
Research Article
Chronic Dis Int. 2015;2(1): 1011.
Tapentadol and Dual Pain Inhibition: A New Strategy for Pain Relief in Australia
Pergolizzi JV¹, Schug SA², Raffa RB³ and Taylor R4*
1Department of Medicine, Johns Hopkins University School of Medicine, USA
1Department of Pharmacology, Temple University School of Medicine, USA
1Department of Anesthesiology, Georgetown University School of Medicine, USA
1Association of Chronic Pain Patients, USA
2Anaesthesiology in Pharmacology, University of Western Australia, Australia
2Anaesthesia and Pain Medicine, Royal Perth Hospital, Australia
3Department of Pharmaceutical Sciences, Temple University School of Pharmacy, USA
4NEMA Research Inc., USA
*Corresponding author: Taylor R, NEMA Research Inc, 3384 Woods Edge Circle, #102, USA
Received: December 09, 2014; Accepted: January 05, 2015; Published: January 07, 2015
Abstract
Although prevalent, pain is often under-treated, in part because pain can involve multiple physiological mechanisms. Pain signals are transmitted via ascending pathways and are modulated via descending pathways. The pathways are influenced by a complex interplay of inhibitory and excitatory actions involving the endogenous opioid and mono aminergic (e.g. nor adrenaline) systems. When pain involves multiple mechanisms, analgesics that inhibit only a single system will likely result in suboptimal pain relief, possibly initiating a vicious cycle of escalating doses, accelerated onset of dependence/tolerance, and excess side effects. Combining two agents with complementary mechanisms of action can be effective in treating such multi mechanistic pain, but taking multiple individual drugs can be inconvenient to the point of compromising compliance, and presaging a potentially dangerous poly pharmacy. The risk of drug-drug interactions increases dramatically as more drugs are added, and many patients who are in moderate to severe pain are already taking several other prescriptions for underlying disorders before analgesics are added. Tapentadol is a new analgesic agent that has a dual mechanism of action-it activates μ-opioid receptors and also inhibits the neuronal reuptake of norepinephrine, making it an attractive match for multi mechanistic pain syndromes. The dual mechanism of action was designed and observed using in vitro and animal testing, but now with 3 years in the clinic, we aim to present and evaluate the successful “translation” of these types of design strategies and preclinical data to the Australian clinic.
Keywords: Tapentadol; Dual inhibition; Analgesia; Pain; Chronic pain
Introduction
More than 10 million Australians (67% of the population = 15 years of age) experience pain at least one time in the prior four weeks, of which 9% characterize the pain as severe to very severe [1]. About 20% of the population (including pediatric patients) have chronic pain, and in the senior population (>65 years), prevalence rates are about 33% [2]. As in other parts of the world, pain increases with advancing age, certain health conditions (for example, osteoporosis), and mental health conditions, such as depression. Risk factors for severe to very severe pain include smoking, obesity, and a sedentary lifestyle [3]. Only cardiovascular disease and musculoskeletal conditions cost the Australian healthcare system more than does chronic pain. Despite the availability of good clinical care, it is estimated that less than 10% of Australians suffering chronic noncancer pain receive adequate analgesia [4], and it is more likely to be available to city-dwelling Australians than to rural citizens [5].
The physiological/biochemical complexities of pain signal transmission cause many pain syndromes to be multi factorial, involving neurological activation of both ascending and descending pain pathways [6]. While the well-known helpful World Health Organization (WHO) pain ladder recommends treating pain based on intensity level [7], WHO treatment recommendations predated modern understanding of the mechanisms of pain and thus do not take multiple underlying pain mechanisms into account [8]. Pain signals are processed and modulated by a variety of interacting excitatory and inhibitory systems; analgesic agents work because they either block excitatory transmissions or activate inhibitory systems. Identifying the underlying mechanisms of chronic pain can be particularly difficult, in that symptoms do not necessarily correlate with the mechanism. In traditional clinical practice, patients tend to be identified by the initial cause or location of their pain, for example low back pain patients, rather than pain mechanism. However, one patient with low back pain might have purely nociceptive pain, while another might have mixed pain, including a neuropathic component. An effective treatment for the first patient will be inadequate for the second. Thus, pain should be treated mechanistically, and it is often necessary to treat it multi mechanistically.
Methodology
This paper is a narrative review based on the most recent literature relating to tapentadol and the experiences and insights of the authors. The authors searched the PubMed database for the broad keyword “tapentadol,” which yielded over 200 results. The literature was then reviewed for those most relevant to a general discussion of the drug’s safety, efficacy, and potential role in the armamentarium against pain. In particular, the authors were seeking clinical trials, reviews, and other literature relating to tapentadol’s dual inhibition.
