
Research Article
Chronic Dis Int. 2015;2(1): 1013.
Influence of Periostin on Synovial Fibroblasts in Knee Osteoarthritis
Ishikawa S¹*, Asano K1,2, Kusayanagi H¹, Takashima M1,3, Yoshida N1,3, Yamasaki E¹ and Hisamitsu T¹
1Department of Physiology, School of Medicine, Showa University, Japan
2Division of Physiology, School of Nursing and Rehabilitation Sciences, Showa University, Japan
3Orthopedic Surgery, Showa University, Fujigaoka Hospital, Japan
*Corresponding author: Ishikawa S, Department of Physiology, School of Medicine, Showa University, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan
Received: December 25, 2014; Accepted: April 07, 2015; Published: April 08, 2015
Abstract
Osteoarthritis (OA) is a slowly progressive degenerative joint disease characterized by joint space narrowing, osteophyte formation, and subchondral sclerosis. Despite extensive efforts, actual breakthroughs in the identification of biochemical biomarkers of OA have been limited. Therefore, we investigated in vivo periostin production in knee synovial fluid of OA patients and assessed its influence on the extracellular matrix using synovial fibroblasts in vitro. The study population included 40 OA patients (mean age, 75.3±6.6 years) who were classified according to the Kellgren–Lawrence system. Our results showed that periostin and IL-13 levels were up regulated along with progression of OA. A second round of in vitro experiments using human fibroblast-like synoviocytes suggested that elevated periostin mediated an increase in matrix metalloproteinase-9, which is an important molecule in bone turnover. Taken together, these observations indicate that periostin may be a useful diagnostic and/or prognostic marker of OA.
Keywords: Periostin; knee osteoarthritis; Synovial fibroblast; Bone turnover
Abbreviations
HFLSs: Human Fibroblast-Like Synoviocytes; IL: Interleukin; MMP-9: Matrix Metalloproteinase-9; OA: Osteoarthritis; RPMI: Roswell Park Memorial Institute; TGF-β: Transforming Growth Factor Beta; TIMP-1: Tissue Inhibitor of Metalloproteinase-1
Introduction
The number of patients with painful knee Osteoarthritis (OA) continues to dramatically increase with the aging of society: the estimated number of patients exceeds 25 million in the United States [1] and 8 million in Japan [2]. OA refers to the clinical syndrome of joint pain characterized by varying degrees of functional limitation and impaired quality of life. It is the most common form of arthritis and one of the leading causes of pain and disability worldwide, which most commonly affects the peripheral joints, especially the knee. OA is a complex chronic progressive disease attacked by biological and mechanical factors, and as a result from the anabolic and catabolic imbalance in chondrocytes, subchondral bone and extracellular matrix. The degradation and destruction of collagen caused by Matrix Metalloproteinases (MMPs) are considered as the core factor in the occurrence and development of OA for remodeling disorder [3].
Periostin is a member of the fasciclin family of proteins based on its homology to fasciclin I, which was initially identified in insects [4]. Periostin, also termed osteoblast-specific factor 2, is a 93.3-kDa, secreted, vitamin K-dependent, glutamate-containing matricellular protein, originally isolated from a mouse osteoblast cell line [5,6], with known functions in osteology, tissue repair, oncology, cardiovascular and respiratory systems, and various inflammatory settings. Periostin is regulated by Interleukin (IL)-4, IL-13 or Transforming Growth Factor Beta (TGF-β) produced in inflammation, which has a role for remodeling [7].
Periostin is considered an important structural mediator, balancing appropriate versus inappropriate tissue adaption in response to insult/injury. However, the paracrine effect of periostin in OA-synovial fibroblast biology remains poorly understood. Therefore, we investigated the in vivo production of periostin in synovial fluid of the knee of OA patients. Furthermore, we evaluated expression levels of Matrix Metalloproteinase-9 (MMP-9) and Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) in synovial fluid from the knees of OA patients [8,9] and examined the influence of these proteins as extracellular matrix modulators of periostin using OAsynovial fibroblasts in vitro.
