
Research Article
Austin Chromatogr. 2015;2(3): 1035.
Development of High Performance Liquid Chromatography (HPLC) Method for the Estimation of Fosamprenavir Calcium in Pharmaceutical Formulation
Pekamwar SS¹*, Bhavar GB², Aher KB² and Kakad SJ²
¹Department of Pharmaceutical Chemistry, Swami Ramanand Teerth Marathwada University, Nanded, MS, India
²Department of Pharmaceutical Chemistry, Amrutvahini College of Pharmacy, Sangamner, MS, India
*Corresponding author:Pekamwar SS, Department of Pharmaceutical Chemistry, School of Pharmacy, Swami Ramanand Teerth Marathwada University, Nanded – 431606, Maharashtra, India
Received: July 02, 2015; Accepted: August 06, 2015; Published: August 12, 2015
Abstract
A Novel, rapid, precise, accurate and stability indicating High Performance Liquid Chromatography (HPLC) method was developed for quantitative analysis of Fosamprenavir Calcium in tablet formulation. Detection of Fosamprenavir calcium in the presence of degradation products was achieved on X-Bridge C18, (250 X 4.6 mm, 5μ) column at 30 °C using a mixture of Phosphate Buffer (pH 6.8 ± 0.05) and Acetonitrile in the proportion 70:30, v/v as the mobile phase. A PDA detector set at 265 nm was used for detection. The peak for Fosamprenavir calcium was observed at 7.97 minute. A linear response was observed in the range of 50-150 μg/mL with a correlation coefficient of 0.9991. The investigated validation parameters showed that the method is specific, accurate, precise and robust. The method can be used for routine quality control analysis of Fosamprenavir calcium drug substance. The method was validated as per ICH guideline.
Keywords: Fosamprenavir calcium; HPLC; Forced degradation; Validation
Abbreviations
SD: Standard Deviation; RSD: Relative Standard Deviation
Introduction
Fosamprenavir Calcium, chemically (3S)-Tetrahydro-3-furyl {(aS)-a-[(1R)- 1-hydroxy- 2-(N1-isobutylsulfanilamido) ethyl] phenethyl} carbamate calcium phosphate (Figure 1), is a novel antiretroviral drug. Fosamprenavir calcium is one of the most recently approved HIV-1 protease inhibitor and is rapidly and extensively converted to amprenavir after oral administration. It is administrated orally with a dose of 700 mg two times a day [1-5].
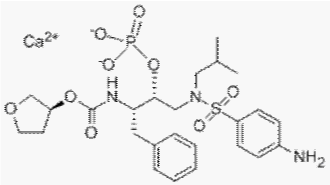
Figure 1: Structure of Fosamprenavir calcium.
The literature review revealed a dissolution method [6] and an electrochemical method [7] for evaluation of Fosamprenavir Calcium in tablet formulation. However, some HPLC and HPLC-MS methods had been reported for estimation of Fosamprenavir Calcium along with other antiretroviral drugs in human blood plasma and body fluids [8-19]. One HPLC method is available for quantitative determination of Fosamprenavir Calcium is in tablet formulation [20]. Further, no official or draft monograph of Fosamprenavir Calcium was published in any of the pharmacopoeia of compendia applications.
It was felt necessary to develop a simple, precise, and rapid HPLC method for the quantitative determination of Fosamprenavir Calcium. The developed method should advantageous to the reported method in terms of chromatographic conditions. Hence the aim of the present work was to develop an economic, precise, accurate, specific, and stability-indicating HPLC method using UV detection for the determination of Fosamprenavir calcium in the presence of its degradation products, in bulk and pharmaceutical dosage form. The method was validated as per International Conference on Harmonization (ICH) guidelines [21-23].
Experimental
Chemicals and reagents
Fosamprenavir calcium working standard was received as a sample from Mylan Laboratories Pvt. Ltd., Hyderabad (India). Acetonitrile used to be of HPLC grade (Qualigens, Mumbai). Millipore water was used throughout the analysis. Pharmaceutical dosage form (Fosamprenavir Calcium tablets containing 100 mg Fosamprenavir) was prepared in laboratory using Lactose Monohydrate, Microcrystalline Cellulose, Starch and Magnesium Stearate. Water was obtained from a Milli-Q UF-Plus apparatus (Millipore) and was used to prepare all solutions for the method. Analytical reagent grade Potassium dihydrogen orthophosphate and Triethylamine were used. Other chemicals used were analytical or HPLC-grade.