Rationale for mechanism-oriented treatment of pain
When treating multi mechanistic pain, each mechanism must be addressed. Drug efficacy can be mechanism-dependent, for example, a drug that is effective in treating nociceptive pain might provide little or no relief for neuropathic pain. For example, a chronic pain patient with nociceptive pain involving a neuropathic component (a common clinical presentation) is often best treated with a combination of drugs or one drug that has multiple mechanisms of action.
In addition to ascending pathways to the brain for processing and descending pathways for inhibitory modulation [9], peripheral and central sensitization can amplify or alter pain signals in an aberrant way, such that a mild noxious stimulus is perceived as very painful or a non-noxious stimulus is perceived as painful. Multi mechanistic pain may occur, for example, in osteoarthritis where central sensitization creates a neuropathic component that combines with nociceptive pain, creating the so-called “mixed pain state.” In such mixed pain states, both peripheral and central sensitization increase the excitability of the ascending pain pathways and decrease descending pain inhibition. Complicating the picture, psychosocial factors and mental health comorbidities may also come into play. Such chronic pain syndromes may not correlate with the extent of tissue damage or localized inflammatory zones [10].
The μ-opioid receptor system and the mono aminergic system are two important, closely interconnected pain modulatory systems. μ-opioid receptor agonists inhibit transmission of pain signals and influence higher brain centers (thus, both the sensation and perception of pain), whereas the noradrenergic system primarily modulates (attenuates) pain signal transmission via descending pathways that synapse in the dorsal horn of spinal cord [11,12]. For a schematic on the ascending and descending pain pathways please see reference [13]. μ-opioids inhibit presynaptic vesicular neurotransmitter release (by inhibiting Ca2+ influx) and hyperpolarize post-synaptic neurons (by altering K+ flux). Inhibition of neuronal noradrenaline reuptake results in an increased synaptic level of noradrenaline and an enhanced inhibitory action.
As pain persists, one system might become more dominant. For example, if acute pain transitions into neither chronic pain (chronification), noradrenaline-mediated inhibition can be increasingly important [14]. Chronic pain can also result in alteration of the opioideric system which, in turn, alters responsiveness to opioid analgesics [15]. Several possibilities are receptor up- or downregulation, opioid-induced hyperalgesia, and others [16]. Altered activity of the neither opioidergic system means that the balance shifts toward noradrenaline-mediated pain inhibition assuming greater importance [17]. The role of another monoamine in the descending pathways, 5-HT (5-hydroxytryptamine, serotonin) is less clear. For example, fluoxetine, a selective serotonin reuptake inhibitor (SSRI) has no clinically meaningful analgesic effect [18]. 5-HT can also have a pro-emetic effect, decreasing tolerability. An agent that targets the μ-opioid and noradrenaline, but not 5-HT, systems might strike the proper balance, with greater efficacy and reduced adverse effects.
Barriers to multi mechanistic pain therapy
In a survey of 415 physicians, 60% ‘agreed’ or ‘totally agreed’ that there is insufficient knowledge within the medical community about the pharmacological characteristics of different analgesic regimens, and 81% agreed or totally agreed with the statement that pain with a neuropathic component is often more severe and more difficult to treat [19]. While the identification of the underlying mechanism of pain can be important in the planning of a treatment strategy, identifying the specific mechanisms can be challenging, particularly since symptoms are not always reliable indicators of pain mechanism [20]. A single medication that could target multiple pain components (e.g., neuropathic and nociceptive) would eliminate the need to pinpoint exact pain mechanisms, simplifying prescribing.
Multidrug therapy guidelines
Practice guidelines for pain therapy advocate the use of combination therapy [21]. The American Society of Anesthesiologists Task Force on Acute Pain Management writes, “The literature supports the administration of two analgesic agents that act by different mechanisms via a single route for providing superior analgesic efficacy with equivalent or reduced adverse effects [22]”. Although the use of multidrug analgesic regimens is less well studied for chronic pain, the strategy has been endorsed for a variety of chronic pain syndromes [23].
The interruption of more than one mechanism of pain signaling increases the likelihood of successful pain treatment. For this, combination drug therapy should involve using agents with different, but complementary, mechanisms of action. Plus, when two or more agents are taken together, there is the possibility that they will have a synergistic effect (where the total effect is greater than the additive effects of the individual agents) [24,25]. Figure 1 (A. and B., it is also possible that combination therapy will amplify adverse effects).
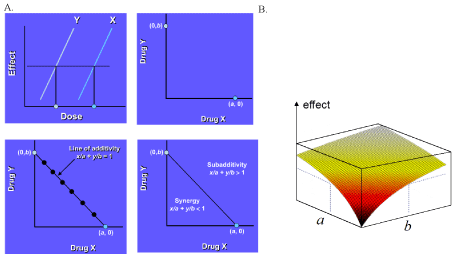
Figure 1: (a) Isobolographic analysis of combination drug therapy. (b) A
response surface is described by 4E=EB{(a/R+b)/[(a/R+b)+CB]} (see reference
for details). Figure used from reference [58] with permission by the publisher
Elsevier and Copyright Owner American Pain Society.
