
Research Article
Austin Chromatogr. 2015;2(3): 1034.
Comparison of the Detection Limit and the Quantification Limit of Nicotinamide on Different Chromatographic Sorbents in TLC
Malgorzata Dolowy¹, Alina Pyka¹* and Milena Pluta²
¹Department of Analytical Chemistry, Medical University of Silesia in Katowice, Poland
²School of Pharmacy and the Division of Laboratory Medicine in Sosnowiec, Medical University of Silesia, Poland
*Corresponding author:Alina Pyka, Department of Analytical Chemistry, School of Pharmacy and the Division of Laboratory Medicine in Sosnowiec, Medical University of Silesia in Katowice, 4 Jagiellonska St., PL- 41-200, Sosnowiec, Poland
Received: April 09, 2015; Accepted: May 28, 2015; Published: June 04, 2015
Abstract
TLC combined with densitometry was successfully applied to determine the Detection Limit (LOD) and also Quantification Limit (LOQ) of nicotinamide. Average values of LODs and LOQs were determined on the basis of the standard deviation of the response and the slope of specific calibration curve according to the procedure recommended by ICH guideline. Influence of chromatographic conditions such as stationary phase (sorbent) and also mobile phase composition on determining of LOD and LOQ values of examined nicotinamide by NP-TLC and RP-TLC systems was estimated. Of all chromatographic plates and mobile phases used, those which give the best results (the lowest LOD and LOQ values) by NP-TLC system is silica gel 60F254 plates developed with methanol as mobile phase: LOD=0.088 μg/spot, LOQ=0.265 μg/spot. In the case of RP-TLC method the three of four applied mobile phases: dioxane, methanol, and ethanol enable detection of nicotinamide at the lowest level on chromatographic plates precoated with RP18F254: LOD ranges from 0.077 to 0.183 μg/spot. The LOQ value is placed in the range from 0.233 to 0.553 μg/spot.The results of LODs and LOQs indicate that the proposed TLC-densitometric method is suitable for the detection and also quantification of nicotinamide at different level.
Keywords: Nicotinamide; Vitamin PP; TLC-densitometry; LOD; LOQ
Introduction
Nicotinamide (known as Vitamin PP) is as member of vitamin B group (vitamin B3) [1]. This bioactive compound is commercially available in various pharmaceutical dosage forms (i.e., tablets, intramuscular and intravenous injection) and also diet supplements. It is widely used for the treatment of central nervous system disorders, cancer therapy and also in synthesis of some steroid hormones [2-6]. Different analytical methods, such High Performance Liquid Chromatography in Reversed Phase System (RP-HPLC), spectrophotometry, photochemical spectrofluorometry and also micellar chromatography were found to be satisfactory to assay of nicotinamide in different simple and complex tablets [7- 10]. These methods are useful for the determination of nicotinamide in pharmaceutical formulations but they are tedious or expensive because required large amount of solvents like for example HPLC. Therefore, it is very important to find a simple and sensitive analytical method for determining nicotinamide in appropriate amount, like for example Thin-Layer Chromatography combined with densitometry (TLC-densitometry). The measure of sensitivity of each analytical method including TLC-densitometry is Detection Limit (LOD) and also Quantification Limit (LOQ). Many of scientific papers and guidelines are focused on the procedure of calculations of the two parameters [11-16]. According to the guideline on Validation of analytical procedures (ICH Harmonised Tripartite Guidelines) numerous methods for determining of LOD and LOQ values are possible [11]. The choice of calculating procedure depends on the type of analytical method used: if is it instrumental or non-instrumental. Generally, the following approaches for determining of LODs and LOQs are acceptable [11]:
- Based on visual evaluation, which is suitable for noninstrumental methods;
- Based on signal to noise (usually applied to analytical procedure which indicates signal noise). LOD and LOQ values are determined by comparison of measured signals from samples (having accurately known low concentration of analyte) with blank sample. To determine the LOD a signal to noise ratio between 3 or 2:1 is acceptable. A typical signal to noise ratio in the case of determining of LOQ value is 10:1;
- Based on the standard deviation of the response and the slope. Using this procedure LOD may be calculated as:
- Standard deviation of intercept of specific regression line (sa)
- Residual standard deviation of regression line (sxy).
