
Research Article
Austin Chromatogr. 2015;2(1): 1026.
Effect of Enzymatic Mash Fermentation on the Galacturonic Acid Content of Sound & Decayed Apples
Sadiye Arpac Yangel1, Sebahattin Nas2 and Cetin Kadakal2*
1Graduate School of Natural and Applied Sciences, Pamukkale University, Turkey
2Department of Food Engineering, Pamukkale University,Turkey
*Corresponding author: Cetin Kadakal, Food Engineering Department, Faculty of Engineering, Pamukkale University, Kinikli-Denizli, P.O. Box 20200 Turkey
Received: December 29, 2014; Accepted: February 18, 2015; Published: February 26, 2015
Abstract
In this study, the effects of apple decay proportions (sound, 50 and 100 % by surface), dosages of pectolytic enzymes (80, 100 and 150 mg/kg) and mash fermentation period (0, 15, 30 45 min) on brix, pH, acidity, Hunter (L, a, b) and the galacturonic acid values were determined. Galacturonic acid concentrations were done by High Performance Liquid Chromatography (HPLC). Statistical analysis of the data showed significant differences (P<0.01) between the galacturonic acid level and decay proportion, enzyme dosage and mash fermentation period. Increasing the decay proportion of apple decreased the galacturonic acid while the enzyme dosage and mash fermentation period increased the galacturonic acid concentration. Galacturonic acid levels in sound, 50% decayed and 100% decayed apples were determined as 106.9- 1845.5 mg/kg, 73.7-977.3 mg/kg and 33.2-370 mg/kg, respectively. The highest galacturonic acid concentration (1854.5 mg/kg) was observed in sound apples with 150 mg/kg enzyme application for 45 minutes.
Keywords: Decayed apple; Galacturonic acid; HPLC; Mash fermentation
Introduction
Apple (Malusdomestica), belonging to the family Rosaceae, is one of the most nutritious and popular among all the fruits [1]. Apples are one of the most consumed fruits worldwide and are consumed fresh or in processed forms such as jam, juice or dried. Apples contain over 84% water, a variety of vitamins (except vitamin B complex), minerals (K, Mg, Ca, and Na), trace elements (Zn, Mn, Cu, Fe, B, F, Se, Mo) and have high fiber content [2]. Over one million tons of apple pomaceis produced in the process of apple juice concentration production. The apple pomace resources contain the availble rich pectins (10-15% calculated in terms of dried qualities) [3].
Consumption of fresh fruit is often replaced by the intake of fruit juices, due to their convenience and ability to quench thirst. According to EU Regulation 1924/2006, it is expected that fresh fruits will be exempt from health and nutritional claims, it is therefore important to evaluate their chemical composition and biological value [4].
In Europe, apple juice is a highly-consumed product, in second place after orange juice [5]. The concentrated fruit juices were obtained from fresh or natural juices by the extraction of at least 50% of their water content and they can be preserved by physical procedures. These concentrates, diluted in fresh water and returned to their original density have to show the same characteristics as the natural juices. Many of the concentrated juices are clarified juices containing only water and soluble solids [6]. In the conventional manufacturing process of this type of juice, there is a clarification stage where the insoluble solids are removed. The aim of the clarification is to remove the juice components causing the turbidity. The clarifying agents used mostly are bentonite, gelatine and silica gel and so on, their purpose being to form aggregates with the protein fraction of the juice to force its precipitation [7]. The depectination stage is simultaneous to the clarification stage and pectinolytic enzymes degrading the juice pectins are used to destroy the protection of the pectins in the suspended juice substances [8].
In the fruit juice industry, to stabilize the cloudiness of cloudy juices during storage is the most important technological problem. Pectin methyl esterase (PME, E.C. 3.1.1.11) has been shown to induce cloud loss and texture modifications of food products from fruits (juice, nectar) by action on pectins [4].