The optimal combinations are those that both increase analgesia and decrease adverse effects. There is no benefit to combination pharmacological therapy if it lessens analgesic benefits, reduces tolerability, or provides no net clinically meaningful benefit. Examples of beneficial combination drug therapies include a low-dose opioid with a non-opioid, such as codeine plus either an NSAID or acetaminophen or tramadol plus an NSAID or acetaminophen; opioid plus a peripherally-restricted antagonist to reduce gastrointestinal side effects; or non-opioid agent plus a gastric protective agent, such as diclofenac plus misoprostol.
Combination drug therapy can be administered either as a “loose-dose,” (i.e., individual agents are taken separately) or in fixeddose combination products (that combine agents in a single tablet). While loose-dose regimens offer clinicians maximum flexibility, they create a pill burden, which has been associated with lower rates of patient adherence to therapy [26]. The use of loose-dose regimens may also result in unpredictable or variable pharmacokinetic profiles. Validated fixed-dose combination products offer benefits of convenience, a reduced pill burden, and known complementary pharmacokinetics. On the other hand, it is not always possible to find the fixed-dose combination that is optimal for a particular patient.
Rational poly pharmacy and potential risks
Poly pharmacy is the situation in which a patient is prescribed, or self-medicates, with multiple prescription or Over-The-Counter (OTC) drugs [27]. Many pain patients have just had surgery, experienced trauma, and/or present with comorbid conditions, and must take several drugs. In fact, taking multiple drugs daily is increasingly the norm for chronic pain patients and the elderly. Moreover, many patients also take nutritional remedies or supplements. The inherent danger of taking more than one drug is the potential risk of a pharmacokinetic and/or pharmacodynamic drug-drug interaction (DDI). The probability that a DDI will occur is related to, and increases rapidly with, the number of drugs [28-30] Figure 2.
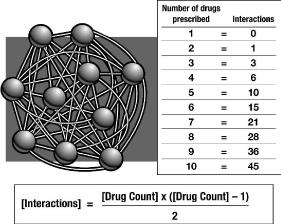
Figure 2: The probability of drug interactions increases rapidly with the
number of agents. (Figure is original artwork).
The cytochrome P450 (CYP450) enzyme family is essential for the metabolism of many common medications, such as statins, warfarin, beta-blockers, opioids, Selective SSRIs, and OTC ingredients. The CYP450 family includes at least 50 isozymes, of which CYP3A4 and CYP2D6 metabolize the majority of current drugs [31]. CYP450 enzymes may be inhibited or induced by certain drugs, resulting in potentially significant DDIs, which can lead to therapeutic failure or unanticipated adverse events. The likelihood of a CYP450-mediated DDI increases with poly pharmacy; the prevalence of CYP450- mediated DDIs in geriatric patients on poly pharmacy is estimated at 80% [32]. The addition of a new medication to an existing five-drug regimen increases the risk of a CYP-mediated DDI by about12% for each new agent [33].
Combination therapy can be an effective treatment of multi mechanistic pain involving both a nociceptive and a neuropathic component, but poly pharmacy may expose patients to potentially dangerous DDIs. Monotherapy at higher doses may not be as effective or well tolerated. The analgesic efficacy of opioids in some pain syndromes may be limited, for example sustained activation of the opioidergic system may lead to decreased opioid responsiveness, which leads to a cycle of increased doses with decreased effectiveness. No currently available single agent effectively treats both nociceptive and neuropathic components in moderate to severe pain syndromes.
Tapentadol
Tapentadol is a novel, centrally acting analgesic agent that combines two mechanisms of action in a single molecule, namely μ-opioid receptor agonist (MOR) and Noradrenaline Reuptake Inhibition (NRI) [34]. The tapentadol molecule has been described as “one key for two locks,” in that it has a chemical structure that can simultaneously interact with (‘fit’) both the opioidergic and the mono aminergic systems (‘locks’) Figure 3. It also has no analgesically-active metabolites. The availability of such a single-agent multi mechanistic molecule presents an important option for first-line treatment for patients who require both nociceptive and neuropathic pain relief [35].
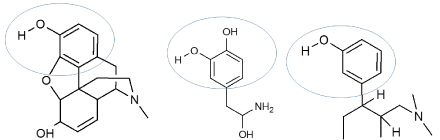
Figure 3: From left to right, morphine, noradrenaline, and tapentadol.