Patients and Methods
Patient selection
Synovial fluid was collected from 40 OA patients (mean age, 75.3±6.6 years; age range, 59-82 years) who underwent medical examinations at Showa University Fujigaoka Hospital (Yokohama, Japan). The Institutional Review Board of our teaching hospital approved the study protocol (authorization number: 2013027) and signed informed consent was obtained from all subjects before study participation. Radiographs were reviewed to determine the size and stage of progression of the OA lesions. Radiographic findings were classified according to the Kellgren–Lawrence system [10], as follows: grade 0 = no radiographic features of OA; grade 1 = doubtful Joint Space Narrowing (JSN) and possible osteophytic lipping; grade 2 = the presence of definite osteophyte and possible JSN on anteroposterior weight-bearing radiograph; grade 3 = multiple osteophyte, definite JSN, sclerosis, possible bony deformities; and grade 4 = large osteophyte, marked JSN, severe sclerosis and definite bony deformities.
Materials
Periostin (recombinant human periostin/OSF-2, CF) was purchased from R&D systems, Inc. (Minneapolis, MN, USA) and dissolved in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich Corporation, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal calf serum (RPMI-FCS; Nihon Bio- Supply Center, Tokyo, Japan), sterilized by passing through 0.2-μm pore filters, and stored at 4°C until use.
Culture of fibroblasts
Human Fibroblast-Like Synoviocytes (HFLSs) were purchased from Cell Applications, Inc. (San Diego, CA, USA) and resuspended at a density of 5×105 cells/mL in RPMI-FCS and cultured with different concentration of periostin in 24-well plates in triplicate. After 24 h, culture supernatants were obtained and stored at -80°C until use.
Collection of synovial fluid
Synovial fluid was collected from the OA patients using an18- gauge needle and then stored at -80°C until assayed.
Assay for biologically active substance
Periostin content in synovial fluid was measured using a commercially available Enzyme-Linked Immuno Sorbent Assay (ELISA) kit (catalog no.: EK-074-41; Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA). Concentration of the inflammatory cytokines IL-13 and TGF-β in synovial fluid were measured using commercially available ELISA test kits (catalog nos.: D1300B and DLAP00; R&D Systems, Inc.) according to the manufacturer’s recommendations. Additionally, MMP-9 and TIMP-1 concentrations in culture supernatants were measured with commercially available ELISA test kits (catalog nos.: RPN2614 and RPN2611; GE Healthcare, Ltd., Chalfont St Giles, Buckinghamshire, UK) according to the manufacturer’s recommendations. The minimum detectable level of these ELISA kits was 0.14 ng/mL for human periostin, 57.4 pg/mL for human IL-13, 3.4 pg/mL for human TGF-β, 0.6 ng/mL for human MMP-9, and 1.25 ng/mL for human TIMP-1.
Statistical analysis
Data are expressed as means ± standard deviations. All assays were repeated three times to ensure reproducibility. Statistical significance between the control and experimental groups was analyzed by oneway analysis of variance followed by the Scheffe test. A probability (p) value <0.05 was considered statistically significant.
Results
Analysis of biologically active substances in synovial fluid
We conducted analysis of synovial fluid samples to identify correlations between OA grade and periostin concentration. As shown in Figure 1, periostin concentration significantly increased with OA. Next, we examined levels of cytokines and periostin production in synovial fluid samples. As shown in Figure 2A, IL-13 levels were significantly increased in the samples along with progression of knee OA. As shown in Figure 2B, no significant difference was found in TGF-β content between each stage of OA.
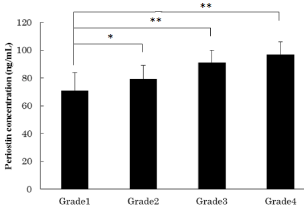
Figure 1: Periostin concentrations in synovial fluid.
This experiment was performed to identify correlations between OA grade
(each group: n=10) and periostin levels in synovial fluid. Asterisks indicate
statistically significant differences (*p<0.05, **p<0.01). Error bars denote ±
standard deviation.