Equipments
Chromatography was performed with Agilent 1100 Series HPLC system (Agilent Technologies, Waldron, Germany) equipped with a G1311A quaternary pump, a G1313A degasser, a G1313A auto sampler, a G1316A thermos tatted column compartment, and a G1314A PDA-detector; data were processed by use of chromeleon software. Chromatography was performed on X-Bridge C18, (250 X 4.6 mm, 5 μm) column (Supplier- HYM Brothers, Mumbai, India). The Balance (Mettler Toledo XP205, Mumbai) was used for weighing and an Ultrasonicator (ENERTECH Electronics Pvt. Ltd., Mumbai) was used for sonication. Thermo Scientific Forma 3960 Series environmental chamber was used for stress testing.
Chromatographic conditions
The analysis was carried out on X Bridge C18, (250 X 4.6mm, 5μ) column at 30 °C using a mixture of Phosphate Buffer (pH 6.8 ± 0.05) and Acetonitrile in the proportion 70:30 as the mobile phase at a flow rate of 1.0 mL/minute. A PDA detector set at 265 nm was used for detection. The injection volume was 20 μL. The peak for Fosamprenavir calcium was observed at 7.97 minutes and the total run time was 15 minutes.
Preparation of phosphate buffer (pH 6.8)
Accurately weighed 6.8 g of potassium dihydrogen orthophosphate was transferred to a 1000 mL volumetric flask and dissolved in about 980 mL of water. The solution was diluted to volume with water. The pH was adjusted to 6.8 ± 0.05 with diluted triethylamine. The solution was filtered through 0.45μm porosity membrane filter.
Preparation of standard stock solution
An accurately weighed Fosamprenavir calcium working standard equivalent to 100 mg of Fosamprenavir was transferred to a 100 mL volumetric flask and dissolved in methanol by sonication. The solution was diluted to mark with the methanol to give the solution of concentration 1000 μg/mL. Aliquot of 5.0 mL from this solution was diluted to 50 mL with the methanol to give the standard stock solution of the concentration 100 μg/mL. The solution was then filtered through 0.45 μ nylon filter.
Method validation
The developed HPLC method was validated as per the ICH guidelines [21-24].
Linearity
For the linearity study aliquots from the standard stock solution were transferred to series of 100 mL volumetric flasks and volume was made up to the mark with methanol to give solutions of concentrations in the range of 50 - 150 μg/mL. The chromatograms and peak areas of these solutions were measured at 265 nm. The linearity graph of concentration against peak response was plotted and the correlation coefficient was determined.
Specificity
To determine the specificity of the method, the mixture of standard Fosamprenavir calcium and the degradation products was injected and chromatogram was recorded. The sample chromatogram was compared with the standard chromatogram.
Precision
Precision of the method was studied in terms of repeatability and intermediate precision.
Repeatability
Six test solutions of Fosamprenavir calcium tablets were prepared as per the analytical method. The % RSD of assay of six test solutions was calculated.
Intermediate precision
Six test solutions of Fosamprenavir calcium Tablets were prepared as per the analytical method on different days. These test solutions were analyzed by a different analyst. The % RSD of assay results of twelve test solutions (six samples from method precision and six samples from intermediate precision) was calculated.
Accuracy (Recovery)
Accuracy study was performed by analyzing Fosamprenavir calcium test solutions of concentration 50 μg/mL spiked with a quantity of Fosamprenavir calcium standard to produce three different concentration solutions equivalent to 50 %, 100 % and 150 % of test concentration.
Robustness
To determine the robustness of the method the experimental conditions were deliberately altered and peak area was evaluated. Three test solutions of the same lot of Fosamprenavir calcium from tablets were prepared as per analytical method. These solutions were injected with different chromatographic conditions as Change in flow rate (±0.2 mL/minute), Change in Column Oven Temp (±50C), and change in wavelength (±2 nm) and Change in pH of the mobile phase (±0.2). When the effect of altering one set of conditions was tested, the other conditions were held constant at the optimum values.
