
Research Article
Austin Chromatogr. 2015;2(2): 1030.
Simple Determination of 17a-Ethynylestradiol in Hair Restorer by High-Performance Liquid Chromatography Coupled with Fluorescence Detection After Pre-Column Derivatization 4-(N-Chloroformylmethyl-N Methylamino)-7-Nitro-2,1,3-Benzoxadiazole
Yasuhiko Higashi*
Department of Analytical Chemistry, Hokuriku University, Japan
*Corresponding author:Yasuhiko Higashi,Department of Analytical Chemistry, Faculty of Pharmaceutical Sciences, Hokuriku University, Ho-3, Kanagawa-machi, Kanazawa 920-1181, Japan
Received: February 19, 2015; Accepted: March 16, 2015; Published: March 24, 2015
Abstract
The concentration of 17a-Ethynylestradiol (EE2) in hair restorer was analyzed by High-Performance Liquid Chromatography (HPLC) coupled with fluorescence detection (excitation wavelength, 470 nm; emission wavelength, 540 nm) after pre-column derivatization with 4-(N-chloroformylmethyl-Nmethylamino)- 7-nitro-2,1,3-benzoxadiazole (NBD-COCl). Derivatization with NBD-COCl was performed in borate buffer (pH 9.0) at room temperature for 3 min. The HPLC column was 150 mm×3.0 mm i.d., containing 5 μm particles of C22 packing material, and the mobile phase was prepared by addition of acetonitrile (580 mL) to 420 mL of Milli-Q water containing trifluoroacetic acid (0.1 v/v%). The sample was eluted from the column at room temperature at a flow rate of 0.5 mL/min, and the retention time of NBD-CO-EE2 was 10.7 min. The calibration plot was linear in the range of 0.05 to 1 μg/mL with an r2 value of 0.9952, and the lower limit of detection was 0.010 μg/mL (at a signal-to-noise ratio of 3:1, absolute amount of 0.050 ng/20 μL injection). The coefficient of variation was less than 9.5%. None of three tested estrogens (estriol, β-estradiol, estrone) interfered with the NBD-CO-EE2 peak. It was found that the content of EE2 in hair restorer was 14.3±0.9 μg/mL (range, 13.3 to 15.9 μg/mL). Recovery in addition-recovery tests was within the range of 70.5 to 98.4%.
Keywords: 17a-Ethynylestradiol; High-performance liquid chromatography; Fluorescence; 4-(N-Chloroformylmethyl-N-methylamino)-7-nitro-2,1,3-benzoxadiazole; Derivatization; Hair restorer
Abbreviations
EE2: 17a-Ethynylestradiol; HPLC: High-Performance Liquid Chromatography; FL: Fluorescence Detection; NBD-COCl: 4-(N-Chloroformylmethyl-N-methylamino)-7-nitro-2,1,3- benzoxadiazole; GC: Gas Chromatography; MS: Mass Spectrometry; E3: Estriol; E2: β-Estradiol; E1: Estrone
Introduction
17a-Ethynylestradiol (EE2, 17a-ethynyl-1,3,5(10)-estratriene-3,17β-diol) is a synthetic female hormone that is sometimes usedin hair restorer and other cosmetics to promote hair growth, skin suppleness, and so on. But, it can have serious side effects. For example, it was reported that a 36-month-old girl presented with vaginal bleeding and uterus enlargement after she had played with her mother’s combs and empty vials that had contained hair lotion in which EE2 was present at the concentration of 0.5% [1]. Komori et al. suggested that long-term application of cosmetic cream containing EE2 to the face and body three times a day for 75 years might have been related to development of breast cancer and endometrial hyperplasia in a 93-year-old woman [2]. Since EE2 at extremely low levels may induce gynaecological side effects, a simple and sensitive analytical method for EE2 determination is needed for routine quality control to ensure the safety of personal care products.
Assay
Content (mg/mL)
Recovery (%)
Added (1.50 mg)
Added (3.00 mg)
Day 1
15.9
74.1
89.8
Day 2
13.6
87.7
98.4
Day 3
14.3
84.6
94.8
Day 4
14.2
70.5
93.7
Day 5
14.6
75.7
90.2
Day 6
14.3
76.4
93.5
Ave. ± S.D. (R.S.D.)