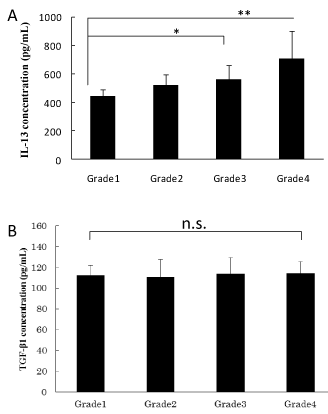
Figure 2: Periostin-related cytokine concentrations in synovial fluid.
Cytokines known to promote periostin production in synovial fluid were
examined.
(A) IL-13 concentrations in synovial fluid (each group: n=10).
(B) TGF-β concentrations in synovial fluid (each group: n=10).
Asterisk indicate statistically significant differences (*p<0.05, **p<0.01, n.s.,
no significant difference). Error bars denote ± standard deviation.
Analysis of biologically active substances in culture supernatant
Next, we evaluated the influence of periostin on MMP-9 and TIMP-1levels, which are OA-associated synovial fibroblast products. As shown in Figure 3, MMP-9 levels increased in a concentrationdependent manner according to periostin levels. However, there were no significant differences in TIMP-1 levels among groups.
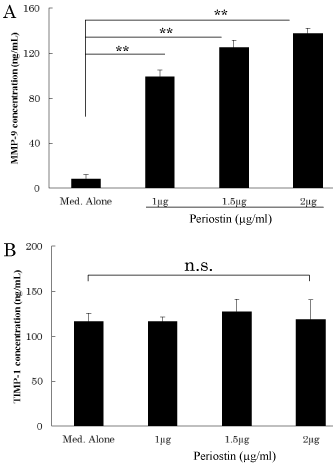
Figure 3: Concentrations of biologically active substances in culture
supernatant.
This experiment was performed to identify correlations between periostin
production and MMP and TIMP levels in synovial fibroblasts.
(A) MMP-9 production by synovial fibroblasts.
(B) TIMP-1 production by synovial fibroblasts.
Asterisks indicate statistically significant differences (**p<0.01, n.s.: no
significant difference). Error bars denote ± standard deviation.
Discussion
OA is a chronic disease that causes cartilaginous degeneration and bone tissue destruction [11]. Symptomatic knee OA is highly prevalent among people aged 50 years and over in most parts of the world and has become a serious healthcare burden worldwide [12,13]. Periostin is transiently up regulated during physiological or pathological changes in OA [14]. Extensive research has helped to elucidate the mechanism of periostin action, yet like OA, its mechanism of action remains unresolved. Therefore, the aim of the present study was to examine the influence of periostin in synovial fluid and OA-associated synovial fibroblasts.
First, periostin levels in synovial fluid obtained from the knees of OA patients was measured, which indicated that periostin levels in synovial fluid increased with progression of osteoclasia in OA. Results of preliminary research indicated high expression levels of periostin in osteoblasts and osteocytes in OA [15]. Based on these findings, we investigated periostin levels according to disease stage based on osteoclastic images, which showed that periostin production increased with progression of OA. Additionally, concentration of the inflammatory cytokine IL-13 increased in synovial fluid along with increasing periostin levels with progression of OA. Tsuchida et al. [16] stated that IL-13 is a potentially useful biomarker to monitor the efficacy of therapeutic treatments for OA. Because IL- 13 was found to be associated with periostin concentration, IL-13- associated up regulation of periostin may contribute to progression of OA. Periostin is a matricellular protein with known functions in osteology, tissue repair, oncology, cardiovascular and respiratory systems, and in various inflammatory settings [14]. The protein is mainly expressed by periosteal osteoblasts and osteocytes [17], and its signaling pathways appear to enhance osteoblast differentiation and bone formation via Wnt/β-catenin signaling [18]. Periostin deficiency alters bone material properties and may adversely impact bone metabolism, which occurs in osteoporosis [19]. Matricellular proteins, such as periostin, interact with cell surface receptors, such as integrins, and are able to bind growth factors as well as structural components of the cellular collagen matrix. In fact, periostin is not only involved in the regulation of bone formation and BMD, but plays a role in the regulation of collagen cross linking [19]. However, the paracrine effect of periostin as a matricellular protein remains poorly understood in OA of the knee. Therefore, we secondly investigated the influence of periostin on MMP-9 expression by fibroblasts in vitro. The MMP-9 concentration increased in a periostin-dependent manner, but TIMP-1 concentration did not change in response to periostin. In vitro studies on HELSs suggested that elevated periostin mediated an increase in MMP-9 concentration. Several studies have reported increased MMP-9 expression levels in knee synovial fluid with progression of OA [20,21]. When MMP-9 coexists with collagenase, it cleaves type I collagen in the bone matrix in concert with other proteases. MMP-9 has been recognized as an important molecule in bone turnover [22-24]. Our results support a correlation between expression of periostin and MMP-9 in synovial fluid.