Limit of detection and quantitation
Limit of Detection and Quantitation is established by injecting six times very low concentration of Fosamprenavir calcium standard preparation i.e. 5.0 μg/mL and 10 μg/mL respectively.
System suitability parameters
Six replicate injections of system suitability solution (Fosamprenavir calcium standard working solution) were injected. The retention time, areas, theoretical plates, peak asymmetry and resolution were calculated for standard solutions
Analysis of pharmaceutical dosage form
To determine the content of Fosamprenavir Calcium in tablet dosage form (Fosamprenavir Calcium tablets containing 100 mg Fosamprenavir), twenty tablets were weighed and finely powdered. An accurate quantity of the powder equivalent to 100 mg Fosamprenavir was weighed and transferred into a 100 mL volumetric flask containing 50 mL methanol, sonicated for 30 min, and diluted to 100 mL with methanol. The solution was filtered through 0.45 μ nylon filter. The aliquot of 5.0 mL from this solution was transferred into a 50 mL of volumetric flask and diluted to volume with the methanol. The chromatogram was developed using previously described chromatographic conditions. Six replicates injections of this solution were injected and chromatogram and peak area were recorded. The concentration of tablet solution was determined using linear regression equation of calibration graph and amount of drug in tablet was determined. The possibility of excipient interference in the analysis was studied.
Forced degradation studies
The forced degradation studies were performed to establish the stability indicating nature and specificity of the assay method and to observe any degraded compounds. Fosamprenavir calcium working standard and Sample (Fosamprenavir calcium Tablets, 50 mg) were subjected to stress with 5 N hydrochloric acid, 5 N sodium hydroxide, 3 % v/v hydrogen peroxide, UV degradation at 254 nm and thermal degradation at 60 °C in the presence of 80 %RH (Table 1). Chromatograms were recorded for all the above solutions.
Stress condition
Description of stress condition
Acid-induced degradation
5 N HCl heated at about 60 °C for 10 min on water bath.
Base-induced degradation
5 N NaOH heated at about 60 °C for 10 min on a water bath.
Oxidative degradation
3 %v/v H2O2 heated at about 60 °C for 10 min on a water bath.
UV degradation
254 nm for 7 days in UV chamber.
Thermal degradation in presence of humidity
60 °C at 80 %RH for 7 days in a humidity chamber.
Table 1: Forced degradation conditions.
Result and Discussion
Method validation
Linearity: The average peak area of Fosamprenavir calcium peak at each concentration level was determined and the linear graph was plotted over the range of 50 - 150 μg/mL. The results of linearity study are as given in Table 2.
Linearity range (μg/mL)
50 - 150
Slope
23.96
Y-intercept
20.90
Correlation coefficient (r2)
0.999
Table 2: Linearity data for Fosamprenavir calcium.
Specificity: The spectra obtained from tablet solution (Figure 2) were identical with that obtained from standard solution containing an equivalent concentration of standard Fosampenavir calcium (Figure 3). This showed that there was no any interference from Excipients. The peaks due to degradation products were found to be well separated from the peak due to Fosamprenavir calcium. The peak purity criteria of Fosamprenavir calcium were found to pass at each condition of degradation.
Figure 2: Typical chromatogram of Fosamprenavir calcium sample.
Figure 2: Typical chromatogram of standard Fosamprenavir calcium.
Precision: Precision was expressed in terms of % R.S.D. All values for precision were within recommended limits.
Repeatability: Analysis of six separate solutions of Fosamprenavir calcium showed the repeatability of the method. The results of assay obtained from six test solutions preparations are given in Table 3.
% Assay (Mean ± SD)*
101.01 ± 0.49
(%) RSD
0.48
Table 3: Results of repeatability.
Intermediate precision: The % RSD of assay results of analysis carried out on two different days as summarized in Table 4 indicated that the method is precise and reproducible.
% Assay (Mean ± SD)*
100.12 ± 0.46
(%)RSD (n=12)
0.46
*Mean of twelve determinations.