Because tapentadol is not a prodrug, both its pharmacokinetics and analgesic efficacy are independent of metabolic activation [36], and there is low risk of drug-drug interactions via the cytochrome (CYP) 450 metabolic pathways [37]. Its mechanisms of action resides in the parent drug, which based on animal studies, are agonist action at the μ-opioid receptor and inhibition of neuronal reuptake of NA [38- 40]. The main pathway of tapentadol metabolism is conjugation with glucuronic acid; 99% of tapentadol and its metabolites are eliminated from the body in the urine [41]. Tapentadol has low plasma-protein binding and has not been shown to induce or inhibit CYP450 enzymes. None of the metabolites of tapentadol contribute to its analgesic effects [42]. Population pharmacokinetic studies describe the tapentadol immediate-release formulation as best fit by a two-compartment model with zero-order release followed by firstorder absorption and elimination [43].
Tapentadol has been evaluated in a number of clinical trials Table 1.The Immediate-Release (IR) and Extended-Release (ER) formulations have been compared to oxycodone IR and Controlled- Release (CR) and found to be non-inferior [44,45]. The primary advantage of tapentadol over oxycodone in these studies is the reported reduced rate of side effects, particularly gastrointestinal (GI) effects, such as nausea, vomiting, and constipation [46,47]. This advantage may be more pronounced in geriatric patients [48], which is of particular clinical interest, since revised guidelines issued by the American Geriatric Society for the management of moderate to severe pain in the elderly state that nonselective NSAIDs and COX-2 inhibitors should be used rarely and only with extreme caution [49], thus limiting the analgesic options for this important, and increasing, age group.
Study
Patients
Agents
Results
Safety
Mercadante [46]
Open-label, 4 wk study
50 opioid-naïve cancer patients
Slow-release TP 50 mg twice daily
Significant reduction in pain intensity vs. baseline for all weeks (p<0.005)
No significant change in AEs
Kavanagh [47]
Post-hoc analysis of 2 multicenter, randomized, double-blind trials, 10 and 90 days
1338 patients with moderate to severe OA pain
TP IR 50 and 75 mg vs. oxycodone IR 10 mg in 10 day trial; TP IR 50 or 100 mg vs. oxycodone IR 10 or 15 mg in 90-day trial
Pain reduction 30% or 50% with no treatment-emergent AEs was endpoint (PRT). In 10-day study, 30% PRT was significantly greater for 50 mg TP vs. oxycodone. In 90 day study, TP patients had significantly more days meeting PRT criteria (30%).
The PRT endpoint included tolerability.
Steigerwald [48]
Open-label, phase 3b study
196 patients with severe chronic LBP with and without a neuropathic component
TP-PR 50-250 mg BID during 5-wk titration and 7-wk maintenance periods. TP IR was used for acute episodes such that combined dose = 500 mg/day.
TP was associated with significant improvements in pain over baseline and significant reduction of neuropathic pain symptoms
AEs = 10% were nausea, dizziness, headache, dry mouth, fatigue, constipation, diarrhea, nasopharyngitis, and somnolence
Vorsanger [39]
Post-hoc analysis of 90-day phase 3, double-blind, clinical trial
849 patients with moderate to severe pain (elderly and non-elderly)
Flexible dose, TP IR 50 and 100 mg vs. oxycodone IR 10 and 15 mg, every 4-6 h, as needed
Pain relief was similar between TP and oxycodone groups, both age groups (efficacy not primary endpoint); no age-based efficacy differences
Constipation and nausea/vomiting were significantly lower in elderly patients taking TP IR than oxycodone IR
Etropolski [36]
Randomized, double-blind, placebo-controlled study
596 patients with end-stage joint disease
TP IR 50 mg, TP IR 75 mg, oxycodone 10 mg, and placebo for 14 days followed by 28 day ER treatment of same active agents or placebo
Efficacy was not an endpoint.
Nausea/vomiting decreased significantly with TP 50 and 75 mg (p<0.001) vs oxycodone and placebo in 14-day phase, with ER formulations in 28-day phase similar
Schwartz [49]
Double-blind, parallel-group, randomized-withdrawal, placebo-controlled study
395 patients with painful diabetic peripheral neuropathy; a 3-mo history of analgesic use (opioid and/or non-opioid), dissatisfaction with current analgesic regimen; and moderate to severe pain
3-wk open-label phase for titration to optimal dose of TP ER; then 12-wk double-blind maintenance phase during which patients were randomized 1:1 to TP ER (at their optimal dose) or placebo. Doses of TP ER were 100-250 mg bid.
TP ER patients had significantly better pain relief using all imputation methods (p<0.001) vs placebo; at end of double-blind phase, 64% of TP ER and 38% of placebo patients reported their status was “much” or “very much” improved (p<0.001)
Rates of treatment-emergent AEs were 71% during open-label and 70% during double-blind phases. Most common (occurring at rates = 10%) were nausea, dizziness, somnolence, and constipation. AE rates were similar for patients over and under 65 years of age.