LOQ value can be estimated by use of formula:
Where:
S - is the slope of the calibration curve
s- is the standard deviation of the response.
The slope (S) could be calculated on the basis of specific calibration curve prepared for series standard solutions of analyte.
To estimate the standard deviation (s) the following parameters are applied:
It is well known that the choice of each analytical method including TLC for assay a proper compound as active ingredient as well as impurity present in pharmaceutical or biological matrix depends strongly on its sensitivity, thus on LOD and LOD values. Therefore the aim of this study was to determine the LODs and LOQs of nicotinamide by the use of TLC-densitometry under different chromatographic conditions (various sorbents and mobile phases) in Normal Phase and Reversed Phase Systems (NP-TLC and RPTLC). Influence of all applied stationary phases and mobile phases on LODs and LOQs, so on sensitivity of proposed method for detection of this compound was estimated. Present study is continuation of previous researches by Pyka et al.[17-23] concerning the application of TLC-densitometry in the determination of chemical stability and also separation of various nicotinic acid derivatives. Our last paper confirms the applicability of RPHPTLC-densitometric method with the use of silica gel 60 RP18WF254 as stationary phase and mixture of methanol-water (3:7, v/v) for the quantification of nicotinamide in selected pharmaceutical formulations [23]. In the present study evaluation of the LOD and LOQ study of examined nicotinamide was conducted by the use of two TLC systems. NP-TLC method was performed on five chromatographic plates: silica gel 60 F254 (Art. 1.05554), silica gel 60 (Art. 1.05553), a mixture of silica gel 60 and kieselguhr F254 (Art. 1.05567), aluminum oxide 60 F254 (Art. 1.05550) and aluminum oxide 150 F254 (Art. 1.05551). The plates were developed using the mixtures containing methanol and acetone, respectively. In the case of RP system two different sorbents (silica gel RP18F254s, silica gel RP8F254s) and four mobile phases consisted of methanol, ethanol, propanol and dioxane was applied. Comparison of LODs and LOQs obtained in both chromatographic systems (NP-TLC and RP-TLC) was done. Present work can be a useful indicator for the choice of chromatographic conditions of all described enabling detection of nicotinamide by TLC-densitometry in RP-TLC and also NP-TLC with appropriate sensitivity (at needed level) which is enough for pharmaceutical analysis preparations containing this compound.
Experimental
Standard and chemicals
Reference standard of investigated nicotinamide (NAM) was procured from Sigma-Aldrich (St. Louis, MO, USA). Ethanol absolute (=99.8%) and other solvents which have been applied as mobile phase compositions, such as dioxane, propanol, methanol, acetone and methanol were obtained from POCh (Gliwice, Poland). All chemicals and reagents were analytical grade.
Materials
The following chromatographic plates were used in this study:
- NP-TLC aluminum plates: 10 cm x 20 cm precoated with 0.20 mm layers of the following sorbents: aluminum oxide 60F254 (Art. 1.05550, lot. # 440148, Merck, Darmstadt, Germany), aluminum oxide 150 F254 (Art. 1.05551, lot. #97278204, Merck, Darmstadt, Germany), silica gel 60 (Art. 1.05553, Merck, lot. #HX743154, Darmstadt, Germany), silica gel 60 F254 (Art. 1.05554, lot. #HX616692, Merck, Darmstadt, Germany) and also a mixture of silica gel 60 and kieselguhr 60 F254 (Art. 1.05567, lot.# 22722797, Merck, Darmstadt, Germany).
- RP-TLC plates: 10 cm x 20 cm, aluminum, precoated with 0.20 mm layer of silica gel RP18F254s (Art. 1.05559, lot. # HX716967, Merck, Darmstadt, Germany) and 10 cm x 20 cm, glass plates precoated with 0.20 mm layer of silica gel RP8F254s (Art. 1.15424, lot. # OB549661, Merck, Darmstadt, Germany).
