
Research Article
Austin Chromatogr. 2015;2(2): 1029.
Application of RP-HPTLC and Oscik’s Equation for the Evaluation of Lipophilic Properties of Selected Biologically Active Compounds
Malgorzata Dolowy1, Alina Pyka1*, Tomasz Raczek2, Katarzyna Kasprzycka2, Izabela Bajorek2 and Patrycja Piecha2
1Department of General and Analytical Chemistry, Institute of Analytical Chemistry, School of Pharmacy and the Division of Laboratory Medicine, Medical University of Silesia in Katowice, Poland
2Student Research Group at the Department of General and Analytical Chemistry, Institute of Analytical Chemistry, School of Pharmacy and the Division of Laboratory Medicine, Medical University of Silesia in Katowice, Poland
*Corresponding author: Alina Pyka, Department of General and Analytical Chemistry, Institute of Analytical Chemistry, School of Pharmacy and the Division of Laboratory Medicine, Medical University of Silesia in Katowice,4 Jagiellonska St, 41-200Sosnowiec, Poland.
Received: February 19, 2015; Accepted: March 11,2015; Published: March 19, 2015
Abstract
The aim of this study was to compare different methods including theoretical based on various computer software as well as simple thin-layer chromatography in reversed phase system in the determination of lipophilicity of various classes of biologically active compounds, such as fatty acids, esters of nicotinic acid and also bile acids. The lipophilicity descriptor expressed as partition coefficient (logP) predicted by five computational algorithms (AlogPS, IAlogP, ClogP, logPKOWWIN, xlogP) and also experimental value of logP (determined by shakeflask method) which is available for selected of examined compounds belonging to esters of nicotinic acid and also bile acids can provide useful information about lipophilic character of these active compounds in their preliminary study. Experimentally determined retention parameter (RM) by means of RP-HPTLC method using binary system methanol-water on silica gel RP-18WF254 is applicable to predict other lipophilicity parameters denoted by RMWS and RMWO which have been calculated accordance with Soczewinski-Wachtmeister’s and Oscik’s equations, respectively. The results obtained in present study demonstrate that RMWO may be a good alternative in describing of the lipophilic character of biologically active compounds with higher lipophilicity (i.e., fatty acids and bile acids). In the case of active substances with lower lipophilicityRMWS proved to be more reliable than RMWO in describing their lipophilic character.
Keywords: Fatty acids; Esters of nicotinic acid; Bile acids; Lipophilicity; LogP; RP-HPTLC; Oscik’s equation
Introduction
Lipophilicity, expressed trough the Partition coefficient (P) or its decimal logarithm (logP) of neutral compound between two immiscible solvents, usually n-octanol and water is one of the most important descriptors which has a significant impact on the behavior (i.e., absorption, distribution, metabolism, excretion) of organic compounds in biological system (ADME system). Because lipophilicity is associated with biological activity and plays important role in the pharmacodynamics and toxicological profile of drugs, it is very often applied in medicinal chemistry, in preclinical study of potential new drugs in order to predict their ADME properties [1]. The traditional procedure of determining of this parameter is shakeflask method. This method is simple to use but it is time-consuming and requires large amount of sample and solvents. Moreover, the logP values determined by shake-flask method are limited to the range of –3 and +3. For this reason, this method cannot be used to very hydrophilic or very hydrophobic compounds.
In recent days, the classical shake-flask method is successfully replaced by the Reversed-Phase Thin-Layer Chromatography (RP-TLC) and the Reversed-Phase High Performance Liquid Chromatography (RP-HPLC) [2,3]. Currently, the chromatographic determination of lipophilicity is most preferred due to less laborious and a wide range of measurable lipophilicity values, comparing to extraction method. Although, in the case of chromatographic methods, especially RP-TLC or RP-HPTLC, respectively a very small amount of sample and not very pure is needed. Numerous papers describe the determination of lipophilicity by both TLC techniques for different classes of biologically active compounds, such as mercaptopurine derivatives, phynylthioamides, alkaloids and others [4-10].
Recently, an alternative in prediction of the lipophilicity parameter (logP) is the use of in silico study [7,11]. Computationally determined partition coefficient has become crucial in preclinical study of newly synthesized drug candidates. Because of the fact that the computed methods of predication of logP are in development until today and show different power of calculation of this descriptor, in order to obtain reliable lipophilicity parameter, the computed logP values should be always compared with experimental values.