Wild [50]
Randomized comparative study
1117 patients with chronic knee or hip OA pain or LBP
TP ER (100 to 250 mg) twice daily or oxycodone CR (20 to 50 mg) up to 1 yr
Mean pain intensity scores were 7.6 at baseline (TP ER and oxycodone groups) and decreased to 4.4 (TP ER) and 4.5 (oxycodone) at endpoint.
Rate of AEs were 86% for TP ER and 91% for oxycodone CR. 22% of TP and 37% of oxycodone patients discontinued drug because of side effects.
Afilalo [51]
Randomized, double-blind, active- and placebo-controlled, parallel-arm, phase 3 study
1023 patients with moderate to severe OA knee pain
TP ER (100 to 250 mg twice daily) oxycodone CR (20 to 50 mg twice daily) or placebo for 3-wk titration then 12-wk maintenance phases
TP significantly reduced pain over baseline compared to placebo for 12 wk; oxycodone significantly reduced pain over baseline compared to placebo for 11 wks (not week 12). 24% of TP ER patients, 17% of oxycodone, and 24% of placebo patients achieved = 50% reduction in pain (TP ER vs. placebo, p=0.027)
Rates of patients who had = 1 AE were 61% (placebo), 76% TP, and 87% (oxycodone)
Buynak [37]
Randomized, double-blind, placebo- and active-controlled, phase 3 study
981 patients with chronic LBP
TP ER 100 to 250 mg BID or oxycodone CR 20 to 50 mg BID or placebo, 15 wk
Both TP and oxycodone significantly reduced pain over baseline.
TP was associated with fewer treatment-emergent AEs than oxycodone. Most common AEs were GI-related events, rates 26% (placebo), 44% (TP), 62% (oxycodone)
Daniels [34]
Randomized, double-blind, placebo-controlled, phase 3 study
901 bunionectomy patients
TP IR 50 or 75 mg, oxycodone IR 10 mg or placebo every 4 to 6 h for 72 h post-surgery
TP 50 and 75 mg and oxycodone 10 mg provided significantly greater pain relief than placebo and TP 50 and 75 mg were non-inferior to oxycodone 10 mg.
TP 50 mg resulted in significantly lower rates of nausea and/or vomiting than oxycodone 10 mg (35% vs. 59%, p<0.001); TP 75 mg had a rate of 51% (p=0.057 vs. oxycodone).
Hartrick [35] Randomized, double-blind, active- and placebo-controlled study
659 patients with end-stage joint disease awaiting joint replacement
TP IR 50 or 75 mg or oxycodone IR 10 mg or placebo every 4-6 hours during waking hours for 10 days
All active agents reduced pain significantly more than placebo (p<0.001) and TP IR 50 and 75 mg were non-inferior to oxycodone IR 10 mg.
GI AEs were significantly lower for both TP IR groups than oxycodone (p<0.001). Discontinuation rates were 18% and 26% for TP IR 50 and 75 mg, respectively, and 35% (oxycodone) and 10% (placebo).
Hale [38]
Randomized, double-blind, active-controlled, phase 3 study
878 patients with LBP or hip or knee OA
TP IR vs. oxycodone IR over 90 days
Pain intensity measurements showed TP IR was similar to oxycodone.
TP compared to oxycodone: 18% vs. 29% nausea; 17% vs. 30% vomiting; 13% vs. 27% constipation, dizziness 18% vs. 17%, headache 12% vs. 10%, somnolence 10% vs. 9%.
Daniels [52]
Randomized, double-blind, active-controlled, phase 3, multiple-dose study
603 bunionectomy paients
TP IR 50, 75 or 100 mg vs. oxycodone IR 15 mg or placebo every 4-6 h for 72 h after surgery
All active agents provided significantly greater pain relief than placebo; post-hoc analysis showed that TP IR 100 mg provided equivalent analgesia as oxycodone 15 mg
Overall rates of AEs were 70% TP 50 mg, 75% TP 75 mg, 85% TP 100 mg, 87% oxycodone, 41% placebo. TP 100 mg had a significantly lower incidence of nausea and/or vomiting than oxycodone 15 mg (53% vs. 70%, respectively, p=0.007)
Stegmann [53]
Randomized, double-blind, multiple-dose, active-controlled phase 2 study
269 bunionectomy patients
TP IR 50 or 100 mg, oxycodone IR 10 mg or placebo every 4-6 h for 72 hours following surgery
All active agents provided significantly greater pain relief than placebo
TP 50 and oxycodone 10 mg rates were 46% vs. 72% for nausea, 33% vs. 57% dizziness, 16% vs. 39% vomiting, 6% vs. 18% constipation, and 28% vs. 27% somnolence.