TIMPs and MMPs activities are mainly regulated through MAP kinases cascade including AP-1 transcription factors [25]. TIMPs are specific inhibitors that bind MMPs in a 1:1 stoichiometric ratio [26,27]. Tissue homeostasis is achieved by a balance of MMP proteolysis to TIMP expression. However MMPs including MMP-9 are activated by multiple factors, including E-26 (Ets) transcription factors, NF-κB, Polyomavirus Enhancer A-binding Protein-3 (PEA3), Specificity Protein 1 (Sp-1), and Serum Amyloid A-Activating Factor (SAF)-1 as well as AP-1 [28].
In addition, it was reported that periostin may activate the NF- κB pathway in fibroblasts synergistically [29]. These reports and our results support that periostin coordinates the MMPs expression by cascade except the MAP kinase. Elevated MMP/TIMP expression ratios are often viewed with arthritis including OA. In the context of disease pathogenesis, MMP and TIMP expression are interpreted with respect to the proteolytic consequences of increased MMP/ TIMP ratios [26,27].
Thus, we speculate that up regulation of periostin in synovial fluid of the knee in OA may confer a cytoprotective effect to promote tissue repair, and propose periostin as a novel biomarker of knee- OA progression. The results in this study showed that periostin had an important role in development of OA. Our findings provide evidence that periostin in OA may reflect tissue changes in this chronic degenerative disease. We showed that periostin affects OAassociated synovial fibroblast, while the articular tissue still contains chondrocytes. It is possible that the chondrocytes contribute to MMP secretion under inflammation. Therefore, we think it is necessary to investigate the influence of periostin towards MMP secretion of either synovial tissue or chondrocytes.
Conclusion
In summary, although it should be noted that the predictive value of periostin in OA has yet to be confirmed in large clinical trials, our findings provide evidence that periostin in OA may reflect tissue changes in this chronic degenerative disease.
References
- 1. Muraki S, Oka H, Akune T, Mabuchi A, En-yo Y, Yoshida M, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage. 2009; 17: 1137-1143.
- 2. Attur M, Belitskaya-Levy I, Oh C, Krasnokutsky S, Greenberg J, Samuels J, et al. Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011; 63: 1908-1917.
- 3. Casagrande D, Stains JP, Murthi AM. Identification of shoulder osteoarthritis biomarkers: comparison between shoulders with and without osteoarthritis. J Shoulder Elbow Surg. 2015; 24: 382-390.
- 4. Ohta N, Ishida A, Kurakami K, Suzuki Y, Kakehata S, Ono J, et al. Expressions and roles of periostin in otolaryngological diseases. Allergol Int. 2014; 63: 171-180.
- 5. Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999; 14: 1239-1249.
- 6. Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993; 294: 271-278.
- 7. Muramatsu K, Asano K, Tokita E, Hisamitsu T. Attenuating effect of a COX-2 inhibitor, meloxicam on the production of matrix meeralloproteinases in vivo. Pharma Medica. 2009; 27: 101-105.