This work is continuation of our previous researches on lipophilicity determination of various biologically active compounds using the thin-layer chromatography methods (RP-TLC and RPHPTLC) as well as comparison of these results with computed logP and also with experimental value of logP obtained by means of shakeflask method [12-14]. In continuation of this assay, the aim of this study was the determination applicability of RP-HPTLC technique and both, Soczewinski-Wachtmeister’s and Oscik’s equations to predict measurable lipophilicity values of selected compounds which belong to three classes: fatty acids, esters of nicotinic acid and also bile acids. Although, comparison of two chromatographic parameters of lipophilicity which have been determined by Soczewinski- Wachtmeister’s and Oscik’s equations (denoted as RMWS and RMWO), respectively with that predicted by computer programs, such as AlogPS, IAlogP, ClogP, logPKOWWIN, xlogP and logPexp was done.
Experimental
Chemicals and reference standards: The reference standards of examined compounds included oleic acid, palmitic acid, elaidic acid, stearic acid belonging to fatty acids, esters of nicotinic acid: methyl nicotinate, ethyl nicotinate, butyl nicotinate, benzyl nicotinate, isopropyl nicotinate, hexyl nicotinate, and selected bile acids, such ascholic acid, deoxycholic acid, lithocholic acid, glycolithocholic acid, glycodeoxycholic acid, glycocholic acid and chenodeoxycholic acid were supplied by Sigma-Aldrich (St. Louis, MO, USA). Methanol which has been used as mobile phase component was purchased from POCh (Gliwice, Poland). Distillated water was from Institute of Analytical Chemistry (School of Pharmacy and the Division of Laboratory Medicine, Medical University of Silesia, Sosnowiec, Poland). Standard solutions of examined compounds at concentration of 5 mg/mL each were prepared in methanol or in the case of fatty acids in chloroform (from POCh, Gliwice, Poland). All reagents were of analytical grade of purity.
RP-HPTLC analysis
The TLC experiment was done by thin-layer chromatography on RP-HPTLC plates: RP-18WF254 (E. Merck, Darmstadt, Germany, and Art. 13124). The solutions of examined compounds were spotted separately onto chromatographic plates using micropipettes in quantity of 2μL. The chromatograms were developed using the mixtures of methanol-water in different volume compositions. The content of organic modifier (methanol) in mobile phase was gradually varied by 5% (v/v) from 40-100 (%, v/v). Fifty mL of mobile phase used was placed into a classical chromatographic chamber (Art. 022.5255, Camag, Muttenz, Switzerland). The chamber was saturated with solvent vapor for 30 minutes. The chromatograms were developed to distance 75 mm at temperature of 18 (±1) °C. After developing, the plates were dried at room temperature. Each chromatogram was done in triplicate. The spots were localized in UV at λ=254 nm with accuracy of ±1 nm in the case of the esters of nicotinic acid. The chromatograms of bile acids were previously sprayed with 10% ethanolic solution of phosphomolybdic acid and next heated at temperature 120oC for 20 minutes. In order to visualize the spots of fatty acids, exposition to iodine vapor was applied.
For subsequent calculations of lipophilicity parameters of all investigated compounds, mean RF values obtained for each chromatographic conditions were used.
Calculations
Chromatographic parameter of lipophilicity RMWS: In order to determine the lipophilicity parameter based on Soczewinski- Wachtmeister’s procedure the RF values obtained under applied chromatographic conditions were converted to RM values according to the expression: (1)Linear relationship between RMand volume content of methanol in mobile phase (ø) permits an extrapolation of calculated RM values to the zero concentration of methanol accordance with Soczewinski- Wachtmeister’s equation (2). The value of intercept (RMWS) represents the lipophilicity parameter of the studied compound [1].
RM = RMWS- S ·ø (2)
Where: RM - is the RM value of the examined compound, RMWS-is the RM value extrapolated to zero concentration of methanol (organic modifier) in mobile phase: methanol-water, S - is the slope of the regression plot, ø - is the volume fraction of methanol in mobile phase.
Chromatographic parameter of lipophilicity RMWO: Measurable lipophilicity value expressed as RMWO was determined according to Oscik’s equation [15-18]:
(3)
Where: RM, RMorg, RMWO are the solute retention factors in: mixed mobile phase, pure organic solvent, and water respectively; xorg is the molar fraction of organic solvent in the mobile phase; a, b are constants in the linear correlation between G(xorg) and xorg in mobile phase used.