Pectin is a complex polysaccharide found in the primary cell walls and intercellular regions of higher plants. Its structure is important in determining plant cell-wall strength and flexibility. Because of its excellent gelling, thickening, and stabilizing properties, the polymer is extensively utilized in the food industry [9,10]. The dominant feature of pectin is a linear chain of a-(1?4) linked D-Galacturonic Acid (GalA) units in which varying proportions of the acid groups are methyl-esterifies [11]. This homogalacturonan backbone is occasionally interrupted by rhamnose-rich regions which can be highly substituted with neutral sugar-rich side chains. Pectins display a large polydispersity with varying levels of methyl esterification and neutral sugar content [12].
In case of clear juice production an essential technological operation is mash enzymation [13]. This process leads to native pectin degradation and decreases of raw juice viscosity and, in consequence, increases in juice yield and reduced pomace volume [14,15] improving production efficiency. Enzyme application conditions: time and temperature largely depend on the enzyme used. Enzymation may affect the phenolic compounds content [16,17]. The application of enzyme treatment may be the key to increase phenolic compounds content as new technologies as pulse electric field treatment do not increase significantly the phenolic in juices [18]. Current trend in processing is towards shortening enzymation time and lowering enzymation temperature to decrease the cost of processing. The temperature of enzyme treatment was suggested by enzyme suppliers ensuring optimal effectiveness of enzyme action [19].
No published data have been found in the literature about the effect of apple decay proportions on galacturonic acid concentrations. Thus, our objectives were to investigate the effect of apple decay proportions (sound, 50, 100 % by surface) and dosages of pectolytic enzymes (80, 100 and 150 mg/kg) with different mash fermentation periods (0, 15, 30 and 45 min) on brix, pH, acidity, Hunter (L, a, b) and galacturonic acid content and to help the apple juice manufacturing industry select an appropriate procedure.
Materials and Methods
Materials
Sampling and preparation procedures: In this research the apples (Malusdomesticacv “Golden delicious”) used for the mash fermentation were obtained from a well-established local factory (Cal town in Denizli, Turkey). In each sampling day, thirty six kilograms of apples were obtained randomly for every decay group when the contents of each truck container of the day were transferred to the receiving pool (each day approximately 50 containers in the factory yard). Each apple group were transferred to the laboratory and processed for mash fermentation. Naturally decayed apples (colonized visibly by mold) were sorted as sound, 50 and 100% based on the surface ratio of mold growth and decay to apple whole surface. In order to estimate 50% of decayed ones, apples were classified by marking on the fruit surface of the decay proportion after dividing them into ten equal parts with a color pen. Each individual apple was examined closely enough to state that its surface was 0, 50 or 100% decayed. The sound and 100% decayed apples were separated visually. Three different sampling for each decay group were carried out during 3 days to obtain sound, 50 and 100% decayed apples.
Production of apple mash: The apples were cut into quarters with stainless steel knives and crushed (Arzum model prokit 444, Istanbul, Turkey) to get apple mash. The apple mash heated to 30 °C and different dosages of pectolytic enzymes and mash period were applied to the each group of apples (sound, 50 and 100% decayed). The apple mash samples were analyzed for their brix, pH, acidity, color values (Hunter L, a, b) and galacturonic acid concentrations. Following heat treatment (up to 30 °C), the apple mash was divided into three lots and nine different enzymation treatments were shown in Figure 1.
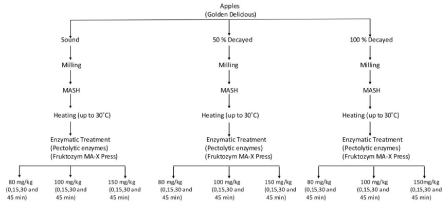
Figure 1: Laboratory scale apple mash processing scheme following different enzymation treatments.
