
Special Article – RP-HPLC
Austin Chromatogr. 2015;2(1): 1028.
Comprehensive Two-Dimensional Liquid Chromatography Methods for Analysis of Polyphenols in Food Samples
Cacciola F1*, Russo M2, Beccaria M2, Donato P1, Farnetti S3, Dugo P2,4,5and Mondello L2,4,5
1Department of Environmental Science, Territorial, Food and Health Security, University of Messina, Italy
2Department of Drug Sciences and Health Products, University of Messina, Italy
3Chromaleont s.r.l., c/o “Department of Drug Sciences and Health Products, University of Messina, Italy
4University Campus Bio-Medico of Rome, Italy
5Chromaleont Ltd, Pharmaceutical Sciences and Health Products Department, University of Messina, Italy
*Corresponding author: Francesco Cacciola, Department of Environmental Science, Territorial, Food and Health Security, University of Messina, Viale F. Stagno d’Alcontres 31, 98166 Messina, Italy
Received: February 19, 2015; Accepted: March 09, 2015; Published: March 11, 2015
Abstract
Phenols and flavonoids presenting an enormous structural variability are receiving special interest because of their broad spectrum of pharmacological effects. The identification of these polyphenol derivatives in food samples is a difficult task due to the complexity of the structures and the limited standards commercially available. So far, one-dimensional chromatography was the most widely applied analytical approach for their analysis. However, when dealing with very complex media, a single separation system often does not provide sufficient resolving power for attaining rewarding results. Comprehensive twodimensional Liquid Chromatography (LC×LC) is a technique of great analytical impact, since it offers much higher peak capacities than separations in a single dimension. The present review describes the applications carried out in the field of LC×LC for polyphenol separation in food and food-related products.
Keywords: Polyphenols; Food; Comprehensive liquid chromatography; Mass spectrometry
Abbreviations
LC: Liquid Chromatography; MS: Mass Spectrometry; LC×LC: Comprehensive Liquid Chromatography; ESI: Electro Spray Ionization; APCI: Atmospheric Pressure Chemical Ionization; GC: Gas Chromatography; CE: Capillary Electrophoresis
Introduction
Phenols and flavonoids presenting an enormous structural variability (5000 derivatives are known today), are receiving special interest because of their broad spectrum of pharmacological effects [1]. The identification of these polyphenol derivatives in food samples is a difficult task due to the complexity of the structures; from a chemical viewpoint, they can be classified in different groups, i.e. phenolic acids, flavan-3-ols, flavanones, flavones, flavonoids, lignans, and so on. To this regard, the most common separation techniques used to determine these kinds of bioactive compounds in such samples have been Capillary Electrophoresis (CE), Gas Chromatography (GC), and Liquid Chromatography (LC). CE provides high efficiencies in short migration times with small amounts of reagents and sample volumes needed [2], although its main disadvantage is the low concentration sensitivity. GC is the least used technique for this purpose, since a derivatization step is necessary. LC in combination with Mass Spectrometry (MS), using Atmospheric Pressure Chemical Ionization (APCI) and Electro Spray Ionization (ESI) as interfaces under positive and negative ionization modes, is usually adopted, for identification and structural characterization of these compounds. However, when the polyphenol content is very complex, onedimensional LC is not sufficient for attaining rewarding results. As a consequence, to overcome such a limitation multidimensional Liquid Chromatography (2D-LC) techniques, where the two dimensions
are based on different separation mechanisms, could be exploited [3-6]. Multidimensional LC (MD-LC) techniques can be classified into “heart-cutting” and “comprehensive LC”, abbreviated as LC-LC and LC×LC respectively, according to the well-known established notations [7]. In both approaches, the columns are connected by means of a switching valve. The main difference between these two techniques is the amount of the primary column effluent that is transferred. In the former approach, only a few selected fractions of the effluent, containing the analytes of interest are directed from the primary to the secondary column (dimension) [8,9]; in the LC×LC, the entire sample is subjected to separation in both dimensions [10]. The first LC×LC application can be dated back to 1978 thanks to the pioneeristic work by Erni e and Frei [11]; since then, especially in the last decade, an ever continuous increase of papers have been published in this field to suit dedicated case studies and some of them were devoted to polyphenol analysis [12-42].