- the 5 μL Camag micropipettes (Muttenz, Switzerland) were used to apply the solutions to the plates,
- Densitometer TLC Scanner 3 with WinCATS 1.4.2 software, manufacturer: Camag(Muttenz, Switzerland),
- TLC chamber: twin-trough chamber for 20 cm x 10 cm plates (Art. 0.222.5221, Camag, and Muttenz, Switzerland).
Apparatus and instruments
Chromatography
Preparing of standards solutions of nicotinamide for NP-TLC and RP-TLC analysis: Standard solution of examined nicotinamide was obtained by dissolving of 200mg of accurately weighed nicotinamide in 50 mL of ethanol (99.8%). This solution was applied for the preparation of appropriate dilutions. Samples solutions for study at the following concentrations: 0.20 mg/mL, 0.18 mg/mL, 0.16 mg/mL, 0.14 mg/mL, 0.12 mg/mL, 0.10 mg/mL, 0.08 mg/mL, 0.06 mg/mL, and 0.04 mg/mL were spotted on the chromatographic plates in quantity of 5 μL in each case.
NP-TLC analysis of nicotinamide: TLC analysis of nicotinamide in normal phase was performed on chromatographic plates for NPTLC precoated with 0.20 mm layers of various sorbents: aluminum oxide 60 F254 (Art. 1.05550), aluminum oxide 150 F254 (Art. 1.05551), silica gel 60 (Art. 1.05553), silica gel 60 F254 (Art. 1.05554) and also with a mixture of silica gel and kieselguhr 60 F254 (Art. 1.05567). The plates were procured from Merck (Darmstadt, Germany). The amount of 5 μL of respective standard solution was applied in form of spots on chromatographic plates of 10 cm x 20 cm in size which were previously activated at temperature of 120°C during 30 minutes before use. Plates were developed using two mobile phases: methanol and also acetone to a distance of approximately 7.5 cm from baseline in TLC chamber for 20 cm x 10 cm plates (Art. 0.222.5221) obtained from Camag (Muttenz, Switzerland). After developing, the plates were dried at room temperature (21±1°C) in a fume cupboard during 24 hours and next densitometrically analyzed.
RP-TLC analysis of nicotinamide: TLC analysis of nicotinamide in reversed phase was performed on chromatographic plates for RP-TLC precoated with 0.20 mm layers of silica gel RP18F254 (Art. 1.05559) and on glass plates precoated with 0.20 mm layer of silica gel RP8F254s(Art. 1.15424) supported from Merck (Darmstadt, Germany). The amount of 5 μL of standard solutions was spotted on chromatographic plates of 10 cm x 20 cm in size which were previously activated at temperature of 120°C during 30 minutes before use. Plates were developed with the four mobile phases consisted of methanol, ethanol, propanol and dioxane, respectively to a distance of approximately 7.5 cm from baseline in TLC chamber for 20 cm x 10 cm plates (Art. 0.222.5221) obtained from Camag (Muttenz, Switzerland). After developing, the plates were dried at room temperature (21±1°C) in a fume cupboard during 24 hours and next densitometrically analyzed.
Densitometric analysis of nicotinamide
Spectrodensitometric scanning was done by Camag TLC Scanner 3 (Muttenz, Switzerland) equipped with WinCATS 1.4.2 software. All spectrodensitometric measurements were conducted in reflectance absorbance mode at wavelength of 222 nm. This wavelength was found to be suitable for densitometric analysis of nicotinamide under applied chromatographic conditions. The source of radiation was a deuterium lamp. The scanning speed was 20 nm/s and the data resolution was 100 nm/step. The slit dimension was kept at 8.00 mm x 0.60 mm, Macro. The spot areas (AU) obtained under applied chromatographic systems (NP-TLC and also RP-TLC) were used in determination of LOD and LOQ values. Each analysis was repeated three times.