AE=Adverse Event; BID=Bis in Die (twice daily); CR=Controlled-Release formulation; ER=Extended-Release formulation; h=hours; IR=Immediate-Release formulation; LBP=Low Back Pain; OA=Osteoarthritis; PR=Prolonged Release; TP=Tapentadol; wk=week(s); yr=year
Table 1: Key clinical trials reported over the past five years (2008 – 2012) that evaluated the safety and/or efficacy of tapentadol. Studies were selected and presented in order to show use in a variety of pain syndromes. Studies were not omitted based on lack of efficacy or poor safety outcomes.
In a clinical study to establish dosing equivalency between tapentadol’s two formulations, approximately equivalent total daily doses (TDDs) of the IR and ER formulations offered patients equivalent analgesic benefits for the relief of moderate to severe pain associated with low back pain; both formulations are well tolerated and allow direct conversion [50].
Abuse potential
In experiments by the sponsor and reported to regulatory agencies, tapentadol has been evaluated in standard animal models of abuse liability: it substituted fully for morphine-trained rats, produced conditioned place preference that was blocked by naloxone, and was self-administered by rhesus monkeys trained to self-administer morphine [51]. In clinical abuse liability studies, tapentadol produced dose-dependent drug ‘Liking’. The effects peaked 1 – 2 h after dosing and were not different from calculated equianalgesic doses of hydromorphone IR. Negative subjective effects were noted 2-6 h after dosing. In Phase 3 clinical studies a small number of opioidexperienced patients administered more tapentadol IR, although this did not result in adverse events. Opioid-naïve patients first exposed to tapentadol IR are less commonly observed to ‘doctor shop’ as compared to oxycodone IR [52]. In a retrospective cohort study, the reported risk of abuse was 65% less, and the risk of receiving an abuse diagnosis was lower, for tapentadol IR than with oxycodone IR [53,54].The physical barrier technology (INTAC™; Grünenthal GmbH, Aachen, Germany) of the extended release tablets appears to discourage abuse routes of administration, since only 14% of intranasal prescription opioid abusers report they would attempt to snort the drug and only 18% of intravenous prescription opioid users report they would attempt to inject the gel.
The majority of reports about tapentadol from internet websites that share experiences on drug abuse highlight difficulties of breaking down the tapentadol ER tablet, and warn not to snort or smoke crushed tapentadol due to a severe burning sensation. The postings disagree on whether or not the tapentadol effect is worth experiencing. In a survey of online discussions about illicit and prescription drugs among recreational drug users, tapentadol was of little interest to those actively abusing drugs. Over 1.9 million messages were posted between January 1, 2011 and September 30, 2012 on seven recreational drug abuse forums; only 0.03% of posts during that time related to tapentadol, significantly fewer than for comparator drugs (p<0.001) [55]. A study of 113,914 substance abuse treatment patients found tapentadol was significantly less frequently abused than comparator drugs (p<0.001) and lower than abuse for most Schedule II analgesic agents [56]. The risk of the patient’s experiencing withdrawal symptoms upon discontinuation of tapentadol is low compared to other currently marketed products [57]. Though these reports lean towards less abuse for tapentadol, it should be noted that a variety of factors may underestimate tapentadol’s true abuse potential. These include lower prescription rate versus other well marketed opioids, abuse-deterrent technology in tapentadol versus other products as well as the datasets that were used which may not represent national estimates of abuse prevalence. Therefore, to fully understand tapentadol’s true abuse potential, large epidemiological studies would be needed.
Conclusion
Australia, similar to most other parts of the world, has a high prevalence of chronic non-cancer pain and a high rate of severe to non-severe pain of all types and the incidence of pain is expected to rise together with the aging population. While numerous conventional mono-mechanistic analgesics are currently available (e.g., NSAIDs, acetaminophen, and opioids), prescribing the right product or combination of products involves the balancing of risks and benefits. And each of the major analgesic categories is associated with the potential for serious adverse effects. As a result, much pain goes under-treated, because the multiple mechanisms that underlie many pain syndromes is in effectively covered by mono-mechanistic drugs; complete analgesia requires that all mechanisms be addressed. Combination pharmacotherapy can help address multi mechanistic pain, but it may open the door to poly pharmacy and its associated problems and increase the risk for potentially serious drug-drug interactions. Thus, while a very strong case for rational poly pharmacy can be made; rational poly pharmacy has its limits, particularly for a clinician who is not a pain specialist. The novel agent tapentadol appears to provide a promising approach, in that it combines dual mechanisms of action (opioid and non opioid) in a single molecule, with no analgesically active metabolites. And it has been shown in clinical trials to be effective and well tolerated. Its recent approval in Australia appears to offer a promising first-line agent for patients suffering from multi mechanistic pain.