Methods
GalA Standard solution: Water used in all the experiments was doubly distilled and deionized. The galacturonic acid standard was obtained from Sigma (Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany). Stock and standard solutions of galacturonic acid were prepared in mobile phase. A stock standard solution (500 mg/L) of GA (0.0125 g) was prepared and stored at -20°C for further dilutions. For preparing calibration curve, seven different concentrations (0.2, 0.5, 1.0, 5.0, 10.0, 25.0 and 50.0 mg/L) of galacturonic acid standard were used. To 10 ml aliquots of the standard mixtures, a 0.5 g quantity of PVP was added and the mixtures were stirred for 15 min and subsequently centrifuged for 15 min at 3500 rpm. The supernatants were filtered through 0.2 μm syringe filters and three replicate injections of each mixture were made into the HPLC.
Sample treatment: The determination of galacturonic acid in the samples was carried out by using Shimadzu Class-VP V5.01 high pressure liquid chromatography apparatus (Shimadzu Corp., Kyoto, Japan), as suggested by Zotou et al. [20]. A 0.5 g quantity of PVP was added to 10 g of sample in a conical flask. The sample was stirred for 15 min and then filtered through a single layer pod filter. The filtrate was then centrifuged for 15 min at 3500 rpm. The supernatant was filtered through a 0.2 μm syringe filter, then diluted 1:2 with water and adjusted to pH 9.0 ±0.5 with 1 M NaOH. This final solution was kept in test tubes (20 ml) at - 18°C in a deep freezer until the chromatographic measurements were made.
Apparatus for HPLC: For the analysis, a reversed-phase inersil ODS -2 (250X4.6 ID mm) column, a photodiode array detector (Shimadzu, model SPD-M10A) set at 230 nm, a LC-10AT-VP Shimadzu HPLC pump, a column oven (Shimadzu, CTO-10AS) set at 25 °C and a Software program (Shimadzu) were used. The sample (20 μL) was injected with a syringe (Hamilton Co., Reno, NV, USA) into the HPLC. Coefficient of determination (r2) was found to be 99.99% for galacturonic acid. The detection limit (S/N=3) [21] was found 0.1 mg/kg. The mobile phase was 0.02 M KH2PO4 (adjusted to pH 2.88 ± 0.02 with H3PO4) and methanol (98/2; v/v) with a flow rate of 0.6 ml/ min. The experiments were performed in triplicate for all parameters analyzed.
Recovery of galacturonic acid: In the recovery experiment, samples, for which galacturonic acid concentrations were predetermined, were spiked with the different concentrations of galacturonic acid, using aliquots at 1, 5, 10, 25 and 50 mg/L, to determine the recovery of the extraction procedure in the initial step. Three determinations were carried out for each addition level.
Further Determinations (Brix, pH, total acidity, color): Brix (the soluble solids) from apple mash were determined by refractive index measurements using a digital refractometer (Kruss A. Krussoptronic/ electronischerPeltier Thermostat PT31, Germany). The pH was measured with a pH meter (WTW GmbH & Co, Model 537, Weilheim-Germany).Hunter L (lightness, 0=black to 100=White), a [red (positive reading) to green (negative reading)] and b [yellow (positive reading) to blue (negative reading)] values of the mash samples were measured by reflectance with a Minolta chroma meter (Model CR 300, Minolta Co., Osaka, Japan). Titrable acidities were determined using AOAC method [22] and expressed as g malic acid/L. Results were expressed as the average of duplicate samples.
Results and Discussion
Galacturonic acid determination in apple mash samples was done using an HPLC method and expressed as mg/kgof sample weight. Typical chromatogram of a standard galacturonic acid is given in Figure 2.The procedure requires approximately 1 h to complete. The analytical method for galacturonic acid proved reliable with a detection limit of 0.1 mg/kg.
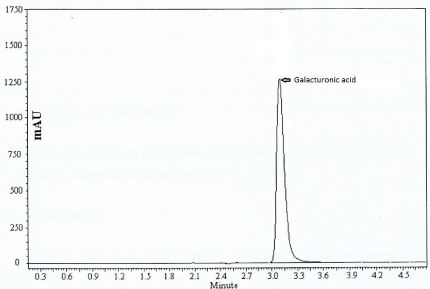
Figure 2: HPLC chromatogram of a standard galacturonic acid.