In Oscik’s procedure various RMWO values are fitted numerically to Equation 3 in order to obtain linearity between G(xorg) and xorg. Other parameters, such as RM and RMorg are measured experimentally at 0.1 increments of xorg (in respective range of methanol) for appropriate compound. The linear relationship between G (xorg) vs. xorg allows an estimation ofRMWO of examined compound.
Determining the theoretical partition coefficients (logP): The computationally calculated logP values expressed as AlogPS, ClogP, IAlogP, logPKOWWIN, xlogP and also experimental logPexp for selected of examined compounds were determined by online software (available at VCCLAB.org website) [19].
Regression and simple cluster analysis (CA): Regression and simple cluster analysis of obtained results were performed with the use of computer program STATISTICA v. 10.0 (StatsoftPoland, Kraków, Poland).
Results and Discussion
The main aim of this study was to estimate the applicability of simple thin-layer chromatography method (RP-HPTLC) and also both, Soczewinski-Wachtmeister’s and Oscik’s equations to evaluate lipophilicity of selected biologically active compounds belonging to the three groups of organic compounds: fatty acids, esters of nicotinic acid and also bile acids. In order to predict the measurable values of lipophilicity parameters expressed as RMWS (accordance with Soczewinski-Wachtmeister’s equation) for the members of the three examined classes of organic compounds, the experimental data of RM values which have been obtained on silica gel RP-18WF254 using methanol-water in different volume compositions as mobile phase were extrapolated to an methanol content of zero by Equation 2. In all cases the equations were linear with correlation coefficient above 0.9. As was presented in experimental part, the intercepts in these equations can be considered as a measure of lipophilicity. All calculated intercepts have been accepted as lipophilicity descriptors (RMWS). Obtained lipophilicity parameters with the use of Soczewinski-Wachtmeister’s equation are listed in Table 1. As shown the lipophilicity of tested compounds is in agreement with their structure (non-polar character). RMWS results in Table 1 indicate that in the group of investigated fatty acids this parameter is the highest of all and is placed in the range of 4.07 to 5.06. In the case of bile acids the lipophilicity descriptor RMWS exists in the range from 2.43 to 5.29. For esters of nicotinic acid the RMWS is lower (from 1.25 to 2.87).
Substance
Lipophilicity parametrs
logPexp
AlogPS
IAlogP
ClogP
logP
KOWWIN
xlogP
RMWO
RMWS
Fatty acids
Oleic acid
-
7.51
7.48
7.79
7.73
6.50
7.15
4.07
Elaidic acid
-
7.51
7.48
7.79
7.73
6.50
8.88
5.06
Palmitic acid
-
6.90
7.00
7.21
6.96
6.09
7.26
4.20
Stearic acid
-
7.91
7.91
8.27
7.94
7.02
7.06
4.94
Esters of nicotinic acid
Methyl nicotinate
0.83
0.61
0.82
0.77
0.64
0.71
1.42
1.25
Ethyl
nicotinate
1.32
1.27
1.33
1.30
1.33
1.13
2.05
1.56
Isopropyl
nicotinate
-
1.65
1.64
1.61
1.55
1.59
2.20
1.69
Butyl
nicotinate
2.27
2.16
2.29
2.35
2.11
2.06
3.06
1.96
Hexyl
nicotinate
3.51
3.12
3.27
3.41
3.10
3.19
3.14
2.87
Benzyl
nicotinate
2.40
2.25
2.00
2.60
2.35
2.42
2.50
2.18
Bile acids
Lithocholic
acid
-
4.38
5.31
6.60
6.19
6.57
4.57
5.29
Deoxycholic
acid
3.50
3.30
3.26
4.51
5.06
5.76
3.76
3.96
Chenodeoxycholic acid
4.15
3.01
3.68
4.51
5.06
4.91
3.57
3.72
Glycolithocholic
acid
-
3.71
4.11
5.89
5.08
5.75
3.22
3.62
Cholic acid
2.02
2.26
2.12
2.43
3.52
4.09
2.91
4.42
Glycodeoxycholic acid
2.25
2.69
2.40
3.80
3.95
4.93
2.68
3.76
Glycocholic
acid
1.65
1.70
1.09
1.71
2.41
3.27
1.92
2.43
Table 1: Summary of lipophilicity study of examined compounds.