LC×LC: General aspects and method development
2D-LC methods can be operated either under “off-line” or “online” mode depending on the way to transfer the first dimension (1D) effluent to the second column (2D). In the former case, fractions of the 1D effluent are collected manually or via a fraction collector, and afterwards re-injected on the 2D column. “Off-line” approaches are time consuming, difficult to automate, non-reproducible and prone to sample loss and contamination and artefact formation. “On-line” approaches are faster and more reproducible, but more difficult to operate because of the use of special interfaces. Whatever “off-line” or on-line” approach employed, a 2D-LC separation is considered “orthogonal” if the two separation mechanisms provide complementary selectivity [43]. Orthogonal separations can be achieved when suitable mobile and stationary phases are selected, taking into account the physicochemical properties of the sample components including size and charge, hydrophobicity and polarity. In particular, LC techniques offer a wide variety of separation mechanisms, such as Normal Phase (NP), Reversed Phase (RP), Size Exclusion (SEC), Ion Exchange (IEX) or Affinity Chromatography (AC), characterized by different selectivities.
The development and optimization of an LC×LC method requires the adjustment of many parameters. An LC×LC system is composed of at least two pumps, two columns, an injector, an interface and a detector. The interface has the function to hyphen the two dimensions and in the most common set-up, small volume fractions of the effluent from the 1D are transferred via a multi-port switching valve into the 2D. Before coupling, the methods in both dimensions should be optimized, considering the sample characteristics and the parameters that affect the peak capacity. For the 2D analyses, separation time should be fast enough to ensure both complete fraction elution before the subsequent transfer and adequate 1D sampling [44]; this time is slightly enhanced if a regeneration step needs to be considered when running gradient programs. Analysis speed in the 2D, can be increased in different ways. For polyphenol analysis, monolithic columns were the first to be used, due to their short regeneration characteristics and high permeability, thus allowing operation at high flow rates without loss in resolution [12,16-18]. Another way to speed up the 2D analysis is to use conventional columns packed with stationary phases of reduced particle size such as partially or superficially porous stationary phases [19-25] or sub-2μm particle packed columns [33]; in the latter case, a more sophisticated hardware capable of withstanding such high pressures (PMAX= 440 bar) viz. Ultra High Pressure Liquid Chromatography (UHPLC) is required [33]. To speed up the 2D analysis, the use of elevated temperatures in the 2D have been also exploited aiming to strongly increase the flow rate thanks to the reduced mobile phase viscosity, even though the stability of the stationary phase and analytes must be taken into account [14].
Both dimensions can be operated under either isocratic or gradient conditions. As an interface for the automatic transfer of the 1D effluent to the 2D, many configurations have been proposed. The most widely used interface involves the use of a 2-position/10-port switching valve [12-37], despite also two 2-position/6-port switching valves have been used as well [31,33,42]. The loops are alternately emptied and filled with the 1D effluent prior to the 2D analysis in a “continuous” way. The empty storage loops can be replaced by loops packed with stationary phase. Under these conditions, the 1D mobile phase is preferably a weak solvent and the solutes are focused in the loop prior to be transferred to the 2D; fast desorption by a strong solvent is afterwards performed by the 2D mobile phase [12,16]. Also, two parallel 2D columns have been employed in absence of storage loops using columns of the same batch and adapted tubing system [14,15,29]. As far as columns are concerned, the 1D consists usually of micro (1.0 mm ID)- or narrow-bore (2.1 mm ID) columns providing flow rates compatible with conventional 4.6 mm i.d. columns in the 2D. An issue of utmost importance is the compatibility of the mobile phases used in the two dimensions. The mobile phase eluting from the 1D column should preferably consist of a weak solvent constituent of the 2D mobile phase, in order to create a satisfactory peak compression thus achieving “peak focusing”. Such a requirement is a must whatever combination of LC mobile phases is employed, especially if the solvents or solvent mixtures are not completely miscible. In the case of polyphenol analysis being most RP-LC×RP-LC separations, fully compatible 2D solvents are employed. A recent promising column combination in LC×LC employs the use of Hydrophilic Interaction Liquid Chromatography (HILIC) and RP conditions in the 1D and 2D, respectively. In the last two separation mode combination, the mobile phase used in the 1D has a higher elution strength than the one used in the 2D. For such a reason, the employment of micro-flowrates in the 1D is highly beneficial in order to minimize dilution and provide flow rates compatible with 2D injection volumes [36-41].