Recovery
The recovery rates of galacturonic acid in the pulp samples for 5 different concentration added to samples ranged from 95.7% to 99.2% with an average percent recovery of 96.8 (±0.58). Therefore, the level of galacturonic acid in apple mash samples was corrected for the average recovery rate.
Precision
Six determinations of the same apple mash sample (364.5 mg/kg) were performed using the same reagents and apparatus to evaluate the method precision in apple mash. The precision of the method for determination of galacturonic acid in apple mash was found as 4.26 RSD (%) (Relative standard deviation).
Linearity and detection limits
The coefficient of correlation for the standard calibration curve was found to be 0.9999. The detection limit (S/N = 3) was found as 0.1 mg/kg.
Data for brix, pH, acidity and Hunter L, a and b values for the apple mash samples produced from sound, 50 and 100% decayed apples were given in Table 1. It was found that enzyme dosage and mash fermentation perion had no significant effect on brix, pH, acidity and Hunter L, a and b values. In contrast, decay proportion showed significant effect on the brix, pH, acidity and Hunter L, a and b values. Increasing the decay proportion decreased the pH, Hunter L, a and b values, but decreased the acidity value of the samples. On the other hand, the brix values of 50%decayed apples were higher than the sound and the 100% decayed ones. In addition, brix values of sound apples were lower than the 50 and 100% decayed ones. Least Significant Difference (LSD) test results of various mash fermentation periods with pectolytic enzyme dosages on brix, pH, titrable acidity and Hunter L, a and b values of the apple mash samples produced from sound (0%), 50 and 100% decayed apples were given in Table 1.
Enzyme
Dosage mg.kg-1
DecayProprotion (%)
Time (min)
Brixy
pHValuey
TitrableyAcidity
(g.L-1)
Huntery
L
a
b
80
(0 %)
Sound
0
11.31 ±0.85
3,32 ±0.04
0.30
23.56 ±0.83
7.05 ±0.64
9.00 ±1.11
15
12.92 ±0.28
3,38 ±0.04
0.36
24.54 ±1.44
7.28 ±0.06
9.42 ±0.13
30
12.58 ±0.69
3,25 ±0.12
0.34
23.28 ±1.88
7.45 ±0.64
10.08 ±1.36
45
11.64 ±0.12
3,25 ±0.08
0.30
22.97 ±1.71
7.98 ±0.98
10.15 ±0.77
50 %
Decayed
0
15.49 ±0.13
3,19 ±0.02
0,51
22.69 ±2.46
8.07 ±0.29
10.48 ±0.45
15
15.41 ±0.15
3,21 ±0.01
0,49
24.23 ±1.19
7.81 ±0.27
10.59 ±0.36
30
15.26 ±0.08
3,17 ±0.01
0,50
25.28 ±1.20
7.18 ±0.16
9.30 ±0.16
45
15.14 ±0.21
3,16 ±0.05
0,49
24.44 ±1.90
7.24 ±0.47
9.38 ±0.42
100 %
Decayed
0
14.43 ±0.24
3,12 ±0.01
0,99
19.32 ±4.44
6.95 ±0.34
9.59 ±0.43
15
14.48 ±0.58
3,11 ±0.01
0,99
18.88 ±5.27
6.44 ±0.77
8.76 ±1.15
30
14.30 ±0.79
3,14 ±0.03
0,99
23.71 ±0.19
7.65 ±0.48
10.92 ±1.11
45