Table 4: Results of intermediate precision.
Accuracy (Recovery): The results of recovery studies showed the accuracy of the method. The recoveries were ranged between 98.46- 99.66 %, Results obtained are given in Table 5.
Level
Amount added (μg/mL)
Amount found (μg/mL)
(Mean ± SD)
%Recovery*
(%)RSD
50
25
24.61 ± 0.01
98.46
0.04
100
50
49.52 ± 0.04
99.05
0. 09
150
75
74.74 ± 0.08
99.66
0.11
*Mean of three determinations.
Table 5: Results of recovery studies.
Robustness: Robustness of the method was indicated by the small % RSD observed for the assay of Rilpivirine HCl sample at deliberately altered experimental conditions. For all changes of conditions the sample was assayed in triplicate. Assay of Rilpivirine HCl for all deliberate changes of conditions was within 98.91– 101.78%. The results are shown in Table 6.
Condition
% Assay*
% RSD
Flow Rate
0.8 mL/min
101.78
0.18
1.2 mL/min
98.91
0.57
Column Temperature
25 °C
101.63
0.95
35 °C
99.14
1.06
Wavelength
263 nm
100.49
0.15
267 nm
100.12
0.31
pH
pH 6.6
101.46
0.34
pH 7.0
98.98
0.72
*-Mean of three determinations.
Table 6: Results of robustness studies.
Limit of detection and quantitation: Limit of Detection and Quantitation was observed to be 5.0 ppm and 10.0 ppm respectively. The % RSD for the peak response of Fosamprenavir calcium obtained for six replicate solutions was less than 5%.
System suitability parameters: System suitability parameters were tested for the chromatographic conditions and results are as shown in Table 7.
Parameter
Average
% RSD
Retention time
7.97 minute
0.63
Peak Area
2455.59
0.35
HETP
5814
Tailing Factor
1.28
Table 7: System suitability parameters.
Assay of fosamprenavir calcium in tablets: The concentration of tablet solution was determined using linear regression equation (using slope and Y-intercept) and amount of drug in tablet was determined. The results of the assay in tablets are summarized in Table 8.
Amount taken (mg)
100
Amount found* ± SD (mg)
100.80 ± 0.55
% Assay
100.80
% RSD
0.55
*Mean of six determinations.
Table 8: Results of assay of Fosamprenavir calcium in tablets.
Forced degradation: The purity of Fosamprenavir Calcium was unaffected by the presence of its degradation products thus method can be said to be stability-indicating. The percent assay of the all degraded samples varied between 98.9 and 100.2%. The forced degradation results are summarized in Table 9.
Stress condition
% Assay* of Fosamprenavir
Peak Purity
Acid-induced degradation
94.2
Passes
Base-induced degradation
96.1
Passes
Oxidative degradation
92.2
Passes
Thermal with humidity degradation
99.7
Passes
*Mean of three determinations.
Table 9: Results of degradation study of Fosamprenavir calcium.
The numbers of degradation peaks observed in different stress condition were as follows
Acid-induced degradation: Two very small degradation peaks were observed in acid degradation of standard preparation (Figure 4) as well as sample preparation.
Figure 4: Typical chromatogram of Acid-induced degradation of
Fosamprenavir calcium.
Base-induced degradation: One degradation peak was observed in alkali degradation of standard preparation (Figure 5) and sample preparation.
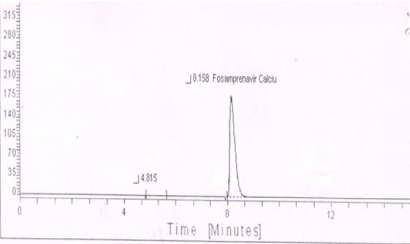
Figure 5: Typical chromatogram of Alkali-induced degradation of
Fosamprenavir calcium.
Oxidative degradation: One degradation peak was observed in peroxide degradation of standard preparation (Figure 6) as well as sample preparation.