LC×LC: Instrumentation and data handling
Commercial ready-to-use LC×LC systems are currently available from various manufactures, making the methodology much easier for practical uses, allowing matching specific 2D-LC requests. All conventional LC detectors, such as UV Photo-Diode-Array (PDA), Mass Spectrometric (MS) and Evaporative Light Scattering (ELS) detectors can be used in LC×LC. Usually, a single detector is installed after the 2D column, although an additional detector can be used to collect the 1D data, with the 1D separation monitored only during the optimization step. The high speed of the 2D analysis requires a very fast detector acquisition rate to ensure the adequate sampling which is critical for quantification purposes otherwise loss in resolution, due by a low number of data points, may occur. MS detection can be considered a third dimension to the LC×LC system, since the MS can be capable of identifying the co-eluting non-isobaric peaks when they are not resolved by chromatography. ESI and APCI MS interfaces receiving the 2D effluent are preferably used for effective ionization of all polyphenolic compounds. For both visualization and quantification of 2D data, at least two commercially available software, specially designed are available on the market (https://www. chromaleont.it/chromsquare.html; https://www.gcimage.com/lcxlc/).
Comprehensive LC techniques for polyphenol separation in food analysis
On-line LC-LC have been successfully used for analysis of food polyphenols in only two works [15,45]. In the first case, natural polyphenolic antioxidants were separated using a C18 column in the 1D and two parallel Zirconia Carbon columns working in alternating cycles in the 2D. The combination of the two columns, run under temperature and solvent gradients, provided great differences in separation selectivity in each dimension and an almost orthogonal 2DLC system. Temperature gradients provided shorter separation times in comparison with solvent gradients (Figure 1). Such an approach was applied to the analysis of beer and wine samples. The more recent example was investigated for simultaneously determination of 12 major components, namely seven flavonoids, four phenylpropanoid glycosides, and N-trans-feruloyltyramine in tartary buckwheat.
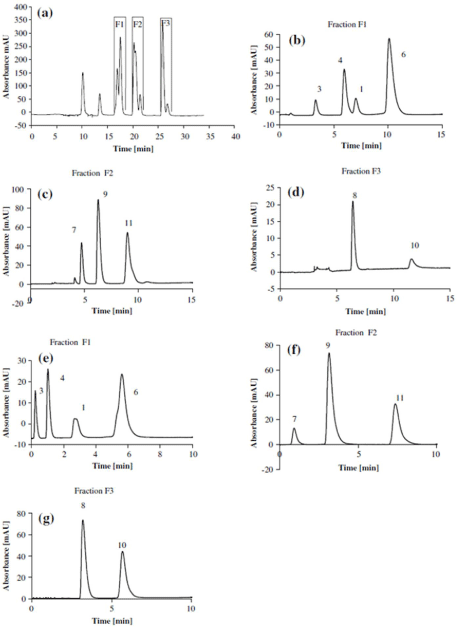
Figure 1: Heart-cutting of a mixture of phenolic antioxidants on a Purosphere Star RP-18e column (250x4.6 mm; 5 μm) in the first dimension and on a ZR-Carbon
column (50x2.1 mm; 5 μm) in the second dimension. For details see ref [15].
As far as comprehensive LC approaches are concerned, both HILIC×RP-LC and RP-LC×RP-LC set-up by using different stationary phase chemistries have been employed for analysis of polyphenols in various food-related products [12-24,30,32,36-42].