Determination of Detection Limit and Quantification Limit of nicotinamide (LOD and LOQ)
In order to estimate the LODs and LOQs of nicotinamide, the method based on standard deviation value of the response and also on the slope of a specific regression line was applied. The lowest amount of examined nicotinamide in a studied sample that can be detected by applied TLC-densitometry (designated as LOD) was calculated according to formula (1). The LOQ value of nicotinamide which is defined as the lowest amount of nicotinamide that might be quantitatively determined by the use of TLC-densitometry (under applied chromatographic conditions) with good accuracy was calculated according to the equation (2). It could be noted that in determining of LOD and LOQ values, the standard deviation (s) could be calculated in two ways using standard deviation of intercept of specific regression line (sa) and with the use of residual standard deviation of regression line (sxy), respectively. Statistical evaluation of the obtained results (i.e., regression plots) was prepared by Statistics v 10.0 PL (StatSoft, Kraków, Poland).
Additionally, the correctness of LOD values obtained in each case was checked with the use of the following relationships (Eqs. 3 and 4) which can be observed between calculated LOD value and the concentration of examined substance [16]:
10 × LOD > C (3)
LOD < C (4)
where:
LOD - Limit of Detection,
C - Concentration of analyzed substance in the reference samples.
Results and Discussion
In present study the limit of detection and quantification of nicotinamide by TLC-densitometry on various sorbents and using different mobile phases was determined. Average values of LOD and LOQ calculated on the basis of standard deviation of intercept of specific regression line (sa) and using residual standard deviation of regression line (sxy) - Eqs. 1 and 2 for NP-TLC systems are listed in Table 1. Comparison of all obtained results shows Figures 1&2. The results obtained using TLC-densitometry in normal phase system indicate that the lowest LOD and LOQ values, thus the best detection shows the chromatographic plates precoated with silica gel 60 F254 (Art. 1.05554) and developed by methanol (LOD=0.088 μg/ spot, LOQ=0.265 μg/spot). The highest LOD and LOQ values have been obtained using this mobile phase on the chromatographic plates precoated with aluminum oxide 60F254and 150 F25 (Art. 1.05550, Art. 1.05551): LOD=0.264÷0.328 μg/spot and LOQ=0.801÷0.994 μg/spot. This fact confirms the worst results of the detection and quantification limit of nicotinamide predicted with the use of methanol as mobile phase.
TLC system
Chromatographicconditions
LOD [μg/spot] calculated on the basis of
Average value of LOD
[μg/spot]
Type of chromatographic plates
Mobile phase
sa
sxy
NP-TLC
1.05550
methanol
0.449
0.079
0.264
1.05551
0.567
0.089
0.328
1.05553
0.161
0.073
0.117
1.05554
0.120
0.055
0.088
1.05567
0.243
0.049
0.146
1.05550
acetone
0.539
0.353
0.446
1.05551
0.455
0.298
0.377
1.05553
0.137
0.038
0.088
1.05554
0.346
0.080
0.213
1.05567
0.294
0.046
0.170
RP-TLC
1.05559
dioxane
0.304
0.061
0.183
1.15424
0.897
0.588
0.743
1.05559
propanol
0.200
0.056
0.128
1.15424
0.184
0.064
0.124
1.05559
ethanol
0.114
0.040
0.077
1.15424
0.180
0.062
0.121
1.05559
methanol
0.158
0.055
0.107
1.15424
0.167
0.058
0.113
Where:
1.05550 – aluminum oxide 60 F254
1.05551 – aluminum oxide 150 F254
1.05553 – silica gel 60
1.05554 – silica gel 60 F254
1.05567 – mixture of silica gel 60 and kieselguhr F254
1.05559 – silica gel RP18F254s
1.15424 - silica gel RP8F254s
sa–standard deviation of calibration curve, sxy–residual standard deviation of calibration curve
Table 1: The LODs of nicotinamide obtained using different chromatographic conditions in NP-TLC and RP-TLC systems.
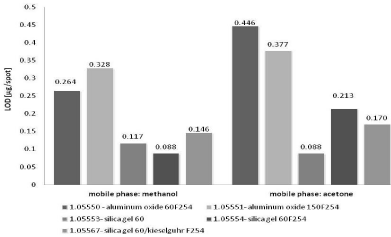
Figure 1: The comparison of average LOD values of nicotinamide obtained
by NP-TLC using various chromatographic conditions.