14.3 ± 0.9 (6.3%, n=6)
85.8 ± 9.4% (11.0%, n=12)
Table 3: Level of EE2 in hair restorer and recovery of spiked EE2.
Various methods for EE2 determination have been reported, including High-Performance Liquid Chromatography (HPLC) or Gas Chromatography (GC) coupled with Mass Spectrometry (MS) [3-9]. The systems using MS are very sensitive, and have been employed for determination of EE2, other female hormones, and endocrine disrupters in river, sea, and waste water. However, MS is relatively expensive and complicated. In addition, some of the reported methods needed large volumes of sample (more than 1 L) [4,6]. An enzyme-linked immunosorbent assay for quantification of EE2 was also developed [10]. This method is simple and sensitive, but suffers from cross-reactivity, large standard deviation, and the lack of commercially available antiserum. Recently, micro fluidic immunoassay methodology for analysis of EE2 was established for sensitive determination of EE2 in river water samples [11]. In addition, sensitive methods for EE2 analysis by HPLC with Fluorescence detection (FL) have been developed [12-14], including a method employing dansyl chloride derivatization for EE2 analysis in pharmaceutical tablets [14].
However, for determination of EE2 in routine quality control of cosmetic products, we required a rapid, simple, convenient and inexpensive method. It was considered that an HPLC–FL method would be most appropriate, since the equipment is inexpensive, widely available and easy to use, and derivatization with a fluorescent labeling agent generally offers good selectivity and sensitivity. For derivatization, it was focused on 4-(N-chloroformylmethyl-Nmethylamino)- 7-nitro-2,1,3- benzoxadiazole (NBD-COCl), because it has already been used as a fluorescent labeling agent of primary and secondary amino groups and phenolic hydroxyl group for HPLC–FL [15-17], and it should also react with the phenolic hydroxyl group of EE2. In this paper, a simple HPLC–FL method for determination of EE2 in hair restorer after pre-column derivatization with NBD-COCl is presented. The derivatization scheme is illustrated in Figure 1.
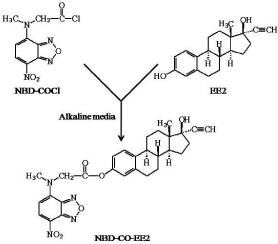
Figure 1: Scheme of EE2 derivatization with NBD-COCl.
Material and Methods
Reagents
EE2, estriol (E3), β-Estradiol (E2), and Estrone (E1) were purchased from Sigma-Aldrich Co. (St. Louis, MO, U.S.A.). NBDCOCl was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Hair restorer was purchased from a market in Kanazawa city, Ishikawa Pref., Japan. Although EE2 was stated to be present on the container label, the concentration was not given. Other general reagents were obtained from Wako Pure Chemical Industries (Osaka, Japan).
Equipment
The HPLC system comprised a model L-6200 pump (Hitachi, Tokyo, Japan), a Rheodyne injection valve (Cotati, CA, USA) with a 20- μL loop and a model RF-10A fluorometer (Shimadzu, Kyoto, Japan) operating at an excitation wavelength of 470 nm and an emission wavelength of 540 nm. The HPLC column (C22-MS-II, Nacalai tesque, Kyoto, Japan) was 150 mm×3.0 mm i.d., containing 5 μm particles of C22 packing material. Quantification of peaks was performed using a Chromatopac Model C-R3A integrator (Shimadzu). The mobile phase was prepared by the addition of acetonitrile (580 mL) to 420 mL of Milli-Q water containing trifluoroacetic acid (0.1 v/v%). The samples were eluted from the column at room temperature at a flow rate of 0.5 mL/min.
Derivatization
Ultrapure water was from a Milli-Q water purification system (Simplicity® UV, Millipore Corporation, Bedford, MA, USA). A standard solution of EE2 (2 mg) in methanol (50 mL) was prepared and stocked at 4oC. Working standard solutions (0, 0.05, 0.1, 0.25, 0.5, and 1 μg/mL) were prepared by dilution with 10% methanol. Borate buffer (0.1 M) was adjusted to pH 9.0 by the addition of NaOH. Borate buffer (50 μL) was added to a diluted standard sample (50 μL), then NBD-COCl solution in acetonitrile (50 μL, 3 mg/mL) was added. The mixture was vortexed and allowed to react for 3 min at room temperature, and then ice-cold HCl solution (50 μL, 0.1 M) was added to stop the reaction. An aliquot of 20 μL was injected into the HPLC system.