14.70 ±0.47
3,21 ±0.04
1,01
24.72 ±3.08
7.25 ±0.30
9.51 ±0.04
100
Sound
0
11.91 ±1.22
3,25 ±0.01
0,40
22.50 ±2.41
7.42 ±0.38
9.81 ±0.39
15
12.61 ±0.13
3,33 ±0.03
0,36
24.28 ±1.61
7.02 ±1.24
9.28 ±3.03
30
10.57 ±2.45
3,28±0.01
0,31
26.03 ±0.67
7.86 ±0.81
10.34 ±0.70
45
12.29 ±0.92
3,32 ±0.01
0,33
24.15 ±1.00
8.44 ±1.82
10.73 ±1.59
50 %
Decayed
0
15.17 ±0.34
3,23 ±0.01
0,49
22.83 ±0.47
7.68 ±0.11
10.41 ±0.16
15
15.00 ±0.01
3,21 ±0.01
0,49
21.49 ±0.01
7.54 ±0.32
10.04 ±0.16
30
15.57 ±0.37
3,22 ±0.01
0,51
24.10 ±2.57
7.09 ±0.57
9.64 ±0.22
45
15.81 ±0.39
3,23 ±0.01
0,50
20.73 ±0.52
7.45 ±0.28
9.65 ±0.45
100 %
Decayed
0
14.67 ±0.23
3,12 ±0.01
1,02
26.76 ±5.22
6.97 ±0.23
9.66 ±0.28
15
14.46 ±0.25
3,10 ±0.00
0,98
19.99 ±4.28
6.93 ±0.56
9.33 ±0.59
30
14.36 ±0.08
3,10 ±0.00
0,98
21.23 ±0.18
6.61 ±0.06
8.84 ±0.44
45
14.54 ±0.36
3,11 ±0.05
0,97
22.36 ±0.34
7.27 ±0.54
9.93 ±0.76
150
Sound
0
11.81 ±1.06
3,34±0.01
0,34
22.31 ±2.36
7.39 ±0.35
9.77 ±0.83
15
13.71 ±0.33
3,25±0.02
0,40
22.34 ±2.81
6.31 ±0.37
9.28 ±0.01
30
11.59 ±2.14
3,38±0.07
0,32
23.64 ±1.63
8.17 ±1.25
10.92 ±1.58
45
12.29 ±0.37
3,40±0.11
0,37
22.27 ±0.67
6.81 ±0.21
8.80 ±0.30
50 %
Decayed
0
16.09 ±0.76
3,24 ±0.00
0,54
20.99 ±0.21
7.97 ±0.07
10.09 ±0.06
15
14.84 ±0.43
3,24 ±0.00
0,48
21.93 ±0.01
7.75 ±0.03
10.53 ±0.21
30
15.14 ±0.38
3,21 ±0.01
0,48
21.01 ±0.45
7.91 ±0.03
9.89 ±0.01
45
15.19 ±0.50
3,23 ±0.01
0,51
22.22 ±0.39
7.79 ±0.33
10.61 ±0.24
100 %
Decayed
0
14.21 ±0.40
3,10 ±0.01
0,95
20.42 ±1.33
7.41 ±0.51
9.51 ±0.38
15
14.48 ±0.01
3,16 ±0.01
0,98
20.14 ±4.57
7.41 ±0.54
10.12 ±0.62
30
15.02 ±0.83
3,12 ±0.05
1,01
22.99 ±3.95
6.93 ±0.07
9.25 ±0.11
45
13.96 ±0.04
3,16 ±0.05
0,96
19.80 ±0.54
7.00 ±0.48
9.53 ±0.47
y Values are the mean of three determinations in two replicates.
Table 2: LSD test results of various mashfermentation periods with pectolyticenzymedosages on galacturonic acid, brix, pH, titrable acidity and Hunter L, a and b values of the apple mashsamples produced from sound (0%), 50 and 100% decayed apples.
Table 1: Effect of various mashfermentation periods with pectolyticenzymedosages on brix, pH, titrable acidity and Hunter L, a and b values of the apple mashsamples produced from sound (0%), 50 and 100% decayedapples.
Effect of various mash fermentation periods with pectolytic enzyme dosages on galacturonic acid values of the apple mash samples produced from sound (0%), 50 and 100% decayed apples were given in Figure 3. In addition LSD test results of various mash fermentation periods with pectolytic enzyme dosages on galacturonic acid values of the apple mash samples produced from sound (0%), 50 and 100% decayed apples were given in Table 2, Figure 3.