Acknowledgement
Dr. Pergolizzi is a consultant for Grünenthal, Baxter, and Hospira. Prof. Schug has received research and travel funding and speaking and consulting honoraria from Eli Lilly, bio CSL, Grünenthal, Janssen, Munipharma, Pfizer, Phosphagenics and iX Biopharma. He is a member of the international and Australian advisory board for tapentadol. Dr. Taylor and Dr. Raffa are speakers for a number of pharmaceutical companies, but receive no compensation for the sale of any product. Funding for manuscript preparation was provided by BioCSL through an educational research grant. This article was prepared with editorial and graphic assistance from LeQ Medical, Angleton, Texas. Authors paid LeQ Medical directly for their services.
References
- Australian Bureau of Statistics. Facts at your Fingertips: Health. Australia: Australian Bureau of Statistics. 2011.
- Pain Australia. Pain Australia, working to prevent and manage pain. Australia: Pain Australia. 2014.
- National Rural Health Alliance Inc. Chronic Pain - A Major Issue in Rural Australia. Australia: National Rural Health Alliance, Inc. 2013.
- Raffa RB, Pergolizzi JV, Tallarida RJ. The determination and application of fixed-dose analgesic combinations for treating multimodal pain. J Pain. 2010; 11: 701-709.
- World Health Organization. WHO's pain ladder for adults. Geneva, Switzerland: World Health Organization. 1988.
- Varrassi G, Muller-Schwefe G, Pergolizzi J, Oronska A, Morlion B, Mavrocordatos P, et al. Pharmacological treatment of chronic pain - the need for CHANGE. Curr Med Res Opin. 2010; 26: 1231-1245.
- Fong A, Schug SA. Pathophysiology of pain: a practical primer. Plast Reconstr Surg. 2014; 134: 8S-14S.
- Gottschalk A, Smith DS. New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician. 2001; 63: 1979-1984.
- Benarroch EE. Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology. 2008; 71: 217-221.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003; 349: 1943-1953.
- Gambert SR, Garthwaite TL, Tate PW. Clinical implications of the endogenous opiates: Part I. Physiological. Psychiatr Med. 1983; 1: 93-105.
- Joo DT. Mechanisms of opioid tolerance: merging evidence and therapeutic implications. Can J Anaesth. 2007; 54: 969-976.
- Bannister K, Bee LA, Dickenson AH. Preclinical and early clinical investigations related to monoaminergic pain modulation. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2009; 6: 703-712.
- Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001; 26: 30-36.
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999; 353: 1959-1964.
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007; 132: 237-251.
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2004; 100: 1573-1581.
- Practice guidelines for chronic pain management. A report by the American Society of Anesthesiologists Task Force on Pain Management, Chronic Pain Section. Anesthesiology. 1997; 86: 995-1004.
- Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 2001; 26: 257-264.
- O'Connor JL, Gardner EM, Mannheimer SB, Lifson AR, Esser S, Telzak EE, et al. Factors Associated With Adherence Amongst 5295 People Receiving Antiretroviral Therapy as Part of an International Trial. The Journal of infectious diseases. 2013; 208: 40-49.
- DeSevo G, Klootwyk J. Pharmacologic issues in management of chronic disease. Prim Care. 2012; 39: 345-362.
- Pergolizzi JV, Labhsetwar SA, Puenpatom RA, Joo S, Ben-Joseph R, Summers KH. Exposure to potential CYP450 pharmacokinetic drug-drug interactions among osteoarthritis patients: incremental risk of multiple prescriptions. Pain practice: the official journal of World Institute of Pain. 2011; 11: 325-336.
- Pergolizzi JV, Labhsetwar SA, Puenpatom RA, Joo S, Ben-Joseph RH, Summers KH. Prevalence of exposure to potential CYP450 pharmacokinetic drug-drug interactions among patients with chronic low back pain taking opioids. Pain practice: the official journal of World Institute of Pain. 2011; 11: 230-239.
- Kohler GI, Bode-Boger SM, Busse R, Hoopmann M, Welte T, Boger RH. Drug-drug interactions in medical patients: effects of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000; 38: 504-513.
- Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007; 76: 391-396.
- Doan J, Zakrzewski-Jakubiak H, Roy J, Turgeon J, Tannenbaum C. Prevalence and risk of potential cytochrome P450-mediated drug-drug interactions in older hospitalized patients with polypharmacy. Ann Pharmacother. 2013; 47: 324-332.
- Pergolizzi J, Alon E, Baron R, Bonezzi C, Dobrogowski J, Galvez R, et al. Tapentadol in the management of chronic low back pain: a novel approach to a complex condition? J Pain Res. 2011; 4: 203-210.
- Smith HS, Raffa RB, Pergolizzi JV, Taylor R, Tallarida RJ. Combining opioid and adrenergic mechanisms for chronic pain. Postgrad Med. 2014; 126: 98-114.
- Drugs.com. Tapentadol. New Zealand: Drugsite Trust. 2014.