The second of discussed lipophilicity parameters (RMWO) was determined according to the methodology presented by Janicka et al. [16,17] on the basis of previously obtained RM values on silica gel RP-18WF254 using methanol-water. The RMWO values have been predicted for examined compounds in the three steps which are accurately illustrated in Figure 1, for example for ethyl nicotinate. In the first step (Figure 1(a)) various RMW values were fitted to Equation 3 with respective step in order to check if a linear relationship exists between the two variables Gorg and xorg. The second step examines the determination coefficient R2 (from Figure 1(b)) depending on RMWO. As can be seen, the values of R2 grow exponentially, next moderately, and finally they could be changed insignificantly in order to achieve unity. During the last step the relation R2 vs RMWO is derivated. Lipophilicity parameter RMWO is calculated by extrapolation of linear part of obtained graph (Figure 1(c)) in direction of zero changes of R2.
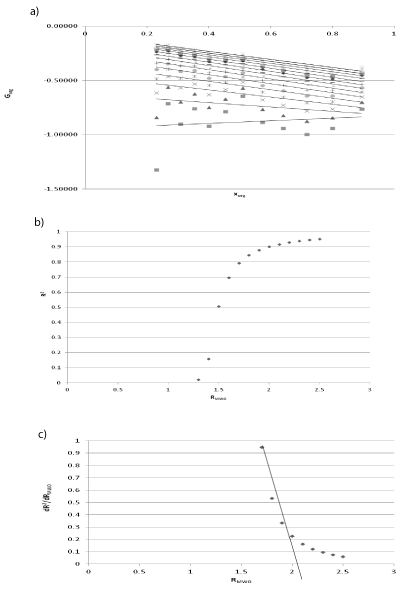
Figure 1: The relationship between G(xorg) and xorg (a), dependence between
R2 values and different values of RMWO (b), relationship between dR2/dRMW0
and RMWO (c) obtained for ethyl nicotinate.
Similar plots and procedures were done for other examined compounds. The values of RMWO estimated for all compounds studied are presented in Table 1.
The results of obtained RMWO values similarly like above mentioned RMWS confirm that the most lipophilic properties of all studied compounds show fatty acids and bile acids, which RMWO is placed in the range of 7.06 to 8.88 and from 1.92 to 4.57,respectively. The lipophilicity parameter RMWO determined for the esters of nicotinic acid ranged from 1.42 to 3.14.
In order to evaluate the possibility of the applying of both lipophilicity descriptors: RMWS and RMWO which have been calculated using Soczewinski-Wachtmeister’s and Oscik’s equations for the determination of the lipophilicity of all examined compounds, the results of obtained RMWS and RMWO values were compared with partition coefficient (logP) predicted by means of computer software: AlogPS, ClogP, IAlogP, logPKOWWIN, xlogP available at VCCLAB.org website (as Interactive analysis logP prediction). Moreover, in the case of the selected esters of nicotinic acids and also some bile acids this program enabled determine the experimental value of partition coefficient (logPexp) which was found by shake-flask method. The computationally calculated lipophilicity values expressed as logP and also experimental logPexp for appropriate compounds are summarized in Table 1.
To compare different calculation methods (AlogPS, ClogP, IAlogP, logPKOWWIN, xlogP) and also chromatographically determined RMWS and RMWO parameters (using Soczewinski-Wachtmeister’s and Oscik’s equations), simple cluster analysis of all obtained lipophilicity parameters for three groups of analyzed compounds was done.
Dendrogram in Figure 2(a) represents the results of cluster analysis (Euclidean distance) of lipophlicity parameters which have been determined for four examined fatty acids: oleic, elaidic, palmitic and also stearic. Figure 2(a) suggests that generally all theoretical lipophilicity parameters (logP) and also RMWO calculated using Oscik’s equation form one subgroup which confirms their similarity. The second, single subgroup in this dendrogram forms RMWS. Among partition coefficients predicted using different calculation methods the biggest similarity show IAlogP and AlogPS. Observed big similarity between both theoretical parameters of lipophilicity of studied fatty acids confirms that IAlogP could be successfully replaced by AlogPS in lipophilicity study of these substances. Of two chromatographically determined lipophilicity descriptors, the lipophilicity parameter RMWO indicates better agreement with theoretical partition coefficients than RMWS calculated using Soczewinski-Wachtmeister’s equation. Thus, it could be concluded that simple thin-layer chromatography in reversed phase system (RP-HPTLC) and experimentally determined (using Oscik’s equation), lipophilicity parameter RMWO is a good alternative to the theoretical values of lipophilicity parameters and also to other experimental partition coefficients whose determination by extraction method, especially in the case of very lipophilic compounds, like for example fatty acids is too difficult or impossible.