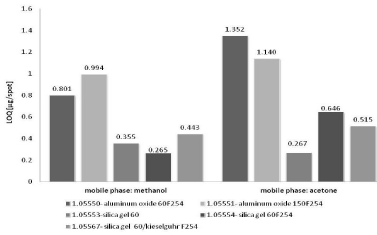
Figure 2: The comparison of average LOQ values of nicotinamide obtained
by NP-TLC using various chromatographic conditions.
Application of acetone as mobile phase enables obtain higher LOD and LOQ values for studied compound, especially in the case of described sorbent – aluminum oxide. The best results of detection and quantification limit of nicotinamide by the use of TLC-densitometry and acetone are observed for silica gel 60 (Art. 1.05553): LOD=0.088 μg/spot and LOQ=0.267 μg/spot.
Similar analysis of the detection and quantification limit of nicotinamide under various chromatographic conditions including two different sorbents (silica gel RP18F254s, silica gel RP8F254s) and four mobile phases consisted of methanol, ethanol, propanol and dioxane, respectively was done in the case of applied RP-TLC system (Table 2).
TLC system
Chromatographicconditions
LOQ [μg/spot] calculated on the basis of
Average value of LOQ
[μg/spot]
Type of chromatographic plates
Mobile phase
sa
sxy
NP-TLC
1.05550
methanol
1.362
0.239
0.801
1.05551
1.719
0.269
0.994
1.05553
0.487
0.222
0.355
1.05554
0.364
0.166
0.265
1.05567
0.737
0.148
0.443
1.05550
acetone
1.634
1.069
1.352
1.05551
1.377
0.902
1.140
1.05553
0.417
0.116
0.267
1.05554
1.047
0.245
0.646
1.05567
0.891
0.139
0.515
RP-TLC
1.05559
dioxane
0.921
0.185
0.553
1.15424
2.720
1.780
2.250
1.05559
propanol
0.605
0.169
0.387
1.15424
0.558
0.193
0.376
1.05559
ethanol
0.346
0.120
0.233
1.15424
0.547
0.189
0.368
1.05559
methanol
0.478
0.166
0.322
1.15424
0.506
0.175
0.341
Where:
1.05550 – aluminum oxide 60 F254
1.05551 – aluminum oxide 150 F254
1.05553 – silica gel 60
1.05554 – silica gel 60 F254
1.05567 – mixture of silica gel 60 and kieselguhrF254
1.05559 – silica gel RP18F254s
1.15424 - silica gel RP8F254s
sa–standard deviation of calibration curve, sxy–residual standard deviation of calibration curve
Table 2: The LOQs of nicotinamide obtained using different chromatographic conditions in NP-TLC and RP-TLC systems.
The results performed in Figures 3&4 show that the best detection and also quantification limit (the lowest LOD and LOQ values) have been determined using the three of four applied mobile phases: methanol, ethanol, dioxane and chromatographic plates – silica gel RP18F254s (Art. 1.05559). The LOD value ranges from 0.077 to 0.183 μg/spot (Figure 3). The LOQ value is placed in the range from 0.233 to 0.553 μg/spot (Figure 4). Satisfactory results of LOD and LOQ obtained on silica gel RP18F254s are observed especially in the case of dioxane. For silica gel RP8F254s the LOD and LOQ values are about four times higher than for discussed silica gel RP18F254s.
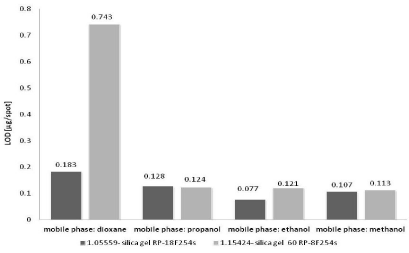
Figure 3: The comparison of average LOD values of nicotinamide obtained
by RP-TLC using various chromatographic conditions.