- 8. Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012; 303: G1175-1187.
- 9. Fotopoulos VC, Tzinia A, Tzurbakis M, Kalfakakou V, Levidiotou-Stefanou S, Georgoulis A. Expression levels of matrix metalloproteinase (MMP)-9 and its specific inhibitor TIMP-1, in septic and aseptic arthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012; 20: 1159-1167.
- 10. Kumar D, Souza RB, Singh J, Calixto NE, Nardo L, Link TM, et al. Physical activity and spatial differences in medial knee T1rho and T2 relaxation times in knee osteoarthritis. J Orthop Sports Phys Ther. 2014; 44: 964-972.
- 11. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003; 81: 646-656.
- 12. Busija L, Bridgett L, Williams SR, Osborne RH, Buchbinder R, March L, et al. Osteoarthritis. Best Pract Res Clin Rheumatol. 2010; 24: 757-768.
- 13. Fautrel B, Hilliquin P, Rozenberg S, Allaert FA, Coste P, Leclerc A, et al. Impact of osteoarthritis: results of a nationwide survey of 10,000 patients consulting for OA. Joint Bone Spine. 2005; 72: 235-240.
- 14. Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, et al. The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci. 2014; 71: 1279-1288.
- 15. Chou CH, Wu CC, Song IW, Chuang HP, Lu LS, Chang JH, et al. Genome-wide expression profiles of subchondral bone in osteoarthritis. Arthritis Res Ther. 2013; 15: R190.
- 16. Tsuchida A, Beekhuizen M, T Hart MC, Radstake T, Dhert W, Saris D, et al. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res Ther. 2014; 16: 441.
- 17. Merle B, Bouet G, Rousseau JC, Bertholon C, Garnero P. Periostin and transforming growth factor β-induced protein (TGFβIp) are both expressed by osteoblasts and osteoclasts. Cell Biol Int. 2014; 38: 398-404.
- 18. Kido R, Matsumoto T. Space flight/bedrest immobilization and bone. Signaling cascade induced by mechanical stress. Clin Calcium. 2012; 22: 1837-1843.
- 19. Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008; 2: 9-17.
- 20. Ryu JH, Lee A, Huh MS, Chu J, Kim K, Kim BS, et al. Measurement of MMP Activity in Synovial Fluid in Cases of Osteoarthritis and Acute Inflammatory Conditions of the Knee Joints Using a Fluorogenic Peptide Probe-Immobilized Diagnostic Kit. Theranostics. 2012; 2: 198-206.
- 21. Yang CC, Lin CY, Wang HS, Lyu SR. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PLoS One. 2013; 8: e79662.
- 22. Logar DB, Komadina R, Prezelj J, Ostanek B, Trost Z,Marc J. Expression of bone resorption genes in osteoarthritis and in osteoporosis. J Bone Miner Metab. 2007; 25: 219-225.
- 23. Andersen TL, del Carmen Ovejero M, Kirkegaard T, Lenhard T, Foged NT, Delaisse JM. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 2004; 35: 1107-1119.
- 24. Delaisse JM, Engsig MT, Everts V, del Carmen Ovejero M, Ferreras M, Lund L, et al. Proteinases in bone resorption: obvious and less obvious roles. Clin Chim Acta. 2000; 291: 223-234.
- 25. Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013; 13: 649-665.
- 26. Agarwal S, Misra R, Aggarwal A. Synovial fluid RANKL and matrix metalloproteinase levels in enthesitis related arthritis subtype of juvenile idiopathic arthritis. Rheumatol Int. 2009; 29: 907-911.
- 27. Moran EM, Mullan R, McCormick J, Connolly M, Sullivan O, Fitzgerald O, et al. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, Oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009; 11: R113.
- 28. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda). 2013; 28: 391-403.
- 29. Taniguchi K, Arima K, Masuoka M, Ohta S, Shiraishi H, Ontsuka K, et al. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1a/IL-6 loop. J Invest Dermatol. 2014; 134: 1295-1304.