HILIC×RP-LC combinations have been investigated for polyphenol analysis in beverages and plant extracts [30,32,36- 41], allowing to separate compounds based on polarity and hydrophobicity, respectively. Such an approach have been used for elucidation of Phenolics and Procyanidins (PC) in apples and cocoa samples [30,40,41] and tea [32,36] extracts. The first application was reported for separation of phenolic compounds in apples and cocoa samples where an off-line LC×LC system was designed based on HILIC with a diol stationary phase and RP-LC conditions in the 1D and 2D, respectively. As detection, fluorescence, diode-array and negative ESI-MS systems, were employed for unravelling of the complex composition of low molecular weight phenolic compounds. The HILIC×RP-LC system was characterized by a very high practical peak capacity (over 2000) due to the low degree of correlation (R2<0.2) between the selected separation mechanisms. The proposed methodology demonstrated its suitability for the analysis of various groups of phenolic compounds including proanthocyanidins, phenolic acids, flavonols and flavonol derivatives, all of which cannot be separated in a single analysis by conventional 1D-LC methods. More recently, three different LC×LC configurations namely online, off-line, or stop-flow were compared in terms of practical peak capacities, analysis times and peak production rates [40]. In a separate contribution, the experimental verification of the findings of this study was reported for analysis of cocoa procyanidins [41]. The results showed that while optimisation procedures based on theoretical considerations remain largely valid in practice, several important experimental considerations had to be taken into account to achieve maximum performance in all three modes of HILIC×RPLC. On the one hand, the on-line analysis provided an effective tool for the screening of procyanidins content within reasonable times, whereas on the other, off-line- and stop-flow HILIC×RP-LC analyses were more suited for the detailed analysis of complex procyanidin fractions (Figure 2). In particular, stop-flow operation had a negligible effect on the 1D band broadening under the optimised experimental conditions used. Finally, off-line and stop-flow analyses provided much higher, and similar, resolving power at peak capacity production rates roughly half those obtained by the on-line system; however, the stop-flow system required the use of additional hardware and was experimentally more complicated but on the other hand it offered the advantages of complete automation and minor risk of sample alteration. Similar set-up for PC analysis was also reported for grape seeds extracts [38,39]. The investigated HILIC×RP-LC separation, followed by PDA and tandem MS detection, allowed the tentative identification of 43 flavan-3-ols, including monomers and procyanidins oligomers till a polymerization degree of 7 units with different galloylation degrees. The same set-up was also employed for the Phenolics profiling of different apple varieties in a frame time of less than 50 min, allowing the tentative identification of ca. 65 compounds on each studied sample, including flavan-3-ol oligomers up to a DP = 8, dihydrochalcones, flavonols and phenolic acids [39]. Such a study opened new possibilities for analysis of target and non-target metabolomics-related studies. As 1D of a comprehensive HILIC×RP for separation of polyphenolic compounds, several polar stationary phases namely PEG, DIOL, Amide, Phenyl and sulfobetaine were compared with gradients of decreasing concentration of acetonitrile in buffered aqueous-organic mobile phases, and subsequently coupled on-line with short non-polar or weakly polar monolithic or porous shell columns in the 2D, with fast RP-LC analyses (1-2 min) [37]. For optimum performance of HILIC×RP systems, micro-bore or capillary columns and low flow-rates were used in the 1D, whereas short (3 or 5 cm) core-shell columns with larger diameters was tested at high flow-rates in the 2D. 5-cm 2D columns allowed the transfer of larger fraction volumes to the 2D with respect to the 3-cm columns, without significant band broadening. For 1D a new monolithic sulfobetaine polymethacrylate capillary column was tested under HILIC conditions, providing better orthogonality and good efficiency especially for low molecular compounds; moreover, the 0.53 mm I.D. allowed decreasing the fraction volumes close to the optimum, for separations within 1 or 2 min fast gradients in the 2D.
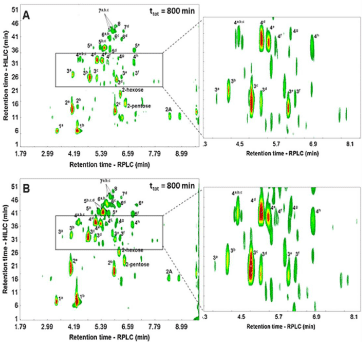
Figure 1: Fluorescence contour plots obtained for the HILIC × RP-LC analysis of a cocoa extract using off-line (A) and stop-flow (B) configurations. Peak numbers
indicate the Degree of Polymerisation (DP) of PC isomers and letters differentiate isomers of the same DP. For details see ref [41].