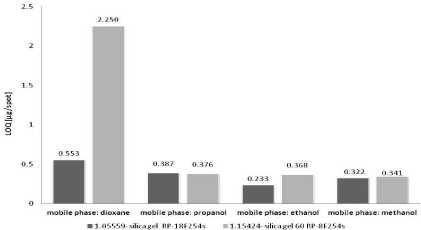
Figure 4: The comparison of average LOQ values of nicotinamide obtained
by RP-TLC using various chromatographic conditions.
Opposite situation can be observed for next applied mobile phase - propanol and the two applied chromatographic plates for RP-TLC. The LOD and LOQ values obtained on silica gel RP8F254s are lower in relation to that determined on silica gel RP18F254s. Generally, it could be concluded that the LOD and LOQ values obtained on the chromatographic plates precoated with silica gel RP18F254s and silica gel RP8F254s and with the use of the following mobile phases: methanol, ethanol and propanol are very similar. They are placed in the following range: LOD: 0.077÷0.128 μg/spot and LOQ: 0.233÷0.387 μg/spot.
Our experiment confirms that appropriate choice of TLC system (RP or NP) and also selection of the chromatographic conditions (kind of sorbent and mobile phase) is important in order to achieve respective level of detection and quantification of nicotinamide by TLC-densitometry. Different chromatographic conditions which are presented in this work allow detecting nicotinamide in amount suitable for pharmaceutical formulations (containing nicotinamide as active ingredient in quantity from 10 mg to 200 mg/tablet) and also in smaller amount which is sufficient for the detection of nicotinamide as impurity of commercial products. Comparison the results of LOD and LOQ values obtained in this study with those presented in other papers which describe the use of various analytical methods, such as (HPLC method) [7], Micellar Liquid Chromatography (MLC) [8] or spectrophotometry [9] for the determination of nicotinamide in different pharmaceutical formulations, confirms the utility of proposed TLC-densitometry method in various chromatographic conditions for the quantitative analysis of formulations containing nicotinamide. Presented TLC-densitometry with the use of appropriate chromatographic conditions (suitable mobile phase and sorbent) enables determine the nicotinamide in the range from few micrograms to few nanograms per spot which is enough for pharmaceutical analysis of nicotinamide. The lowest detection level of nicotinamide obtained in this study is comparable to that performed in the paper concerning HPLC analysis of nicotinamide [7]. In addition to this, the main advantage of proposed TLC-densitomtric method in relation to HPLC is lower cost of analysis and its simplicity.
Conclusion
On the basis of obtained LODs and LOQs of nicotinamide determined by NP-TLC and also RP-TLC systems, it can be concluded that:
- the kind of stationary phase (sorbent) and also the mobile phase composition has a significant impact on LOD and LOQ values of nicotinamide;
- silica 60F254 (for NP-TLC) and methanol as mobile phase enable detection the smallest amount of nicotinamide, the worst detection and quantification limit in NP-TLC system was achieved on the chromatographic plates precoated with aluminum oxide 60F254 using acetone;
- in the case of RP-TLC system, chromatographic plates precoated with RP18F254and ethanol as mobile phase allow to obtain the best results of LOD and LOQ for examined nicotinamide, the worst results were achieved on the chromatographic plates precoated with silica gel 60 RP8F254 using dioxane as mobile phase;
- The results of LODs and LOQs indicate that the proposed TLC-densitometric method performed under appropriate chromatographic conditions (mobile phase and sorbent) is suitable for the detection and also quantification of nicotinamide with good sensitivity (from μg to ng).
Acknowledgement
This research was financed by the Medical University of Silesia in Katowice as part of statutory research project in 2015 year no. KNW- 1-024/N/5/0.
References
- Zempleni J, Rucker RB, McCormick DB, Suttie JW. Handbook of vitamins; 4th edn. Boca Raton: CRC Press. 2007: 196-223.
- Ieraci A, Herrera DG. Nicotinamide protects against ethanol-induced apoptotic neurodegeneration in the developing mouse brain. PLoS Med. 2006; 3: e101.
- Hirakawa N, Okauchi R, Miura Y, Yagasaki K. Anti-invasive activity of niacin and trigonelline against cancer cells. Biosci Biotechnol Biochem. 2005; 69: 653-658.