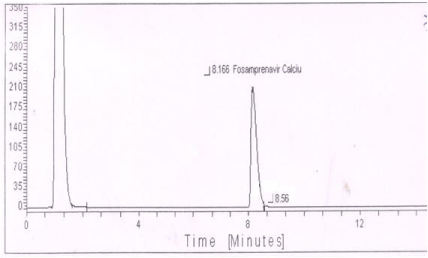
Figure 6: Typical chromatogram of peroxide degradation of Fosamprenavir
calcium.
UV-radiation degradation (at 254 nm): No degradation product was observed after exposure of standard Preparation and sample in to UV light (Figure 7).
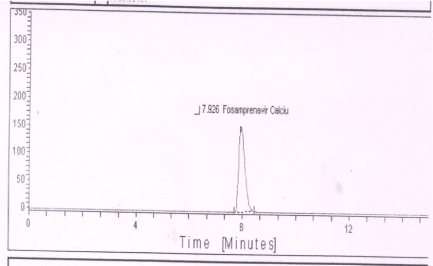
Figure 7: Typical chromatogram of UV degradation of Fosamprenavir
calcium.
Thermal with humidity degradation (60°C/80%RH): One degradation peak was observed in thermal with humidity degradation of standard preparation (Figure 8) and sample.
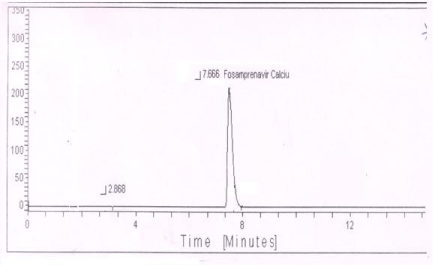
Figure 8: Typical chromatogram of thermal with humidity degradation of
Fosamprenavir calcium.
Conclusion
A simple, rapid and reliable HPLC method has been developed and successfully validated for estimation of Fosamprenavir calcium in the presence of degradation products. As the method separates the drug from its degradation products, it can be employed as a stabilityindicating one. The results of the validation tests indicated that the method was accurate, precise, robust, and stability indicating. The proposed HPLC method is suitable for routine determination of Fosamprenavir Calcium in pharmaceutical formulation in quality control laboratories, where economy and time are essential.
Acknowledgment
Authors are thankful to Mylan laboratories limited, Hyderabad, India, for providing pure drug samples for this study and the Amrutvahini Sheti and ShikshanVikas Sanstha, Sangamner, India, for providing us the research facility.
References
- Sweetman SC. The complete drug reference, Martindale, 36th edition. Pharmaceutical Press-Suffolk. 2009: 877.
- Wire MB, Shelton MJ, Studenberg S. Fosamprenavir: clinical pharmacokinetics and drug interactions of the amprenavir prodrug. Clin Pharmacokinet. 2006; 45: 137-168.
- Corbett AH, Kashuba AD. Fosamprenavir. Vertex Pharmaceuticals/GlaxoSmithKline. Curr Opin Investig Drugs. 2002; 3: 384-390.
- Falcoz C, Jenkins JM, Bye C, Hardman TC, Kenney KB, Studenberg S, et al. Pharmacokinetics of GW433908, a prodrug of amprenavir, in healthy male volunteers. J Clin Pharmacol. 2002; 42: 887-898.
- Ma'ayan A, Jenkins SL, Goldfarb J, Iyengar R. Network analysis of FDA approved drugs and their targets. Mt Sinai J Med. 2007; 74: 27-32.
- Rossi RC, Dias CL, Bajerski L, Bergold AM, Froehlich PE. Development and validation of discriminating method of dissolution for fosamprenavir tablets based on in vivo data. J Pharm Biomed Anal. 2011; 54: 439-444.
- Gumustas M, Ozkan SA. Electrochemical evaluation and determination of antiretroviral drug fosamprenavir using boron-doped diamond and glassy carbon electrodes. Anal Bioanal Chem. 2010; 397: 189-203.
- Rebiere H, Mazel B, Civade C, Bonnet PA. Determination of 19 antiretroviral agents in pharmaceuticals or suspected products with two methods using high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 850: 376-383.
- Ter Heine R, Davids M, Rosing H, Van Gorp ECM, Mulder JW, Van der Heide YT, et al. Quantification of HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in peripheral blood mononuclear cell lysate using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B. 2009; 877: 575-580.