EnzymeDosage
(mg.kg-1)
Gal A*
(mg.kg-1)
MFP
(min)
Gal A*
(mg.kg-1)
Decay
Proportion
(% )
Gal A*
(mg.kg-1)
Bx°
pH
Acidity
L**
a
b
80
292.5c
0
72.3d
Sound
621.4a
12.7c
3.3a
0.36c
23.9a
7.35ab
9.9a
100
386.4b
15
184.9c
50
383.2b
15.4a
3.2b
0.50b
22.4b
7.33a
10.1a
150
484.7a
30
464.9b
100
159.0c
14.2b
3.1c
1.02a
21.9b
7.13b
9.7a
45
829.4a
GalA : Galacturonicacid, MFP : Mashfermantationperiod, Valuesaremean±standarddeviation, n=24
*Valueswithin a columnfollowedbydifferentlettersaresignificant (P < 0.01)
**Valueswithin a columnfollowedbydifferentlettersaresignificant (P < 0.05)
Table 2: LSD test results of various mashfermentation periods with pectolyticenzymedosages on galacturonic acid, brix, pH, titrable acidity and Hunter L, a and b values of the apple mashsamples produced from sound (0%), 50 and 100% decayed apples.
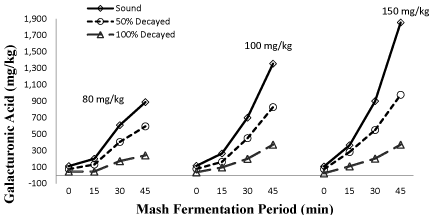
Figure 3: Effect of various mash fermentation periods with pectolytic enzyme
dosages (80, 100 and 150 mg/kg) on galacturonic acid values of the apple
mash samples produced from sound (0%), 50 and 100% decayed apples.
The statistical analysis of the data showed that there were very highly significant differences between the galacturonic acid concentrations of apple mash samples and decay proportions, enzyme dosage and mash fermentation period (P<0.01). As seen in Table 2 and Figure 4, there was a proportionally decreasing relationship between galacturonic acid values with increasing decay proportion of the apples. This result may be due to a decrease in pectin content which is naturally present in their structure. Thus, galacturonic acid content may remain at low level Figure 4.
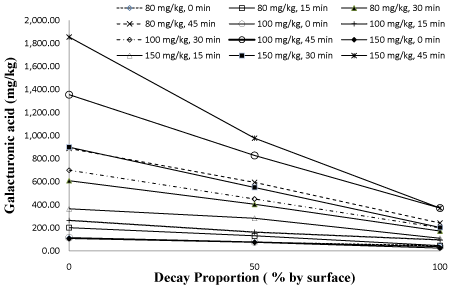
Figure 4: Effect of interaction of decay proportion & enzyme dosage at
different mash enzyme period on galacturonic acid content.
The most significant increase in galacturonic acid was observed in apple mash samples produced with the 150 mg/kg pectolytic enzyme treatment and 45 min mash fermentation in sound apple mash samples (1854.5 mg/kg). Galacturonic acid content in the final apple juice concentration may suggest lower than 1000 mg/kg [23]. High level of galacturonic acid could be miss-used as a sign of a quality indicator. In this study, just only high galacturonic acid was observed in apple mash samples produced with the 100 mg/kg and 150 mg/kg pectolytic enzyme treatment and 45 min mash fermentation in sound apple mash samples.
Galacturonic acid concentration of apple mash increased with the increase of enyzme dosage and mash fermentation period (Table 2). In contrast, galacturonic acid concentration of apple mash decreased with the increase of decay propotion of apple mash (P<0.01).The regression equations for enzyme dosage & mash fermentation period and decay proportion & enzyme dosage were given in Table 3. As shown in Table 3, good correlation coefficients were found with detection limit of 0.1 mg/kg. The statistical analysis yielded the most significant correlation coefficient of 0.9999 (y = -4,9936x + 699,23) indicating a linear regression with 100 mg/kg enzyme treatment and 30 min mash fermentation in sound apple mash samples, when all samples were taken into account (Table 3).