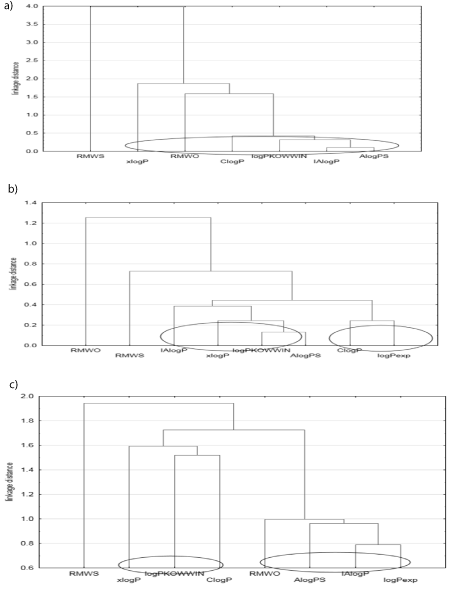
Figure 2: Dendrogram of simple cluster analysis of lipophilicity parameters
determined by different methods for fatty acids (a), esters of nicotinic acid (b)
and also for bile acids (c).
Interpretation of the second dendrogram (Figure 2(b)) which performs the results of simple cluster analysis of the lipophilicity parameters (theoretical and chromatographic) obtained for the next group of investigated organic compounds belonging to esters of nicotinic acid indicates that in this case, the RMWS demonstrates bigger similarity with other parameters of lipophilicity including experimental partition coefficient (logPexp) which is determined by shake-flask method than RMWO. Among computed logP values, great similarity (the smallest Euclidean distance) indicates AlogPS and logPKOWWIN but the most similar to known in literature experimental partition coefficient values (logPexp) for all examined esters (except of isopropyl nicotinate) is ClogP which forms with this theoretical partition coefficient exactly one subgroup.
The last dendrogram in Figure 2(c) refers to cluster analysis of the lipophilicity parameters of the third of examined groups, namely bile acids. Comparison of theoretical logP with chromatographically determined lipophilicity descriptors, such as RMWO and RMWS and also with logPexp (except of lithocholic and glycolithocholic acids) shows that all lipophilicity parameters could be divided into two main subgroups. The first subgroup form xlogP, logPKOWWIN and ClogP. The second one includes AlogPS, IAlogP, logPexp and RMWO. The biggest similarity indicates IAlogP and logPexp. Thus, it suggests that logPexp may be successfully replaced by IAlogP. Moreover, analogously like in the case of fatty acids better correlation with computed logP demonstrates RMWO comparing with RMWS.
Taking into account the observed agreement between the RMWO or RMWS values, respectively and also computational lipophility descriptors (logP) obtained for the three examined groups of biologically active compounds, such as fatty acids, esters of nicotinic acid and bile acids, it may be concluded that RMWO appears to be the most suited lipophilicity descriptor for the class of organic compounds which show very high lipophilicity like for example fatty acids and bile acids(examined in the present study). In the case of active compounds indicating intermediary lipophilicity or very low lipophilicity, such as the esters of nicotinic acid, the second of two proposed lipophilicity parameters denoted as RMWS seems to be most useful. The results of presented study confirm that the RMWS and RMWO may be selected and considered as reliable lipophilicity measures of different classes of biologically active compounds including that which show strong lipophilic properties, like for instance the two examined groups: fatty acids and bile acids.
Conclusion
The results obtained in the present study show that:
- Partition coefficient (logP) predicted by means of different computational algorithms (AlogPS, IAlogP, ClogP, logPKOWWIN, xlogP) can provide useful information about lipophilic character of various classes of biologically active compounds i.e., fatty acids, esters of nicotinic acid and bile acids but in their preliminary study only,
- RP-HPTLC method and lipophilicity parameters denoted by RMWS and RMWO which have been calculated based on retention parameters (RM) accordance with Soczewinski- Wachtmeister’s and Oscik’s equations, respectively may be the alternates to others like for example to logP determined by shake-flask method in describing of lipophilic character of active compounds belonging to the following classes: fatty acids, esters of nicotinic acid and also bile acids,
- The RMWO values could be suitable for the determination of lipophilic character of biologically active compounds with high lipophilicity (i.e., fatty acids and bile acids),
- In the case of active substances with lower lipophilicity, RMWS proved to be more reliable in describing of their lipophilic character than RMWO.