On the other hand, RP-LC×RP-LC methods have been the most widely applied to the analysis of antioxidants and in particular polyphenols occurring in several types of food samples, mainly beverages and plant extracts [12-23,24,31,33,35,42]. However, in almost all RP-LC×RP-LC approaches investigated, peak capacity values were significantly lower with respect to the theoretical ones as a result of the coupling of partially correlated systems. A potential solution to overcome such problem was demonstrated by carefully choosing the LC gradient profile [24]. In particular various types of 2D gradients in LC×LC were compared, namely “full in fraction”, “segment in fraction” and “continuously shifting” gradients. Figure 3 shows the LC×LC separations of phenolic acids and flavones on a PEG column in the 1D and two types of partially porous C18 columns in the 2D (Ascentis Express and Kinetex) by using such gradients are illustrated. The effects of the gradient type on the bandwidths, theoretical peak capacity, and separation time and column pressure in the 2D were investigated. A careful design of 2D gradients could be a valuable tool when partially correlated systems are employed in LC×LC separations.
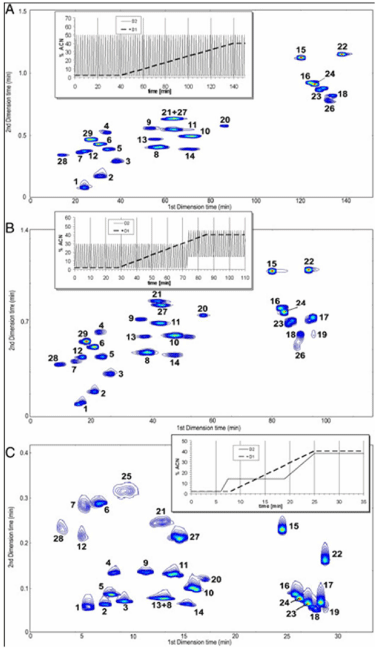
Figure 3: 2-D comprehensive LC×LC separation of phenolic acids and flavones on a PEG column in the first dimension and an Ascentis Express C18 column in
the second dimension. (A) Parallel gradients with the FIF second-dimension gradient 1; (B) parallel gradients with the SIF second-dimension gradient 2; (C) parallel
gradients with the CS second-dimension gradient 3 For details see ref [24].
Conclusion
Comprehensive LC is a valuable tool for analysis of complex food samples. The popularity of such an approach increased with the availability of ready-to-use LC×LC systems equipped with dedicated software for both method development and data handling allowing qualitative and quantitative purposes.
For polyphenol analysis the coupling of hydrophilic interaction liquid chromatography and reversed phase conditions was the most efficient the very recent report describing the coupling of “partially correlated” RP×RP systems turned out to be a viable tool.
References
- Ganzera M. Quality control of herbal medicines by capillary electrophoresis: potential, requirements and applications. Electrophoresis. 2008; 29: 3489-3503.
- Garcia-Canas V, Cifuentes A. Recent advances in the application of capillary electromigration methods for food analysis. Electrophoresis. 2008; 29: 294-309.
- Dugo P, Cacciola F, Kumm T, Dugo G, Mondello L. Comprehensive multidimensional liquid chromatography: theory and applications. J Chromatogr A. 2008; 1184: 353-368.
- Guiochon G, Marchetti N, Mriziq K, Shalliker RA. Implementations of two-dimensional liquid chromatography. J Chromatogr A. 2008; 1189: 109-168.
- Stoll DR, Li X, Wang X, Carr PW, Porter SE, Rutan SC. Fast, comprehensive two-dimensional liquid chromatography. J Chromatogr A. 2007; 1168: 3-43.
- François I, Sandra K, Sandra P. Comprehensive liquid chromatography: fundamental aspects and practical considerations--a review. Anal Chim Acta. 2009; 641: 14-31.
- Schoenmakers P, Marriot P, Beens J. Nomenclature and conventions in comprehensive multidimensional chromatography. LC-GC Eur. 2003; 16: 335-339.
- Davis JM, Giddings JC. Statistical theory of component overlap in multicomponent chromatograms. Anal. Chem. 1983; 55: 418-424.
- Giddings JC. Sample dimensionality: a predictor of order-disorder in component peak distribution in multidimensional separation. J Chromatogr A. 1995; 703: 3-15.
- Mondello L, Lewis C, Bartle KD. Multidimensional Chromatography. John Wiley and Sons, Chichester, UK. 2001; 109.
- Erni F, Frei RW. Two-dimensional column liquid chromatographic technique for resolution of complex mixtures. J Chromatogr. 1978; 149: 561-569.