EnzymeDosage
(mg.kg-1)
Time
(min)
Regressionequation
(y = ax + b)
Correlationcoefficient
Limit of Detection
(LOD)
(mg.kg-1)
80
0
y = -0,7764x + 111,94
R² = 0,9974
0,1
15
y = -1,5539x + 203,00
R² = 0,9969
0,1
30
y = -4,3672x + 613,12
R² = 0,9986
0,1
45
y = -6,4616x + 897,46
R² = 0,9973
0,1
100
0
y = -0,8184x + 115,97
R² = 0,9975
0,1
15
y = -1,6959x + 258,70
R² = 0,9862
0,1
30
y = -4,9936x + 699,23
R² = 0,9999
0,1
45
y = -9,836x + 1342,60
R² = 0,9983
0,1
150
0
y = -0,7452x + 108,25
R² = 0,9961
0,1
15
y = -2,5563x + 379,77
R² = 0,9592
0,1
30
y = -6,9636x + 898,73
R² = 0,9991
0,1
45
y = -14,836x + 1809,4
R² = 0,9890
0,1
DecayProportion
EnzymeDosage
(mg.kg-1)
Regressionequation
(y = ax + b)
Correlationcoefficient
Limit of Detection
(LOD)
(mg.kg-1)
Sound (0 %)
80
y = 273,86x - 232,8
R² = 0,9519
0,1
100
y = 415,38x - 430,2
R² = 0,9308
0,1
150
y = 577,75x - 638,08
R² = 0,9315
0,1
50 % Decayed
80
y = 182,94x - 156,4
R² = 0,9482
0,1
100
y = 253,64x - 255,0
R² = 0,9359
0,1
150
y = 297,8x - 273,75
R² = 0,9726
0,1
100 % Decayed
80
y = 75,171x - 65,055
R² = 0,9245
0,1
100
y = 111,88x - 105,04
R² = 0,9542
0,1
150
y = 110,95x - 98,615
R² = 0,9647
0,1
a: slope, b: interception, Valuesaremean±standarddeviation, n=24, R² : correlationcoefficient
Table 3: Linearityanddetectionlimitsforthegalacturonicacidcontent.
Conclusion
As stated earlier, the galacturonic acid content of apple juice or apple juice concentrate is an important quality parameter for suppliers. Many food processes and pectin ingredient providers need to examine the pectin content in order to control the quality of their products. Indeed, the determination of the galacturonic acid in apple juice is very important because their presence may affect the chemical and sensory characteristics of the matrix such as pH, titratable acidity, microbial stability and the global acceptability therefore provide valuable information on healthy food quality or value necessary to give the specifications of selection.
In this paper, whether the galacturonic acid concentration is affected by the applied process (enzyme dosage and mash fermentation period) or not is studied. As a result of this study, galacturonic acid contents were decreased with increasing decay proportion. It was determined that mg/kg pectolytic enzyme treatment should be applied with 45 min mash fermentation or 100 mg/kg pectolytic enzyme treatment should be applied with 45 min mash fermentation to remain in the desired limit values for galacturonic acid concentration of apple juices. If enzyme treatment exceed 100 mg/L, mash enzymation period should carefully monitored and need to be terminate the process. Therefore pectolytic enzyme treatment should not to exceed 100 mg/kg.
References
- Abid M, Jabbar S, Wu T, Hashim MM, Hu B, Lei S, et al. Effect of ultrasound on different quality parameters of apple juice. Ultrason Sonochem. 2013; 20: 1182-1187.
- Diamante LM, Li S, Xu Q, Busch J. Effects of Apple Juice Concentrate, Blackcurrant Concentrate and Pectin Levels on Selected Qualities of Apple-Blackcurrant Fruit Leather. Foods. 2013; 2: 430-443.
- Peng K, Zhang Y, Wang SJ, Liao XJ, Hu XS. Effect of microwave drying pretreatment on extraction of pectin from apple pomace. T CSAE. 2008; 24: 222-226.