Sample preparation and addition-recovery tests
An aliquot of hair restorer (200 μL) was diluted to 20 ml with 10% methanol, derivatized, and analyzed as described above. Additionrecovery tests were carried out to assess the accuracy of the method by spiking hair restorer (200 μL) with EE2 (1.50 or 3.00 μg), and diluting it in the same manner. An aliquot of 50 μL was analyzed and the EE2 concentration in the sample was determined. Recovery was calculated as follows.
Results and Discussion
For the time course study, the reaction time was set at 1, 2, 3, 5, 7, or 10 min at room temperature. EE2 (50 μL, 1 μg/mL), borate buffer (50 μL, pH 9.0), and NBD-COCl (50 μL, 3 mg/mL) were mixed as described in Derivatization. The derivatization of EE2 reached a maximum at 3 min (Figure 2). Next, pH dependency (pH 8.0 to 10.0) was examined at the derivatization time of 3 min at room temperature. The peak area of NBD-CO-EE2 was maximal at pH 9.0, and it was considerably reduced at pH 8.0 (Figure 3). In addition, the temperature dependency was examined at 3 min and pH 9.0. Peak areas at 4°C (on ice), room temperature, 35°C, 45°C, and 55°C were 1082, 1459, 1360, 1103, and 998 mV×s, respectively (mean values of two experiments). The peak area of NBD-CO-EE2 was maximum when the derivatization was performed at room temperature. Thus, the derivatization time of 3 min at pH 9.0 and room temperature was selected. Additionally, in preliminary test, peak area of NBD-CO- EE2 using C22-MS-II column was tended to slightly increase compared with C18-MS-II column (150 mm×3.0 mm i.d., containing 5 μm particles of C18 packing material, Nacalai tesque), and separated from the later blank noise peak at 11.3 min (data not shown). Therefore, C22-MS-II column was chosen as an analytical column.
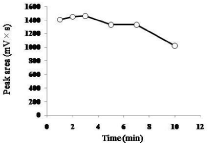
Figure 1: Time course of formation of NBD-CO derivative of EE2. A standard
sample (1 μg/mL, 5 ng/20 μL of injection) was reacted with NBD-COCl in
borate buffer at pH 9.0 at room temperature. Data are expressed as mean
values of two experiments.
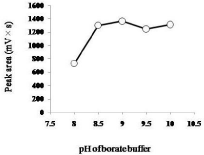
Figure 3: pH dependency of the formation of NBD-CO derivative of EE2. A
standard sample (1 μg/mL, 5 ng/20 μL of injection) was reacted with NBDCOCl
for 3 min in various borate buffers at room temperature. Data are
expressed as mean values of two experiments.
Figure 4 shows typical chromatograms obtained from (A) blank and (B) standard sample (0.5 μg/mL). The retention time of NBDCO- EE2 was 10.7 min.
A standard curve was constructed by plotting integrated peak area vs. concentration of EE2. The plot was linear (y = 1531x - 51.33) in the range of 0.05 to 1 μg/mL with an r2 value of 0.9952. The values of the lower limits of quantification and detection were 0.033 μg/mL (absolute amount of 0.17 ng/20 μL injection, signal-to-noise ratio of 10:1) and 0.010 μg/mL (absolute amount of 0.050 ng/20 μL injection, signal-to-noise ratio of 3:1), respectively. As shown in Table 1, the sensitivity of the author’s method is considerably inferior to those of previous HPLC– or GC–MS methods [3-9], two immunoassays [10,11], and the HPLC–FL method of Matsumoto et al. [13], but is similar to those of previously reported HPLC–FL methods [12,14].