- Tzschentke TM, Christoph T, Kogel B, Schiene K, Hennies HH, Englberger W, et al. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther. 2007; 323: 265-276.
- Vanderah TW, Raffa RB, Lashbrook J, Burritt A, Hruby V, Porreca F. Orphanin-FQ/nociceptin: lack of anti nociceptive, hyperalgesic or allodynic effects in acute thermal or mechanical tests following intracerebroventricular or intrathecal administration to mice or rats. European journal of pain (London, England). 1998; 2: 267-278.
- Nucynta. Atlanta, GA: WebMD. 2011.
- Xu XS, Smit JW, Lin R, Stuyckens K, Terlinden R, Nandy P. Population pharmacokinetics of tapentadol immediate release (IR) in healthy subjects and patients with moderate or severe pain. Clin Pharmacokinet. 2010; 49: 671-682.
- Daniels S, Casson E, Stegmann JU, Oh C, Okamoto A, Rauschkolb C, et al. A randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr Med Res Opin. 2009; 25: 1551-1561.
- Hartrick C, Van Hove I, Stegmann JU, Oh C, Upmalis D. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10-day, phase III, randomized, double-blind, active- and placebo-controlled study. Clinical therapeutics. 2009; 31: 260-271.
- Etropolski M, Kelly K, Okamoto A, Rauschkolb C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, and constipation) of tapentadol compared with oxycodone hydrochloride. Advances in therapy. 2011; 28: 401-417.
- Buynak R, Shapiro DY, Okamoto A, Van Hove I, Rauschkolb C, Steup A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled Phase III study. Expert opinion on pharmacotherapy. 2010; 11: 1787-1804.
- Hale M, Upmalis D, Okamoto A, Lange C, Rauschkolb C. Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, double-blind study. Curr Med Res Opin. 2009; 25: 1095-1104.
- Vorsanger G, Xiang J, Biondi D, Upmalis D, Delfgaauw J, Allard R, et al. Post hoc analyses of data from a 90-day clinical trial evaluating the tolerability and efficacy of tapentadol immediate release and oxycodone immediate release for the relief of moderate to severe pain in elderly and nonelderly patients. Pain research & management: the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2011; 16: 245-251.
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009; 57: 1331-1346.
- Etropolski MS, Okamoto A, Shapiro DY, Rauschkolb C. Dose conversion between tapentadol immediate and extended release for low back pain. Pain Physician. 2010; 13: 61-70.
- McNaughton EC, Black RA, Weber SE, Butler SF. Assessing Abuse Potential of New Analgesic Medications Following Market Release: An Evaluation of Internet Discussion of Tapentadol Abuse. Pain medicine (Malden, Mass). 2014.
- Butler SF, McNaughton EC, Black RA. Tapentadol Abuse Potential: A Postmarketing Evaluation Using a Sample of Individuals Evaluated for Substance Abuse Treatment. Pain medicine (Malden, Mass). 2014.
- Sanchez Del Aguila MJ, Schenk M, Kern KU, Drost T, Steigerwald I. Practical Considerations for the Use of Tapentadol Prolonged Release for the Management of Severe Chronic Pain. Clin Ther. 2014.
- Davis MP. What is new in neuropathic pain? Support Care Cancer. 2007; 15: 363-372.
- Mercadante S, Porzio G, Ferrera P, Aielli F, Adile C, Ficorella C, et al. Tapentadol in cancer pain management: a prospective open-label study. Curr Med Res Opin. 2012; 28: 1775-1779.
- Kavanagh S, Kwong WJ, Hammond GC, Nelson W, Upmalis D, Yang M. Pain relief and tolerability balance of immediate release tapentadol or oxycodone treatment for patients with moderate to severe osteoarthritis or low back pain. Pain medicine (Malden, Mass). 2012; 13: 1110-1120.
- Steigerwald I, Muller M, Davies A, Samper D, Sabatowski R, Baron R, et al. Effectiveness and safety of tapentadol prolonged release for severe, chronic low back pain with or without a neuropathic pain component: results of an open-label, phase 3b study. Curr Med Res Opin. 2012; 28: 911-936.
- Schwartz S, Etropolski M, Shapiro DY, Okamoto A, Lange R, Haeussler J, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Current medical research and opinion. 2011; 27: 151-162.
- Wild JE, Grond S, Kuperwasser B, Gilbert J, McCann B, Lange B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010; 10: 416-427.
- Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Van Hove I, et al. Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clinical drug investigation. 2010; 30: 489-505.
- Daniels SE, Upmalis D, Okamoto A, Lange C, Haeussler J. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Current medical research and opinion. 2009; 25: 765-776.
- Stegmann JU, Weber H, Steup A, Okamoto A, Upmalis D, Daniels S. The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery. Current medical research and opinion. 2008; 24: 3185-3196.