- Blahova E, Jandera P, Cacciola F, Mondello L. Two-dimensional and serial column reversed-phase separation of phenolic antioxidants on octadecyl-, polyethyleneglycol-, and pentafluorophenylpropyl-silica columns. J Sep Sci. 2006; 29: 555-566.
- Cacciola F, Jandera P, Blahová E, Mondello L. Development of different comprehensive two dimensional systems for the separation of phenolic antioxidants. J Sep Sci. 2006; 29: 2500-2513.
- Cacciola F, Jandera P, Mondello L. Temperature effects on separation on zirconia columns: applications to one- and two-dimensional LC separations of phenolic antioxidants. J Sep Sci. 2007; 30: 462-474.
- Cacciola F, Jandera P, Mondello L. Comparison of High-Temperature Gradient Heart-Cutting and Comprehensive LC × LC Systems for the Separation of Phenolic Antioxidants. Chromatographia. 2007; 66: 661-667.
- Cacciola F, Jandera P, Hajdu Z, Cesla P, Mondello L. Comprehensive two-dimensional liquid chromatography with parallel gradients for separation of phenolic and flavone antioxidants. J Chromatogr A. 2007; 1149: 73-87.
- Kivilompolo M, Oburka V, Hyotylainen T. Comprehensive two-dimensional liquid chromatography in the analysis of antioxidant phenolic compounds in wines and juices. Anal Bioanal Chem. 2008; 391: 373-380.
- Dugo P, Cacciola F, Herrero M, Donato P, Mondello L. Use of partially porous column as second dimension in comprehensive two-dimensional system for analysis of polyphenolic antioxidants. J Sep Sci. 2008; 31: 3297-3308.
- Hajek T, Skerikova V, Cesla P, Vynuchalova K, Jandera P. Multidimensional LC x LC analysis of phenolic and flavone natural antioxidants with UV-electrochemical coulometric and MS detection. J Sep Sci. 2008; 31: 3309-3328.
- Dugo P, Cacciola F, Donato P, Airado-Rodríguez D, Herrero M, Mondello L. Comprehensive two-dimensional liquid chromatography to quantify polyphenols in red wines. J Chromatogr A. 2009; 1216: 7483-7487.
- Dugo P, Cacciola F, Donato P, Jacques RA, Caramao EB, Mondello L . High efficiency liquid chromatography techniques coupled to mass spectrometry for the characterization of mate extracts. J Chromatogr A. 2009; 1216: 7213-7221.
- Cesla P, Hajek T, Jandera P. Optimization of two-dimensional gradient liquid chromatography separations. J Chromatogr A. 2009; 1216: 3443-3457.
- Jandera P, Hajek T, Stankova M, Vynuchalova K, Cesla P. Optimization of comprehensive two-dimensional gradient chromatography coupling in-line hydrophilic interaction and reversed phase liquid chromatography. J Chromatogr A. 2012; 1268: 91-101.
- Jandera P, Hajek T, Cesla P. Comparison of various second-dimension gradient types in comprehensive two-dimensional liquid chromatography. J Sep Sci. 2010; 33: 1382-1397.
- Russo M, Cacciola F, Bonaccorsi I, Dugo P, Mondello L. Determination of flavanones in Citrus juices by means of one- and two-dimensional liquid chromatography. J Sep Sci. 2011; 34: 681-687.
- François I, de Villiers A, Sandra P. Considerations on the possibilities and limitations of comprehensive normal phase-reversed phase liquid chromatography (NPLC x RPLC). J Sep Sci. 2006; 29: 492-498.
- Kivilompolo M, Hyotylainen T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: characterisation and quantification of antioxidant phenolic acids. J Chromatogr A. 2007; 1145: 155-164.
- Pol J, Hohnova B, Hyotylainen T. Characterisation of Stevia rebaudiana by comprehensive two-dimensional liquid chromatography time-of-flight mass spectrometry. J Chromatogr A. 2007; 1150: 85-92.
- François I, de Villiers A, Tienpont B, David F, Sandra P. Comprehensive two-dimensional liquid chromatography applying two parallel columns in the second dimension. J Chromatogr A. 2008; 1178: 33-42.
- Kalili KM, de Villiers A. Off-line comprehensive 2-dimensional hydrophilic interaction x reversed phase liquid chromatography analysis of procyanidins. J Chromatogr A. 2009; 1216: 6274-6284.