- Surjana D, Damian DL. Nicotinamide in dermatology and photoprotection. Skinmed. 2011; 9: 360-365.
- Yiasemides E, Sivapirabu G, Halliday GM, Park J, Damian DL. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009; 30: 101-105.
- Murray MF, Langan M, MacGregor RR. Increased plasma tryptophan in HIV-infected patients treated with pharmacologic doses of nicotinamide. Nutrition. 2001; 17: 654-656.
- Khan AR, Khan KM, Perveen S, Butt N. Determination of nicotinamide and 4-aminobenzoic acid in pharmaceutical preparation by LC. J Pharm Biomed Anal. 2002; 29: 723-727.
- Capella-Peiro ME, Carda-Broch S, Monferrer-Pons L, Esteve-Romero J. Micellar liquid chromatographic determination of nicotinic acid and nicotinamide after precolumnKönig reaction derivatization. Anal Chim Acta. 2004; 517: 81-87.
- Hassan RO, Faizullah AT. Determination of nicotinamide by stopped-flow injection method in pharmaceutical formulations. Arab J Chem. 2013; 6: 393-400.
- GuO XQ, Wang DY, Xu JG, Zhao YB. Determination of nicotinamide by photochemical fluorimetryxs. Anal Lett. 1996: 29: 203-219.
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology, Q2(R1), ICH, Geneva, Switzerland. 2005.
- Bievre P, Gunzler H. Validation in chemical measurement. Springer-Verlag, Heidelberg, Berlin. 2005.
- Ferenczi-Fodor K, Renger B, Vegh Z. The frustrated reviewer–recurrent failures in manuscripts describing validation of quantitative TLC/HPTLC procedures for analysis of pharmaceuticals. J Planar Chromatogr - Modern TLC. 2010; 23: 173-179.
- Nagy-Turak A, Vegh Z, Ferenczi-Fodor K. Validation of the quantitative planar chromatographic analysis of drug substances. III. Robustness testing in OPLC. J Planar Chromatogr-Modern TLC. 1995; 8: 188-193.
- Ferenczi-Fodor K, Nagy-Turak A, Vegh Z. Validation and monitoring of quantitative thin layer chromatographic purity tests for bulk drug substances. J Planar Chromatogr –Modern TLC. 1995; 8: 349-356.
- Konieczka P,Namiesnik J. Validation of analytical procedures. In: The estimation and quality control of analytical measurements, WNT, Warsaw. 2007: 225-227; 269-289.
- Pyka A, Klimczok W. Application of densitometry for the evaluation of the separation effect of nicotinic acid derivatives. Part I. Nicotinic acid and its amides. J LiqChromatogr&Relat Technol. 2007; 30: 2317-2327.
- Pyka A, Klimczok W. Application of densitometry for the evaluation of the separation effect of nicotinic acid derivatives. Part II. Nicotinic acid and its esters. J LiqChromatogr & Relat Technol. 2007; 30: 2419-2433.
- Pyka A, Klimczok W. Application of densitometry for the evaluation of the separation effect of nicotinic acid derivatives. Part III. Nicotinic acid and its derivatives. J LiqChromatogr & Relat Technol. 2007; 30: 3107-3118.
- Pyka A, Klimczok W. Use of thin layer chromatography to evaluate the stability of methyl nicotinate.J LiqChromatogr & Relat Technol. 2009; 32: 1299-1316.
- Parys W, Pyka A. Use of TLC and densitometry to evaluate the chemical stability of nicotinic acid and its esters on silica gel. J LiqChromatogr & Relat Technol. 2010; 33: 1038-1046.
- Pyka A. TLC of vitamins including nicotinic acid derivatives. In: Komsta M. Waksmundzka-Hajnos, Sherma J, editors. Thin layer chromatography in drug analysis, Chapter 41, L. CRC Press, Boca Raton, FL, USA. 2014; 773-810.
- Dolowy M, Pyka A. Validation of an RPHPTLC-densitometric method using silica gel 60 RP18WF254for simultaneous determination of nicotinamide in selected pharmaceutical formulations. J Anal Methods Chem. 2015.