.
Conclusion
Based on the results, obtained from the analysis using described method, it can be concluded that the method has linear response in the range of 200-700 ng/spot for FA and 40000 – 140000 for MCZ.
The result of the analysis of pharmaceutical formulation by the proposed method is highly reproducible and reliable and is in good agreement with label claim of the drugs. The additive usually present in the pharmaceutical formulations of the assayed samples did not interfere with MCZ and FA. The method can be used for the routine analysis of MCZ and FA in combined dosage form.
The study involved observation on degradation products formed under different stress condition. The developed method successfully separated drug substances from degradation products formed under various stress conditions. MCZ and FA are prone to alkaline hydrolysis and photolysis.
References
- United States Pharmacopoeia 30, NF 25. United States Pharmacopoeial Convention, Inc. 2007; 2263-2264.
- George CS, Hoffman, Melmon BB, Kenneth L, Nierenberg DW. Melmon and morrelli's clinical pharmacology. 4th edn. Mcgraw Hill Publishers. 2000; 713-733.
- Goyal RK. Derasari and gandhi’s elements of pharmacology. 16th edn. BS Shah Prakashan. 2007; 228, 567.
- Craig CR, Stitzel RA. Modern pharmacology with clinical applications. 6th edn. Lipincott Wiliam & Wilkins Publishers. 2004; 485.
- Rang HP, Dale MM, Ritter JM, Flower RJ. Rang and dale's pharmacology. 6th edn. Elsevier. 2007; 695-696.
- Freedberg I, Eisen A, Wolff K, Austen KF, Goldsmith LA, Katz S. Fitzpatrick's dermatology in general medicine. 6th edn. McGraw-Hill Co. 2003; 407–427, 814–847, 1106, 1164–1204, 1876–1877, 1994–2014.
- Lookingbill D, Marks J. Principles of dermatology. 2nd edn. PA: WB Saunders Co. 1993; 204-214.
- Indian Pharmacopoeia, Government of Indian Ministry of Health and Family Welfare, The Indian Pharmacopoeial Commission. 2007; 771-773: 510-512.
- Kadam SR, Janjale MV. Application of mixed hydrotropic solubilization technique for simultaneous spectrophotometric estimation of metronidazole and miconazole nitrate from different pharmaceutical dosage forms. Int J Pharm Biologic Archi. 2012; 3: 383-390.
- Wrobe K, Wrobel K, de la Garza Rodriguez IM, Lopez-de-Alba PL, Lopez-Martinez L. Determination of miconazole in pharmaceutical creams using internal standard and second derivative spectrophotometry. J Pharm Biomed Ana. 1999; 20: 99-105.
- Aboul-Enein HY, Ali I. Comparative study of the enantiomeric resolution of chiral antifungal drugs econazole, miconazole and sulconazole by hplc on various cellulose chiral columns in normal phase mode. J Pharm Biomed Ana. 2002; 27: 441-446.
- Adlnasab L, Ebrahimzadeh H, Yamini Y, Mirzajani F. Optimization of a novel method based on solidification of floating organic droplet by high-performance liquid chromatography for evaluation of antifungal drugs in biological samples. Talanta. 2010; 83: 370-378.
- Akay C, Ozkan SA, Senturk Z, Cevheroglu S. Simultaneous determination of metronidazole and miconazole in pharmaceutical dosage forms by RP-HPLC. Farmaco. 2002; 57: 953-957.
- Cavrini V, Di Pietra AM, Gatti R. Analysis of miconazole and econazole in pharmaceutical formulations by derivative UV spectroscopy and liquid chromatography (HPLC). J Pharm Biomed Anal. 1989; 7: 1535-1543.
- Cavrini V, Di Pietra AM, Raggi MA. High-pressure liquid chromatographic (HPLC) analysis of imidazole antifungals in commercial dosage forms. Int J Pharm. 1982; 10: 119-124.
- Chmielewska A, Konieczna L, Lamparczy H. Development of a reversed-phase HPLC method for analysis of fluocinolone acetonide in gel and ointment. Acta Chromatogrphy. 2006; 16: 80-91.
- De Zan MM,Camara MS,Robles JC, Kergaravat SV and Goicoechea HC. Development and validation of a simple stability-indicating high performance liquid chromatographic method for the determination of miconazole nitrate in bulk and cream formulations. Talanta. 2009; 79: 762-767.
- Erk N, Altun ML. Spectrophotometric resolution of metronidazole and miconazole nitrate in ovules using ratio spectra derivative spectrophotometry and RP-LC. J Pharm Biomed Anal. 2001; 25: 115-122.
- Gaona-Galdos AA, Garcia PL, Aurora-Prado MS, Santoro MI, Kedor-Hackmann ERM. Simultaneous determination of econazole nitrate, main impurities and preservatives in cream formulation by high performance liquid chromatography. Talanta. 2008; 77: 673-678.
- Di Pietra AM, Cavrini V, Andrisano V, Gatti R. HPLC analysis of imidazole antimycotic drugs in pharmaceutical formulations. J Pharm Biomed Anal. 1992; 10: 873-879.
- Volin P. Simultaneous determination of serum cortisol and cortisone by reversed-phase liquid chromatography with ultraviolet detection. J Chromatogr. 1992; 584: 147-155.
- Carrier A, Parent J. Liquid chromatographic-mass spectrometric determination of itraconazole and its major metabolite, hydroxyitraconazole, in dog plasma. J Chromatogr B Biomed Sci Appl. 2000; 745: 413-420.
- Fredriksson MJ, Petersson P, Axelsson BO, Bylund D. Combined use of algorithms for peak picking, peak tracking and retention modelling to optimize the chromatographic conditions for liquid chromatography–mass spectrometry analysis of fluocinolone acetonide and its degradation products. Analytica Chimica Acta. 2011; 704: 180-188.
- Hou S, Hindle M, Byron PR. Chromatographic and mass spectral characterization of budesonide and a series of structurally related corticosteroids using LC-MS. J Pharm Biomed Anal. 2005; 39: 196-205.
- Ashour S, Kattan N. Simultaneous determination of miconazole nitrate and metronidazole in different pharmaceutical dosage forms by gas chromatography and flame ionization detector (GC-FID). Int J Biomed Sci. 2010; 6: 13-18.
- Gagliardi L, Orsia D, Giudicea MR, Gattaa F, Porraa R, Chimentib P, et al . Development of a tandem thin-layer chromatography–high-performance liquid chromatography method for the identification and determination of corticosteroids in cosmetic products. Analytica Chimica Acta. 2002; 457: 187-198.
- Meshram DB, Bagade SB, Tajne MR. Simultaneous determination of metronidazole and miconazole nitrate in gel by HPTLC. Pak J Pharm Sci. 2009; 22: 323-328.
- ICH. Q 1 A (R2) stability testing of new drug substances and products, in CPMP/ICH/2736/99. Int Confr Harmoniz, EMEA. 2003; 1-20.
- Ferenczi-Fodor K, Vegh Z, Nagy-Turak A, Renger B, Zeller M. Validation and quality assurance of planar chromatographic procedures in pharmaceutical analysis. J AOAC Int. 2001; 84: 1265-1276.
- ICH. Validation of analytical procedures: Definitions and terminology, in Q2 A (R1), International Conference on Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human Use. 1996.
- ICH. Validation of analytical procedures: Methodology, in Q2 B (R1), International Conference on Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human Use. 1996.