Acknowledgment
This research was financed by the Medical University of Silesia in Katowice.
References
- Rutkowska E, Pajak K, Jozwiak K. Lipophilicity--methods of determination and its role in medicinal chemistry. Acta Pol Pharm. 2013; 70: 3-18.
- Tesic ZLj, Milojkovic-Opsenica DM. TLC Determination of Drug Lipophilicity. In: Thin Layer Chromatography in Drug Analysis; Komsta L. Waksmundzka-Hajnos M, Sherma J. Eds. Boca Raton: CRC Press Taylor & Francis Group. 2014; 225-246.
- Tsantili-Kakolidou A. Lipophilicity: Assessment by RP/TLC and HPLC. In: Encyclopedia of Chromatography, 3rd edn. Cazes J, editor. New York, USA, Taylor & Francis. 2010; 1400-1404.
- Kostecka M. Comparison of methods used for estimation of lipophilicity of biologically active phenylthioamides. Ann Univ. Mariae Curie-Sklodowska, Lublin - Poloniae. 2009; 64: 291-299.
- Hawryl A, Kusmierz E, Pisarczyk P, Wujec M, Waksmundzka-Hajnos M. Determination of the lipophilicity of some new thiosemicarbaside derivatives by reversed-phase thin-layer chromatography. Acta Chromatogr. 2012; 24: 271-290.
- Nascu-Briciu RD, Sarbu C. Lipophilicity of oils and fats estimated by TLC. J Sep Sci. 2013; 36: 1317-1326.
- Kalasz H, Benko B, Gulyas Zs,Tekes K. Lipophilicity determination using both TLC and calculations. J Liq Chromatogr Relat Technol. 2009; 32: 1342-1358.
- Odovic JV, Markovic BD, Trbojevic-Stankovic JB. Vladimirov SM, Karljikovic-Rajic KD. Evaluation of ACE inhibitors lipophilicity using in silico and chromatographically obtained hydrophobicity parameters. Hem Ind. 2013; 67: 209-216.
- Komsta L, Skibinski R, Berecka A, Gumieniczek A, Radkiewicz B, Radon M. Revisiting thin-layer chromatography as a lipophilicity determination tool--a comparative study on several techniques with a model solute set. J Pharm Biomed Anal. 2010; 53: 911-918.
- Starek M, Komsta L, Krzek J. Reversed-phase thin-layer chromatography technique for the comparison of the lipophilicity of selected non-steroidal anti-inflammatory drugs. J Pharm Biomed Anal. 2013; 85: 132-137.
- Kujawski J, Popielarska H, Myka A, Drabinska B, Bernard MK. The logP parameter as a molecular descriptor in the computer-aided drug design – an overview. CMST. 2012; 18: 81-88.
- Dolowy M. Comparative study of the lipophilicity of selected tauro-conjugates bile acids determined with use of RPTLC and RPHPTLC methods. J Liq Chromatogr Relat Technol. 2009; 32: 2281-2292.
- Dolowy M, Miszczyk M, Pyka A. Application of various methods to determine the lipophilicity parameters of the selected urea pesticides as predictors of their bioaccumulation. J Environ Sci Health B. 2014; 49: 730-737.
- Dolowy M, Pyka A. Lipophilicity study of salicylic and acetylsalicylic acids using both experimental and calculations methods. J Liq Chromatogr Relat Technol. 2015; 38: 485-491.
- Jaroniec M, Oscik J. Liquid-solid chromatography. Recent progress in theoretical studies concerning the dependence of the capacity ratio upon the mobile phase composition. J High Resol Chromatogr. Chromatogr Commun. 1982; 5: 3-12.
- Janicka M. Application of Oscik’s equation of description of solute retention in RP HPLC and calculation of retention factor in water. J Liq Chromatogr & Rel Technol. 2009; 32: 2779-2794.
- Kwietniewski L. Determination of solute retention factors in RPLC with pure water as eluent using a numerical method based on the Oscik’s equation. J Liq Chromatogr Relat Technol. 2010; 33: 305-323.
- Janicka M, Kwietniewski L, Matysiak J. A new method for estimation log kw values and solute biological activity. J Planar Chromatogr - Modern TLC. 2000; 13: 285-289.
- Tetko IV, Tanchuk VJ. VCC - Lab, Interactive analysis logP prediction. 2002.