- D’Avolio A, Siccardi M, Sciandra M, Lorena B, Bonora S, Trentini L, et al. HPLC–MS method for the simultaneous quantification of the new HIV protease inhibitor darunavir, and 11 other antiretroviral agents in plasma of HIV-infected patients. J Chromatogr B. Analyt Technol Biomed Life Sci. 2007; 859: 234-240.
- Dickinson L, Robinson L, Tjia J, Khoo S, Back D. Simultaneous determination of HIV protease inhibitors amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir and saquinavir in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2005; 829: 82-90.
- Colombo S, Beguin A, Telenti A, Biollaz J, Buclin T, Rochat B, et al. Intracellular measurements of anti-HIV drugs indinavir, amprenavir, saquinavir, ritonavir, nelfinavir, lopinavir, atazanavir, efavirenz and nevirapine in peripheral blood mononuclear cells by liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B. 2005; 819: 259-276.
- Pereira AS, Kenney KB, Cohen MS, Eron JJ, Tidwell RR, Dunn JA. Determination of amprenavir, a HIV-1 protease inhibitor, in human seminal plasma using high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 766: 307-317.
- Verbesselt R, Van Wijngaerden E, De Hoon J. Simultaneous determination of 8 HIV protease inhibitors in human plasma by isocratic high-performance liquid chromatography with combined use of UV and fluorescence detection: Amprenavir, indinavir, atazanavir, ritonavir, lopinavir, saquinavir, nelfinavir and M8-nelfinavir metabolite. J Chromatogr B. 2007; 845: 51-60.
- Keil K, Frerichs VA, DiFrancesco R, Morse G. Reverse Phase High-Performance Liquid Chromatography Method for the Analysis of Amprenavir, Efavirenz, Indinavir, Lopinavir, Nelfinavir and Its Active Metabolite (M8), Ritonavir, and Saquinavir in Heparinized Human Plasma. Ther Drug Monit. 2003; 25: 340-346.
- Justesen US, Pedersen C, Klitgaard A. Simultaneous quantitative determination of the HIV protease inhibitors indinavir, amprenavir, ritonavir, lopinavir, saquinavir, nelfinavir and the nelfinavir active metabolite M8 in plasma by liquid chromatography. J Chromatogr B. 2003; 783: 491-500.
- Turner ML, Reed-Walker K, King JR, Acosta EP. Simultaneous determination of nine antiretroviral compounds in human plasma using liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 784: 331-341.
- Dailly E, Thomas L, Kergueris MF, Jolliet P, Bourin M. High-performance liquid chromatographic assay to determine the plasma levels of HIV-protease inhibitors (amprenavir, indinavir, nelfinavir, ritonavir and saquinavir) and the non-nucleoside reverse transcriptase inhibitor (nevirapine) after liquid–liquid extraction. J Chromatogr B. 2001; 758: 129-135.
- Sarasa-Nacenta M, Lopez-Pua Y, Mallolas J, Luis Blanco J, Gatell JM, Carne X. Simultaneous determination of the HIV-protease inhibitors indinavir, amprenavir, ritonavir, saquinavir and nelfinavir in human plasma by reversed-phase high-performance liquid chromatography. J Chromatogr B. 2001; 757: 325-332.
- Chilukuri M, Narayanareddy P, Hussianreddy K. Stability-indicating HPLC method for determination of fosamprenavir calcium. J Chromatogr Sci. 2014; 52: 781-787.
- International Conference on Harmonisation. ICH – Q2 (R1). Guideline on Validation of Analytical Procedure: Text and Methodology. 2005.
- International Conference on Harmonisation. ICH - Q1B. Photo stability testing of new drug substances and products. International Conference on Harmonization, IFPMA, Geneva, Switzerland. 1996.
- International Conference on Harmonisation. Q1A - (R2). Stability testing of New Drug Substances and Products. 2003.
- Singh S, Bakshi M. Guidance on conduct of stress tests to determine inherent stability of drugs. Pharmaceutical Technology Online. 2000; 24: 1-14.