Method
Limit of detection
Reference
HPLC-MS
1.4 ng/L**
[3]
HPLC-MS
5×10-3 ng*; 0.45 ng/L**
[4]
Ultra HPLC-MS
1.2 ng/L**
[5]
HPLC-MS
6 ng/L**
[6]
HPLC-MS
10.5 ng/L**
[7]
GC-MS
5.0 ng/L**
[8]
GC-MS
1 ng/L**
[9]
Enzyme-linked immunosorbent assay
14 ng/L**
[10]
Microfluidic immunoassay
0.006 ng/L**
[11]
HPLC-FL
0.0566 ng*; 283 ng/L**
[12]
HPLC-FL
6.5×102 ng/L**
[13]
HPLC-FL
0.04 ng*
[14]
HPLC-FL
0.050 ng*; 1.0×104 ng/L**
This paper
The limit of detection was expressed as absolute amount (ng)* and/or concentration (ng/L)**.
Table 1: Sensitivity of various methods for determination of EE2.
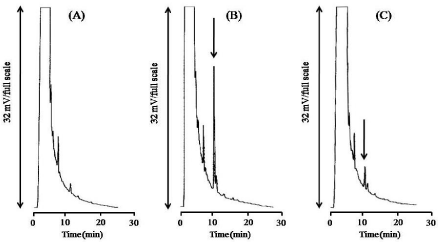
Figure 4C: Typical chromatogram of hair restorer sample (C) after
derivatization with NBD-COCl. The test sample was reacted with NBD-COCl
for 3 min at pH 9.0 at room temperature. NBD-CO-EE2 peak (arrowed peak)
was detected at 10.7 min.
Precision and accuracy for intra-day and inter-day assays of EE2 are shown in Table 2. In the intra-day assay, the range of standard deviation was within 4.5 to 8.4% of the mean, and recoveries were within the range of 90.4 to 98.0%. In the inter-day assay, the range of standard deviation was within 5.8 to 9.5% of the mean, and recoveries were within the range of 88.0 to 96.4%. The accuracy and precision was satisfactory in the tested concentration. However, the data showed the high C.V. value and low recovery on the assays at 0.05 μg/mL. It was considered that a blank peak at 11.3 min slightly would interfere with NBD-CO-EE2 peak.
Concentration
(mg/mL)Measured
(mg/mL, Mean±S.D., n=5)C.V.
(%)Recovery
(%)Intra-day
0.05
0.0452 ± 0.0038
8.4
90.4
0.25
0.232 ± 0.013
5.6
92.8
1
0.980 ± 0.044
4.5
98
Inter-day
0.05
0.0440 ± 0.0042
9.5
88
0.25
0.228 ± 0.015
6.6
91.2
1
0.964 ± 0.056
5.8
96.4
Table 2: Intra- and Inter-day assay reproducibility for determination of EE2.
Interference with the detection of NBD-CO-EE2 by E3, E2, and E1 (each 1 μg/mL) was investigated. Derivatization was performed as described in Derivaization. The retention times of NBD-CO-E2 and NBD-CO-E1 were 8.3 and 11.8 min, respectively. On the other hand, the NBD-CO-E3 peak was not observed, because it overlapped with a large blank peak. None of the three tested estrogens interfered with the NBD-CO-EE2 peak.
Figure 4C shows a typical chromatogram obtained from a sample of hair restorer. The peak of NBD-CO-EE2 was detected at 10.7 min, and the concentration of EE2 in the sample was determined as follows.
The described method was used to determine EE2 in the restorer and in samples spiked with standard compound. As shown in Table 3, the concentration of EE2 in the restorer was found to be 14.3 ± 0.9 μg/mL (range, 13.3 to 15.9 μg/mL). Recovery of spiked EE2 from the restorer was 85.8 ± 9.4% (range, 70.5 to 98.4%). The restorer contained EE2 at the level of 1.72 ± 0.11 mg per package. The present system is able to detect EE2 in hair restorer when the content is more than 108 μg per package (more than 0.9 μg/mL).
Conclusion
An HPLC–FL method for determination of EE2 in hair restorer by using NBD-COCl as a fluorescent labeling reagent has been developed A tested hair restorer was found to contain EE2 at the mean concentration of 14.3 μg/mL using the present system. This method is simple, convenient and inexpensive, and should be suitable for routine quality assessment of cosmetics.
References
- Guarneri MP, Brambilla G, Loizzo A, Colombo I, Chiumello G. Estrogen exposure in a child from hair lotion used by her mother: clinical and hair analysis data. Clin Toxicol (Phila). 2008; 46: 762-764.