- Mnatsakanyan M, Stevenson PG, Shock D, Conlan XA, Goodie TA, Spencer KN, et al. The assessment of pi-pi selective stationary phases for two-dimensional HPLC analysis of foods: application to the analysis of coffee. Talanta. 2010; 82: 1349-1357.
- Kalili KM, de Villiers A. Off-line comprehensive two-dimensional hydrophilic interaction x reversed phase liquid chromatographic analysis of green tea phenolics. J Sep Sci. 2010; 33: 853-863.
- Cacciola F, Delmonte P, Jaworska K, Dugo P, Mondello L, Rader JI. Employing ultra high pressure liquid chromatography as the second dimension in a comprehensive two-dimensional system for analysis of Stevia rebaudiana extracts. J Chromatogr A. 2011; 1218: 2012-2018.
- Scoparo CT, Souza LM, Dartora N, Sassaki GL, Gorin PA, Iacomini M. Analysis of Camellia sinensis green and black teas via ultra high performance liquid chromatography assisted by liquid-liquid partition and two-dimensional liquid chromatography (size exclusion × reversed phase). J Chromatogr A. 2012; 1222: 29-37.
- Fu Q, Guo Z, Zhang X, Liu Y, Liang X. Comprehensive characterization of Stevia Rebaudiana using two-dimensional reversed-phase liquid chromatography/hydrophilic interaction liquid chromatography. J Sep Sci. 2012; 35: 1821-1827.
- Beelders T, Kalili KM, Joubert E, de Beer D, de Villiers A. Comprehensive two-dimensional liquid chromatographic analysis of rooibos (Aspalathus linearis) phenolics. J Sep Sci. 2012; 35: 1808-1820.
- Jandera P, Hajek T, Stankova M, Vynuchalova K, Cesla P. Optimization of comprehensive two-dimensional gradient chromatography coupling in-line hydrophilic interaction and reversed phase liquid chromatography. J Chromatogr A. 2012; 1268: 91-101.
- Montero L, Herrero M, Prodanov M, Ibananez E, Cifuentes A. Characterization of grape seed procyanidins by comprehensive two-dimensional hydrophilic interaction×reversed phase liquid chromatography coupled to diode array detection and tandem mass spectrometry. Anal Bioanal Chem. 2013; 405: 4627-4638.
- Montero L, Herrero M, Ibanez E, Cifuentes A. Profiling of phenolic compounds from different apple varieties using comprehensive two-dimensional liquid chromatography. J Chromatogr A. 2013; 1313: 275-283.
- Kalili K, De Villiers A. Systematic Optimisation and Evaluation of On-Line, Off-Line and Stop-Flow Comprehensive Hydrophilic Interaction Chromatography × Reversed Phase Liquid Chromatographic Analysis of Procyanidins, Part I: Theoretical Considerations. J Chromatogr A. 2013; 1289: 58-68.
- Kalili K, De Villiers A. Systematic Optimisation and Evaluation of On-Line, Off-Line and Stop-Flow Comprehensive Hydrophilic Interaction Chromatography × Reversed Phase Liquid Chromatographic Analysis of Procyanidins. Part II: Application to Cocoa Procyanidins. J Chromatogr A. 2013; 1289: 69-79.
- Larson ED, Groskreutz SR, Harmes DC, Gibbs-Hall IC, Trudo SP, Allen RC, et al. Development of selective comprehensive two-dimensional liquid chromatography with parallel first-dimension sampling and second-dimension separation-application to the quantitative analysis of furanocoumarins in apiaceous vegetables. Anal Bioanal Chem. 2013; 405: 4639-4653.
- Slonecker PJ, Li X, Ridgway TH, Dorsey JG. Informational orthogonality of two-dimensional chromatographic separations. Anal Chem. 1996; 68: 682-689.
- Murphy RE, Schure MR, Foley JP. Effect of Sampling Rate on Resolution in Comprehensive Two-Dimensional Liquid Chromatography. Anal Chem. 1998; 70: 1585-1594.
- Krol-Kogus B, Glod D, Krauze-Baranowska M, Matlawska I. Application of one- and two-dimensional high-performance liquid chromatography methodologies for the analysis of C-glycosylflavones from fenugreek seeds. J Chromatogr A. 2014; 1367: 48-56.