- Markowski J, Baron A, Mieszczakowska M, Plocharski W. Chemical composition of French and Polish cloudy apple juices. J Hortic Sci Biotech. 2009; 68-74.
- Kay-Shuttleworth R. The World and Europe Fruit Juice Industry in Data. AIJN 50th Anniversary Symposium, Brussels, Belgium. 2008.
- Barth SW, Fahndrich C, Bub A, Dietrich H, Watzl B, Will F, et al. Cloudy apple juice decreases DNA damage, hyperproliferation and aberrant crypt foci development in the distal colon of DMH-initiated rats. Carcinogenesis. 2005; 26: 1414-1421.
- Ibarz A, Garza S, Pagan J. Nonenzymatic browning of selected fruit juices affected by D-galacturonic acid. Int J Food Sci Tech. 2008; 43: 908-914.
- Sorrivas V, Genovese DB, Lozano JE. Effect of pectinolytic and amylolytic enzymes on apple juice turbidity. J Food Process Pres. 2006; 30: 118-133.
- Knaup B, Kempf M, Fuchs J, Valotis A, Kahle K, Oehme A, et al. Model experiments mimicking the human intestinal transit and metabolism of D-galacturonic acid and amidated pectin. Mol Nutr Food Res. 2008; 52: 840-848.
- Daas PJH, Meyer-Hansen K, Schols HA, De Ruiter GA, Voragen AGJ. Investigation of the non-esterified galacturonic acid distribution in pectin with endopolygalacturonase. Carbohyd Res. 1999; 318: 135-145.
- Moreira MM, Guido LF, Cruz JM, Baros AA. Determination of galacturonic acid content in pectin from fruit juices by liquid chromatographydiode array detection electrospray ionization tandem mass spectrometry. Cent Eur J Chem. 2010; 8: 1236-1243.
- Qiu N, Tian Y, Qiao S, Deng H. Apple Pectin Behavior Separated by Ultrafiltration. Agr Sci China. 2009; 8: 1193-1202.
- McLellan MR, Acree T. GrapeJuice. Fruit Juice Processing Technology. Nagy S, Chen CS, Shaw PE, editors. In: AgScienceInc. Auburndale FL. 1993; 318-333.
- Voragen AGJ, Schols HA, Beldman G. Tailor-made Enzymes in Fruit Juice Processing. Fruit Process. 1992; 59: 98-102.
- Plocharski W, Szymczak J, Markowski J. Auswirkung enzymatisher Apfelmaischebehandlung auf Saftsbeute und Pektinstoffmenge. Flussigess. Obst. 1998; 6: 325-332.
- Will F, Schulz K, Ludwig M, Otto K, Dietrich H. The influence of enzymatic treatment of mash on the analytical composition of apple juice. Int J Food Sci Tech. 2002; 37: 653-660.
- Mihalev K, Schieber A, Mollov P, Carle R. Effect of mash maceration on the polyphenolic content and visual quality attributes of cloudy apple juice. J Agric Food Chem. 2004; 52: 7306-7310.
- Schilling S, Alber T, Toepfl S, Ludwig M, Dietrich H, Knorr D, et al. Comparative study ofjuice production by pulsed electric field treatment and enzymatic maceration of apple mash. Eur Food Res Tech. 2008; 226: 1389-1398.
- Stocke R. The 3-component stabilization with bentonite, gelatin and silica sol. Fruit Process. 1998; 1: 6-10.
- Zotou A, Loukou Z, Karava O. Method development for the determination of seven organic acids in wines by reversed-phase high performance liquid chromatography. Chromatogr. 2004; 60: 39-44.
- Li HB, Chen F. Determination of silicate in water by ion exclusion chromatography with conductivity detection. J Chromatogr A. 2000; 874: 143-147.
- AOAC. Official methods of analysis of AOAC International (15th edn). Arlington, AOAC International. 1990.
- Maier G. Enzymatic applications in fruit juice industry. MeyveSuyu GelismelerveEgilimlerSemineri. 2003; 1: 121-123.