- Komori S, Ito Y, Nakamura Y, Aoki M, Takashi T, Kinuta T, et al. A long-term user of cosmetic cream containing estrogen developed breast cancer and endometrial hyperplasia. Menopause. 2008; 15: 1191-1192.
- Moreira M, Aquino S, Coutrim M, Silva J, Afonso R. Determination of endocrine-disrupting compounds in waters from Rio das Velhas, Brazil, by liquid chromatography/high resolution mass spectrometry (ESI-LC-IT-TOF/MS). Environ Technol. 2011; 32: 1409-1417.
- Beck IC, Bruhn R, Gandrass J, Ruck W. Liquid chromatography-tandem mass spectrometry analysis of estrogenic compounds in coastal surface water of the Baltic Sea. J Chromatogr A. 2005; 1090: 98-106.
- Sun L, Yong W, Chu X, Lin JM. Simultaneous determination of 15 steroidal oral contraceptives in water using solid-phase disk extraction followed by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2009; 1216: 5416-5423.
- Pailler JY, Krein A, Pfister L, Hoffmann L, Guignard C. Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg. Sci Total Environ. 2009; 407: 4736-4743.
- Mitani K, Fujioka M, Kataoka H. Fully automated analysis of estrogens in environmental waters by in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2005; 1081: 218-224.
- Duong CN, Ra JS, Cho J, Kim SD, Choi HK, Park JH, et al. Estrogenic chemicals and estrogenicity in river waters of South Korea and seven Asian countries. Chemosphere. 2010; 78: 286-293.
- Trinh T, Harden NB, Coleman HM, Khan SJ. Simultaneous determination of estrogenic and androgenic hormones in water by isotope dilution gas chromatography-tandem mass spectrometry. J Chromatogr A. 2011; 1218: 1668-1676.
- Schneider C, Scholer HF, Schneider RJ. A novel enzyme-linked immunosorbent assay for ethynylestradiol using a long-chain biotinylated EE2 derivative. Steroids. 2004; 69: 245-253.
- Martinez NA, Schneider RJ, Messina GA, Raba J. Modified paramagnetic beads in a microfluidic system for the determination of ethinylestradiol (EE2) in river water samples. Biosens Bioelectron. 2010; 25: 1376-1381.
- Yoon Y, Westerhoff P, Snyder SA, Esparza M. HPLC-fluorescence detection and adsorption of bisphenol A, 17beta-estradiol, and 17alpha-ethynyl estradiol on powdered activated carbon. Water Res. 2003; 37: 3530-3537.
- Matsumoto K, Tsukahara Y, Uemura T, Tsunoda H, Kume H, Kawasaki S, et al. Highly sensitive time-resolved fluorometric determination of estrogens by high-performance liquid chromatography using a beta-diketonate europium chelate. J Chromatogr B. 2002; 773: 135-142.
- Roos RW. High-pressure liquid chromatographic analysis of estrogens in pharmaceuticals by measurement of their dansyl derivatives. J Pharm Sci. 1978; 67: 1735-1739.
- Guo X, Fukushima T, Li F, Imai K. Determination of fluoxetine and norfluoxetine in rat plasma by HPLC with pre-column derivatization and fluorescence detection. Biomed Chromatogr. 2003; 17: 1-5.
- Guo X, Fukushima T, Li F, Imai K. Determination of fluoxetine enantiomers in rat plasma by pre-column fluorescence derivatization and column-switching high-performance liquid chromatography. Analyst. 2002; 127: 480-484.
- Higashi Y, Konno K. Simple determination of o-phenylphenol in skin lotion by high-performance liquid chromatography coupled with fluorescence detection after pre-column derivatization with 4-(N-chloroformylmethyl-N-methylamino)-7-nitro-2,1,3-benzoxadiazole. J Cosmet Sci. 2015.
Citation: Higashi Y. Simple Determination of 17a-Ethynylestradiol in Hair Restorer by High-Performance Liquid Chromatography Coupled with Fluorescence Detection After Pre-Column Derivatization 4-(N-Chloroformylmethyl- N-Methylamino)-7-Nitro-2,1,3-Benzoxadiazole. Austin Chromatogr. 2015;2(2): 1030. ISSN: 2379-7975